Abstract
Both theory and empirical evidence support possible associations between two candidate genetic polymorphisms (SLC6A4 5-HTTLPR l/s and COMT Val158Met – rs4680 variants) and emotion-regulation difficulties. One particular form of emotion-regulation difficulty, distress intolerance, has been measured using a behavioral assessment in youth; data indicate a relationship with poor psychological functioning. No prior study has investigated genetic influences on emotion-regulation difficulties in youth. As part of a larger longitudinal study on adolescent risk behaviors, 218 10-14 year-old youths from the metropolitan Washington, D.C., area completed a measure of distress intolerance, the Behavioral Indicator of Resilience to Distress (BIRD), and provided saliva samples for DNA extraction and genotyping. Results indicate that those with one or two copies of the s allele of the 5-HTTLPR polymorphism were more likely to perform poorly on the task (i.e., choose to quit) than were those homozygous for the l allele. Participants who were Val allele carriers of the COMT Val158Met polymorphism were also more likely to quit the task compared to Met homozygotes. A summative risk allele score was created to combine the two polymorphisms, and each risk allele was associated with a 1.75 fold increased likelihood of quitting the task. Exploratory analyses revealed that emotional abuse moderated the relationship between the 5-HTTLPR and BIRD performance, as well as the genetic risk allele and the BIRD. This is the first investigation of genetic predictors of a behavioral measure of tolerance to distress. Results suggest that distress tolerance is at least partially regulated by specific genetic variants. Implications are discussed.
Keywords: COMT, 5-HTTLPR, Distress Tolerance
Distress intolerance, which is defined as limited persistence in goal-directed activity when experiencing aversive emotional states (Brown, Lejuez, Kahler, Strong, & Zvolensky, 2005), is a key component of emotion regulation, and is associated with internalizing and externalizing psychopathology. Adults with distress intolerance evidence increased rates of Axis II psychopathology (Daughters, Sargeant, Bornovalova, Gratz, & Lejuez, 2008; Gratz, Rosenthal, Tull, Gunderson, & Lejuez, 2006), and eating disorders (Anestis, Selby, Fink, & Joiner, 2007), as well as greater substance abuse severity (Quinn, Brandon, & Copeland, 1996) and more frequent relapse during abstinence attempts (Brown, Lejuez, Kahler, & Strong, 2002; Daughters et al., 2005). Recent study of adolescent populations indicate that distress intolerance is associated with externalizing and internalizing features that contribute to the development of future psychopathology such as anxiety and depressive symptoms, delinquent behavior and substance use (Daughters et al., 2009), risk-taking behaviors (MacPherson et al., 2010), and self harm (Nock & Mendes, 2008). Distress tolerance may also be related to traumatic stress and related phenotypes in certain populations (Danielson, Ruggiero, Daughters, & Lejuez, 2010).
The distress intolerance construct originates with negative reinforcement theory, suggesting that the motivational basis of many maladaptive behaviors is the escape or avoidance of affective distress (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Metcalfe & Mischel, 1999). For instance, when an individual is presented with a significant stressor that results in affective distress (e.g., anxiety), the individual in turn narrows his/her focus on this experience. As such, escaping (and over time, avoiding) this affective state becomes a primary motivational concern. Using substance dependence as an example, an individual in the early stages of an abstinence attempt who is experiencing physical and psychological withdrawal symptoms such as irritability and anxiety may turn to substances to relieve these symptoms, thereby continuing the cycle of addiction. The distress intolerance paradigm was developed to assess this process quantitatively, and involves participant engagement in, and persistence on, a computerized task that gradually increases in difficulty, thereby increasing affective distress. The participant has the option to persist (with some small positive reinforcement available for persisting) or to terminate the task, thereby reducing affective distress in the short-term and rewards in the long-term. From this perspective, the measurement of distress intolerance effectively creates a laboratory paradigm with high internal validity by creating a synthetic controlled situation where affective distress and negative reinforcement processes interact, allowing the participant to make the real-world decision of persisting through or avoiding distress.
The genetic mechanisms underlying the development of distress intolerance are relatively poorly understood. This limited understanding represents a gap in the literature as the discovery of genetic predispositions for the development of distress intolerance may aid in the early identification of at-risk individuals. Of particular interest, findings indicate that alleles at both the promoter region of the serotonin transporter gene (SLC6A4) and COMT Val158Met (rs4680) may be risk factors for emotion-regulation difficulties. Pre-clinical data show evidence for large increases in serotonin (5-HT) efflux in the medial pre-frontal cortex in response to uncontrollable, but not controllable, stress in humans (Bland et al., 2003). