Abstract
Self-monitoring of blood glucose is now widely recognized as efficacious to enhance and facilitate diabetes management. More than just a means of recording and storing data, some blood glucose meters (BGMs) are now designed with an embedded automated bolus calculator (ABC) with the goal to propose patients recommendations about insulin dosage. The growing literature in this field tends to claim that these new smart BGMs make patient’s life easier and decision making safer. The main purpose of this review is to verify whether BGMs with a built-in ABC indeed improve the willingness and the ability of insulin-treated patients to make adequate therapeutic decisions and positively impact the metabolic control and the quality of life of ABC users. It appears that, as long as the education provided by caregivers remains a top priority, BGMs with a built-in ABC (more than just electronic gadgets) can be regarded as bringing real value to insulin-treated patients with diabetes.
Keywords: Blood glucose meters, Bolus calculator, Diabetes, Hypoglycemia, Self-monitoring, Quality of life
Introduction
Evidence accumulated over past decades convincingly demonstrates that adequate and sustainable metabolic control in people with diabetes results in better micro- and macrovascular outcomes [1–6]. In addition, when this control occurs early on, it may confer a so-called metabolic memory [6]. However, it must be admitted that such achievement remains elusive in a significant proportion of patients, as more than 60% of them are not reaching the advised glycated hemoglobin (HbA1c) goal of <7% [7].
In addition to marked improvements in the medical treatment of diabetes, increasing evidence indicates that regular assessment of blood glucose (BG) levels may help insulin-treated patients to achieve better glycemic control. Self-monitoring of BG (SMBG) contributes to better adjustment of therapies, and helps to reach treat-to-target goals. More importantly, SMBG can act as an educational tool to support patients to better adhere to their treatment [8–16]. SMBG may provide fruitful feedback about how nutrition therapy, physical activity, and medications influence BG levels and alerts about hypo- and hyperglycemia [15]. However, some patients are reluctant to use SMBG due to the pain associated with finger sticks and the cost associated with SMBG supplies. Additionally, some patients are unable to interpret SMBG data and translate it into appropriate therapeutic decisions [15].
One way to improve SMBG acceptance is to provide therapy algorithms to support patients [especially those treated by multiple daily injection (MDI) or continuous subcutaneous insulin infusion (CSII)] when interpreting their values, and to react accordingly. Such tools are already on the market.
Methods
The question raised in this review is whether BG meters (BGMs) with a built-in automated bolus calculator (ABC) represent an added value in patient performance and ability to make the right therapeutic decisions, which may impact both metabolic control and quality of life. A literature search was carried out using Medline and PubMed to select papers where an ABC was used in addition to SMBG. The papers quoted in this review were selected to bring insights into specific questions concerning improved capability of patients to make therapeutic decisions, treatment satisfaction, improved metabolic control and decreased glycemic variability, and reduced fear and rate of hypoglycemia.
Premeal Short-Acting Insulin Dose Calculations
For SMBG to be considered useful, it should be used regularly and correctly at the very least. This goal is achieved when patients and healthcare providers (HCPs) know how to translate the data into appropriate insulin dose adjustments [16]. To help patients make the right decision, software has been developed to calculate doses of short-acting insulin before meals. This type of software has been available in insulin pumps for over 10 years, but was only recently integrated into BGMs and mobile device applications [17]. MDI- and CSII-treated patients are challenged with complex mathematics before deciding on a premeal short-acting insulin dose, at least three-times daily. Patients are supposed to calculate their insulin dose based on the following formula:
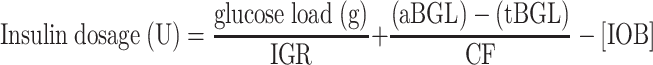 |
Insulin dosage is expressed in units (U). The first part of the equation corresponds to a division between the glucose load and the insulin to glucose (or carbohydrate) ratio (IGR). The glucose load, expressed in grams (g), represents the amount of glucose intended to be consumed, whereas IGR represents how many grams of ingested glucose 1 U of insulin covers. According to Walsh et al., IGR is calculated as: 5.7 × weight (kg)/total daily dose (TDD) [18]. The correction dose, the second part of the equation, is calculated by subtracting the actual blood glucose level (aBGL) from the target blood glucose level (tBGL), divided by the correction factor (CF), also referred to as the insulin sensitivity factor (ISF) in other studies [19–22]. CF represents how much 1 unit of insulin lowers BG and is calculated as: 1,960 mg/dL/TDD, according to Walsh et al. [18]. Insulin on board (IOB), the third part of the equation, corresponds to how much insulin remains theoretically active in the body from the last dose. The IOB amount should be taken into account and subtracted from the correction dose. Of note, King recently proposed rounded formulas for IGR (300/TDD), CF (1,500/TDD), and total basal insulin dosage [TBD = 0.2 × weight (kg)], which gives a slightly higher estimate for bolus insulin and a lower estimate for TBD [23]. The factors, IGR, CF, tBGL, and IOB, should be continuously tailored by the HCP for each patient.
One may easily understand that even well-educated and motivated patients will inevitably consider these calculations time consuming with, on a long-term basis, the risk of mistakes when dealing with so many variables clustered in this equation. In reality, a significant proportion of people with diabetes deal with low literacy and low numeracy skills, which often results in misinterpretation of the recorded information, wrong therapeutic decisions, and low therapeutic compliance, thereby precluding correct metabolic control [24–27]. Thus, one way diabetes device manufacturers sought to facilitate the process of therapeutic decisions was to incorporate an ABC into insulin pumps and BGMs. BGMs with built-in ABCs are mainly designed for MDI-treated patients, as insulin pump users already have calculators integrated into their pump. These “smart” BGMs are also conceived to provide an electronic log book and to store information regarding insulin intake, food consumption, physical activity, and health information. They are sometimes engineered to transmit data to web databases where they are interpreted by specialized software systems [28].
Do BGMs with Built-In ABCs Help Insulin-Treated Patients to Make Appropriate Therapeutic Decisions, While Improving Treatment Satisfaction?
A recently published study reported performances achieved by 205 insulin-treated patients (47.6% with type 1 diabetes and 52.4% with type 2 diabetes) who were asked, based on scenarios of high or normal glucose test results provided by control solutions, to manually calculate mealtime doses of short-acting insulin, followed by the same calculation using a glucose meter with a built-in ABC [29]. Two cohorts of patients, either carbohydrate counters (n = 101) who were using a sophisticated formula, or noncarbohydrate counters (n = 104) who were using a simplified formula, were considered for the study. The results showed that 63% of doses calculated manually by the participants were erroneous, whereas only 6% of incorrect responses were recorded when calculations were performed with an ABC. Eighty-three percent of subjects felt confident about using the ABC and 87% preferred the automated method to the manual calculation. The study was not designed to evaluate the direct impact of ABCs on metabolic control, as the testing was based on control solution values, not on actual blood tests [29].
These results are in line with several previous studies that showed only a small proportion of people with diabetes able to adequately calculate insulin doses, while taking into account glucose load and BG levels. This may explain the low level of treatment compliance and therapeutic inertia over the long duration of diabetes [24, 30, 31]. These data are also reminiscent of those from a 2008 study that demonstrated a benefit of using an ABC in a pediatric population of CSII-treated patients, both in terms of personal satisfaction and improved preprandial and 2-h postprandial BG levels [32]. In one older study [31], an improvement in treatment satisfaction, adherence, and quality of life was shown in 83 adolescents using MDI or CSII. Another study with 49 CSII-treated patients [33] reported better postprandial BG excursions and good confidence in the doses advised by the device (Table 1 [29, 31–33]).
Table 1.
Impact of an ABC on patient satisfaction and quality of life
| References | Study design | Objectives | Results |
|---|---|---|---|
| Sussman et al. [29] | Multicenter study comparing manual versus ABC-assisted calculations of insulin doses | Evaluation of error frequency when insulin dosages calculated either manually or with an ABC | Significant reduction of errors when doses calculated with an ABC (P < 0.001) |
| 205 MDI-treated patients | Improved confidence and preference of using an ABC (P < 0.0001) | ||
| 47.6% type 1 diabetes; 52.4% type 2 diabetes; 104 non-CC; 101 CC | Increased adherence may optimize the use of meal-time insulin | ||
| Glaser et al. [31] | 12-month randomized control trial comparing an IDC device to conventional methods for insulin doses | Impact of an ABC on metabolic control | Higher rate of calculation errors with conventional methods |
| 83 MDI- or CSII-treated type 1 diabetes adolescents | Impact of an ABC on treatment satisfaction, regimen adherence, and quality of life | Improvement in treatment satisfaction, adherence, and quality of life among ABC users | |
| No change in HbA1c among ABC users | |||
| Shashaj et al. [32] | 2-week crossover study comparing an ABC (Bolus Wizard) to conventional methods for insulin doses | Improvement of pre- and postprandial glycemic control | Significant reduction in pre- and 2-h postprandial BG levels and in the number of correction boluses (P < 0.05) |
| 36 CSII-treated type 1 diabetes adolescents | Treatment satisfaction | Higher satisfaction level among ABC users | |
| Gross et al. [33] | 7-day crossover study comparing an ABC to conventional methods for insulin doses | Improvement of postprandial BG levels | Less correction boluses to control postprandial hyperglycemia when using the ABC (P < 0.05) |
| 49 CSII-treated type 1 diabetes subjects | Less supplemental glucose to raise low BG levels when using the ABC (P < 0.05) | ||
| Decreased average deviation of 2-h postprandial BG levels | |||
| ABC easy to use and confidence in advised insulin doses |
ABC automated bolus calculator, BG blood glucose, CC carbohydrate counters, CSII continuous subcutaneous insulin infusion, HbA 1c glycated hemoglobin, IDC insulin dosage calculation, MDI multiple daily injection
Do BGMs with Built-In ABCs Help to Improve Metabolic Control in Insulin-Treated Patients?
A recent study showed a significant improvement in HbA1c values after a 6-month follow-up in 40 consecutive MDI-treated type 1 diabetes patients using an ABC, compared to standard methods (−0.85% vs. −0.007%; P < 0.05) [34]. This ABC-associated improvement in metabolic control was further confirmed in a recent Danish study that reported, after 16 weeks, improved metabolic control (HbA1c −0.7%) and treatment satisfaction in a study group of MDI-treated patients (called the CarbCountABC arm) that received a 3-h educational program, flexible intensive insulin therapy (FIIT) and an ABC as compared to a group that only received FIIT education (HbA1c −0.1%) [35]. The patients in the CarbCountABC arm also experienced less glycemic variability than those in the control group and spent more time in the normal BG range. They also needed less insulin due to more appropriate dosing and less correction of hyperglycemia. The results are in line with those reported in a study of insulin guidance software loaded into a personal data assistant. In this group of 123 MDI-treated adult subjects with type 1 diabetes, there was an improvement in glycemic control, but no change in insulin dose and no weight gain over a 12-month period [36]. In addition, a higher proportion of ABC users reached HbA1c values <7.5%, while remaining within target limit BG levels (70–150 mg/dL). Quite recently, a prospective study performed over a period of 1 year where 30 type 1 diabetic patients were asked to use an ABC, showed a significant decrease in diurnal glucose variability (P < 0.005), and improved HbA1c (P = 0.007) and postprandial BG (P < 0.05) values. The frequency of hypoglycemia was not increased [37].
In a small study (n = 18) published in 2008 comparing ABC users to nonusers, improved metabolic control did not occur [34]. Although mean postprandial BG levels in the ABC users were significantly lowered compared to nonusers, HbA1c values were not significantly improved [38]. Noteworthy, this was an observational study, not a randomized study. Furthermore, the decision whether to use the ABC or not was left to the patient’s discretion. Two other quite recent studies in CSII-treated young type 1 diabetic patients reached the same conclusion. There was an improvement in 2-h postprandial BG levels and glucose variability, but no significant improvement of HbA1c values [39, 40]. The discrepancies between these studies can be understood considering differences in study design and duration (Table 2 [34–40]).
Table 2.
Impact of an ABC on HbA1c values
| References | Study design | Objectives | Results |
|---|---|---|---|
| Maurizi et al. [34] | 3- to 6-month randomized trial comparing patients using an ABC to a control group | Effect of an ABC on metabolic control at 3 and 6 months | At 3 months: nonsignificant improvement in HbA1c levels (−0.61%) |
| 40 consecutive adult type 1 diabetes patients | At 6 months: significant improvement in HbA1c levels (−0.85%; P < 0.05) | ||
| Schmidt et al. [35] (The BolusCal study) | 16-week randomized, controlled, open-label, three-arm parallel trial | Effect of FIIT and an ABC on metabolic control | Significant improvement of HbA1c in the arm of CarbCountABC (P < 0.0001) |
| 51 adult MDI-treated type 1 diabetes patients | Effect of FIIT and an ABC on treatment satisfaction | Significant improvement in treatment satisfaction (DTSQs and DTSQc) in the arm of CarbCountABC (P < 0.001) | |
| Garg et al. [36] | 1-year open-label, randomized, controlled trial | Improvement of HbA1c values | HbA1c improvement by >0.6% at 12 months (P < 0.02) |
| 123 adult type 1 diabetes patients randomized on a 1:1 basis to either an ABC or control group | Higher proportion of ABC users achieving HbA1c <7.5% (P < 0.01) | ||
| Within-target values higher among ABC users | |||
| Without weight gain or changes in insulin dosages | |||
| More severe hypoglycemia among ABC users (P = 0.04) | |||
| Lepore et al. [37] | 1-year prospective observation study | Improvement of HbA1c values | Significant improvement in HbA1c (P < 0.007) |
| 30 CSII-treated type 1 diabetes patients already trained to CH counting and ISF and invited to use an insulin pump with an ABC | Significant improvement of postprandial BG levels (P < 0.05) | ||
| Significant improvement of daily glucose variability (P < 0.005) | |||
| Improved aptitude to calculate proper dose of insulin bolus | |||
| No increased frequency of hypoglycemia | |||
| Klupa et al. [38] | Observational study | Improvement of HbA1c values when using an ABC (in an insulin pump) | No significant improvement in HbA1c values |
| 18 CSII-treated type 1 diabetes patients | Significant decrease in 2-h postprandial values (P < 0.05) | ||
| Enander et al. [39] | 1-year multicenter study involving 40 CSII-treated type 1 diabetes patients in three arms: control, CC, CC plus ABC (in an insulin pump) | Improvement in HbA1c levels and in meal-related BG levels | No difference in HbA1c values |
| Decreased BG levels fluctuations and increase in postmeal BG levels within target (P = 0.014) | |||
| Błazik and Pańkowska [40] | 3-month randomized, open-label study | Changes in HbA1c, postprandial glucose and glucose variability | No change in HbA1c values |
| 48 CSII-treated type 1 diabetic children randomly allocated to a group using diabetics software, and a control group using caloric tables and mental calculations | Significant decrease in 2-h postprandial BG levels and in glucose variability parameters (P < 0.05) |
ABC automated bolus calculator, BG blood glucose, CC carbohydrate counters, CSII continuous subcutaneous insulin infusion, DTSQc and DTSQs diabetes treatment satisfaction questionnaires (change and status versions, respectively), FIIT flexible intensive insulin therapy, HbA 1c glycated hemoglobin, IDC insulin dosage calculation, ISF insulin sensitivity factor, MDI multiple daily injection
Are There Other Advantages to Using BGMs with Built-In ABCs?
Another relevant advantage of using an ABC, besides easier bolus calculation and the likely improvement in metabolic control, or at least in glucose variability, is the reduction in fear and rate of hypoglycemia. This was shown in a recent study that surveyed 1,412 MDI-treated type 1 diabetes patients, of which 588 responded positively [41]. The vast majority of them (76.7%) claimed to use the ABC quite often or always. In 52% of respondents, the fear of hypoglycemia was reduced and most of them (78.8%) reported a high confidence in the insulin dose calculation. In addition, 89.3% reported that bolus calculation was made easy or very easy when the bolus advisor was used. Although reduced fear of hypoglycemia is not a parameter systematically reported in the literature, most of the studies point out patient satisfaction and the improved confidence in insulin dosage when using an ABC. One may therefore presume that the number of patients who skip insulin dosages or commit calculation errors is likely to be lower among those using an ABC compared to those without. It is also more likely that ABC users are more prone to follow insulin titration instructions, which are key in maintaining adequate metabolic control over time.
Even though data are contradictory about hypoglycemic events, with at least one study reporting more severe hypoglycemia among ABC users [36], a recent study by Bergenstal et al. [42], where an insulin support decision algorithm was tested both in subjects with type 1 and type 2 diabetes, rather supports the idea that the frequency of hypoglycemia is lower among patients using an ABC. Thus, less hypoglycemic episodes occurred in the groups using the algorithm despite the higher rate of insulin adjustment. Another important piece of information brought forward by this paper is related to the fact that besides subjects with type 1 diabetes, two groups of subjects with type 2 diabetes (treated either with basal–bolus therapy or twice-daily biphasic insulin) also appeared to show benefit from the computer decision system. This indicates that smart BGMs should not be reserved only for subjects with type 1 diabetes, but rather offered to all MDI-treated patients, regardless of the type of diabetes (Table 3 [41, 42]).
Table 3.
Impact of an ABC on hypoglycemia
| References | Study design | Objectives | Results |
|---|---|---|---|
| Barnard et al. [41] | Survey of 588 MDI-treated type 1 diabetic patients using an ABC | Reduced fear of hypoglycemia | Mild or significant reduction in fear of hypoglycemia in 52% of respondents |
| Prognosis of patients to achieve improved glycemic control | Improvement or significant improvement in the confidence in the insulin dose calculation in 78.8% of respondents | ||
| Bolus calculation made easy or very easy by the ABC in 89.3% of respondents | |||
| Bergenstal et al. [42] | 12-week intervention period (testing the Diabetes Insulin Guidance system following a 4-week baseline run-in period | Primary: fraction of software dosage adjustment approved by the study team | Improvement in average BG levels (P < 0.03) |
| 20 MDI-treated CC type 1 diabetes patients, 20 MDI-treated non-CC type 2 diabetes patients, and six twice-daily biphasic-treated type 2 diabetes patients | Secondary: improved glycemic control | Improvement in mean HbA1c (P < 0.03) | |
| Reduction by 25.2% of hypoglycemic events (P = 0.02) |
ABC automated bolus calculator, CC carbohydrate counters, BG blood glucose, HbA 1c glycated hemoglobin, MDI multiple daily injection
Discussion
Although a consensus is emerging that SMBG increases awareness of diabetes, as well as patient’s empowerment and reassurance, there are also papers reporting worsened quality of life among patients using the SMBG method. Of note, these studies were performed with type 2 diabetes patients who reported increased anxiety and depression, and even obsessive behaviour [43–47]. These feelings were often associated with HCPs lack of interest to actively check and use SMBG results that were otherwise carefully collected by patients. This highlights the pivotal educational role of HCPs about the importance they bring to the interpretation of SMBG results. From this view point, there is little doubt that improved quality of life and patient empowerment resulting from an increased capability to use structured SMBG data and translate it into appropriate therapeutic decisions are two hallmarks of BGMs with built-in ABCs. HCPs in combination with an ABC can educate patients to interpret SMBG values and make appropriate therapeutic decisions to meet metabolic targets. Patients must deal with an overload of variables such as BG levels, ICR, glucose intake, target values, IOB, and physical activity before making insulin dose decisions. When correctly implemented and tailored to each patient, the main advantage of an ABC is to provide appropriate means to quickly make daily decisions, which may contribute to a reduction in hypoglycemic events and metabolic instability. The literature reveals that BGMs with built-in ABCs are used more often and with increased confidence by patients, which could be viewed as a real advantage, especially for those who are confronted to low numeracy and/or low literacy issues. One may reasonably suppose that, because of improved metabolic performances and quality of life, the increasing use of BGMs with built-in ABCs may result in reduced risks of long term micro- and macrovascular complications. This medical improvement may of course have positive economic consequences because of long-term improved HbA1c and a reduced rate of diabetes complications [13].
To be efficacious, smart BGMs that are expected to increase patient adherence to BG monitoring, should be used by patients who form a cohesive team with caregivers. It is of course important that all patients understand the relevance of SMBG and how to use it. Noteworthy, this should be done while always questioning the suggestions delivered by the machine. For instance, a BGM cannot exempt the patient to always predict changes in the physical activity or diet habits in the hours that follow the injection of insulin. Other parameters, such as concurrent illnesses and special medications (e.g., corticoids), must also be taken into account. For all of these reasons, ABC devices should be proposed to insulin-treated patients who already have strong skills to calculate bolus manually. This is the price to pay for them to really understand and critically review dosage recommendations proposed by the software. According to the principle that nothing is definitively fixed in the life of patients and that everything may change overtime, the customization of the device must be continuously proposed and supervised by HCPs who should keep explaining to patients what to do with recorded data. Thus, the key aspect for a successful use of a smart BGM is education. Regular assessments must be foreseen to make sure that recommendations provided by the system are done in a safe way and always in close connection with the patient needs. As long as the principle of ongoing education is kept in mind, one may expect that using an automated decision support algorithm will bring a great relief to patients, especially to those who are carbohydrate counters, because they must deal with more difficult math than those treated with fixed doses of insulin eventually corrected by CF.
The reduced risk of hypoglycemia is certainly another relevant advantage of BGMs with built-in ABCs. The fear of hypoglycemia, just as a low level of education, often precludes the ability of patients to make changes and is a source of therapeutic inertia. Hypoglycemia is one of the main causes of alteration of the patient’s quality of life. The ongoing technical improvements and, for instance, the recent progresses in telemedicine should make us more confident in the ability of support decision software to alert patients in real time about hypoglycemia [48]. This is of course crucial as, most of the time, actual systems do not warn enough patients about hypoglycemia and about the importance of managing it before injecting the next dose of insulin. In addition, they should also encourage patients to inject insulin as soon as BG levels are getting back to normal, a requirement that is often neglected by patients when recovering from hypoglycemia.
Decision softwares were initially designed to help patients dealing with doses of short- or rapid-acting insulin. Unfortunately, fasting values, that are also known to influence HbA1c values [49], are not yet included enough in the calculation process. It should be easy for manufacturers to propose ABC devices able to counsel doses of long-acting insulin based on few days of fasting BG levels. Positive or negative trends in relation with chosen target values would then be recorded and translated into suggestions to increase or decrease the dosages of basal insulin.
Conclusion
BGMs with an embarked ABC are effective motivational tools that should be considered more than just gadgets. They do bring a real value in patient empowerment that is now considered essential in diabetes management. But this statement remains pertinent as long as the principle of ongoing education and the tight cohesion with the HCPs team are preserved.
Acknowledgments
This article was reviewed by Abbott for scientific accuracy before submission. Dr. Colin is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
All authors have nothing to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53 (Erratum in: Lancet. 1999;354:602). [PubMed]
- 2.Diabetes Control and Complications Trial (DCCT) Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial (DCCT) Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–983. doi: 10.2337/diabetes.44.8.968. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group: intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 7.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 8.Strowig SM, Raskin P. Improved glycemic control in intensively treated type 1 diabetic patients using blood glucose meters with storage capability and computer-assisted analyses. Diabetes Care. 1998;21:1694–1698. doi: 10.2337/diacare.21.10.1694. [DOI] [PubMed] [Google Scholar]
- 9.Levine BS, Anderson BJ, Butler DA, Antisdel JE, Brackett J, Laffel LMB. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001;139:197–203. doi: 10.1067/mpd.2001.116283. [DOI] [PubMed] [Google Scholar]
- 10.Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111:1–9. doi: 10.1016/S0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 11.Davidson P, Hebblewhite H, Bode B, Steed RD. Increased frequency of self blood glucose monitoring improves A1c in non-insulin-using persons with diabetes. Diabetes. 2004;53:A101. doi: 10.2337/diabetes.53.5.1403. [DOI] [Google Scholar]
- 12.Haller MJ, Stalvey MS, Silverstein JH. Clinical and laboratory observations: predictors of control of diabetes: monitoring may be the key. J Pediatr. 2004;144:660–661. doi: 10.1016/j.jpeds.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 13.Klonoff DC. Benefits and limitations of self-monitoring of blood glucose. J Diabetes Sci Technol. 2007;1:130–132. doi: 10.1177/193229680700100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, American Diabetes Association Tests of glycemia in diabetes. Diabetes Care. 2004;27(Suppl 1):S91–S93. doi: 10.2337/diacare.27.2007.s91. [DOI] [PubMed] [Google Scholar]
- 15.Blonde L. SMBG and glycemic control: examining the evidence. Medscape website. http://www.medscape.org/viewarticle/532933_2. Accessed 4 Oct 2012.
- 16.American Diabetes Association Standards for medical care in diabetes. Diabetes Care. 2011;35(Suppl 1):S11–S63. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demidowich AP, Lu K, Tamler R, Bloomgarden Z. An evaluation of diabetes self-management applications for Android smartphones. J Telemed Telecare. 2012;18:235–238. doi: 10.1258/jtt.2012.111002. [DOI] [PubMed] [Google Scholar]
- 18.Walsh J, Roberts R, Bailey T. Guidelines for optimal bolus calculator settings in adults. J Diabetes Sci Technol. 2011;5:129–135. doi: 10.1177/193229681100500118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloomgarden ZT. Inpatient diabetes control: approaches to treatment. Diabetes Care. 2004;27:2272–2277. doi: 10.2337/diacare.27.9.2272. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura T. The importance of carbohydrate counting in the treatment of children with diabetes. Pediatr Diabetes. 2007;8(Suppl 6):57–62. doi: 10.1111/j.1399-5448.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 21.Kerr D, Marden S. Numeracy and insulin pump therapy. Diabet Med. 2010;27:730–731. doi: 10.1111/j.1464-5491.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 22.Zisser H, Wagner R, Pleus S, et al. Clinical performance of three bolus calculators in subjects with type 1 diabetes mellitus: a head-to-head-to-head comparison. Diabetes Technol Ther. 2010;12:955–961. doi: 10.1089/dia.2010.0064. [DOI] [PubMed] [Google Scholar]
- 23.King AB. How much do I give? Reevaluation of insulin dosing estimation formulas using continuous glucose monitoring. Endocr Pract. 2010;16:428–432. doi: 10.4158/EP09308.OR. [DOI] [PubMed] [Google Scholar]
- 24.Cavanaugh K, Huizinga MM, Wallston KA, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148:737–746. doi: 10.7326/0003-4819-148-10-200805200-00006. [DOI] [PubMed] [Google Scholar]
- 25.Cavanaugh K, Wallston KA, Gebretsadik T, et al. Addressing literacy and numeracy to improve diabetes care: two randomized controlled trials. Diabetes Care. 2009;32:2149–2155. doi: 10.2337/dc09-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyna VF, Nelson WL, Han PK, Dieckmann NF. How numeracy influences risk comprehension and medical decision making. Psychol Bull. 2009;135:943–973. doi: 10.1037/a0017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavanaugh KL. Health literacy in diabetes care: explanation, evidence and equipment. Diabetes Manag (Lond) 2011;1:191–199. doi: 10.2217/dmt.11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klonoff DC. Improved outcomes from diabetes monitoring: the benefits of better adherence, therapy adjustments, patient education, and telemedicine support. J Diabetes Sci Technol. 2012;6:486–490. doi: 10.1177/193229681200600301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sussman A, Taylor EJ, Patel M, et al. Performance of a glucose meter with a built-in automated bolus calculator versus manual bolus calculation in insulin-using subjects. J Diabetes Sci Technol. 2012;6:339–344. doi: 10.1177/193229681200600218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahola AJ, Mäkimattila S, Saraheimo M, FinnDIANE Study Group et al. Many patients with type 1 diabetes estimate their prandial insulin need inappropriately. J Diabetes. 2010;2:194–202. doi: 10.1111/j.1753-0407.2010.00086.x. [DOI] [PubMed] [Google Scholar]
- 31.Glaser NS, Iden SB, Green-Burgeson D, et al. Benefits of an insulin dosage calculation device for adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17:1641–1651. doi: 10.1515/JPEM.2004.17.12.1641. [DOI] [PubMed] [Google Scholar]
- 32.Shashaj B, Busetto E, Sulli N. Benefits of a bolus calculator in pre- and postprandial glycemic control and meal flexibility of paediatric patients using continuous subcutaneous insulin infusion (CSII) Diabet Med. 2008;25:1036–1042. doi: 10.1111/j.1464-5491.2008.02549.x. [DOI] [PubMed] [Google Scholar]
- 33.Gross TM, Kayne D, King A, Rother C, Juth S. A bolus calculator is an effective means of controlling postprandial glycemia in patients on insulin pump therapy. Diabetes Technol Ther. 2003;5:365–369. doi: 10.1089/152091503765691848. [DOI] [PubMed] [Google Scholar]
- 34.Maurizi AR, Lauria A, Maggi D, et al. A novel insulin unit calculator for the management of type 1 diabetes. Diabetes Technol Ther. 2011;13:425–428. doi: 10.1089/dia.2010.0190. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt S, Meldgaard M, Serifovski N, et al. Use of an automated bolus calculator in MDI-treated type 1 diabetes: the BolusCal Study, a randomized controlled pilot study. Diabetes Care. 2012;35:984–990. doi: 10.2337/dc11-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg SK, Bookout TR, McFann KK, et al. Improved glycemic control in intensively treated adult subjects with type 1 diabetes using insulin guidance software. Diabetes Technol Ther. 2008;10:369–375. doi: 10.1089/dia.2007.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepore G, Dodesini AR, Nosari I, Scaranna C, Corsi A, Trevisan R. Bolus calculator improves long-term metabolic control and reduces glucose variability in pump-treated patients with Type 1 diabetes. Nutr Metab Cardiovasc Dis. 2012;22:e15–e16. doi: 10.1016/j.numecd.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Klupa T, Benbenek-Klupa T, Malecki M, Szalecki M, Sieradzki J. Clinical usefulness of a bolus calculator in maintaining normo-glycaemia in active professional patients with type 1 diabetes treated with continuous subcutaneous insulin infusion. J Int Med Res. 2008;36:1112–1116. doi: 10.1177/147323000803600531. [DOI] [PubMed] [Google Scholar]
- 39.Enander R, Gundevall C, Strömgren A, Chaplin J, Hanas R. Carbohydrate counting with a bolus calculator improves post-prandial blood glucose levels in children and adolescents with type 1 diabetes using insulin pumps. Pediatr Diabetes. 2012;13:545–51. doi:10.1111/j.1399-5448.2012.00883.x. [DOI] [PubMed]
- 40.Błazik M, Pańkowska E. The effect of bolus and food calculator diabetics on glucose variability in children with type 1 diabetes treated with insulin pump: the results of RCT. Pediatr Diabetes. 2012;13:534–539. doi: 10.1111/j.1399-5448.2012.00876.x. [DOI] [PubMed] [Google Scholar]
- 41.Barnard K, Parkin C, Young A, Ashraf M. Use of an automated bolus calculator reduces fear of hypoglycemia and improves confidence in dosage accuracy in T1DM patients treated with multiple daily insulin injections. J Diabetes Sci Technol. 2011;6:144–149. doi: 10.1177/193229681200600117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergenstal RM, Bashan E, McShane M, Johnson M, Hodish I. Can a tool that automates insulin titration be a key to diabetes management? Diabetes Technol Ther. 2012;14:675–682. doi: 10.1089/dia.2011.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335:132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon J, Gray A, Clarke P, Wade A, Neil A, Diabetes Glycemic Education and Monitoring Trial Group Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008;336:1177–1180. doi: 10.1136/bmj.39526.674873.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Kane MJ, Bunting B, Copeland M, ESMON study group Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336:1174–1177. doi: 10.1136/bmj.39534.571644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Kane MJ, Pickup J. Self-monitoring of blood glucose in diabetes: is it worth it? Ann Clin Biochem. 2009;46:273–282. doi: 10.1258/acb.2009.009011. [DOI] [PubMed] [Google Scholar]
- 47.Klonoff DC. New evidence demonstrates that self-monitoring of blood glucose does not improve outcomes in type 2 diabetes-when this practice is not applied properly. J Diabetes Sci Technol. 2008;2:342–348. doi: 10.1177/193229680800200302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klonoff DC, True MW. The missing element of telemedicine for diabetes: decision support software. J Diabetes Sci Technol. 2009;3:996–1001. doi: 10.1177/193229680900300501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006;12:42–46. doi: 10.4158/EP.12.S1.42. [DOI] [PubMed] [Google Scholar]


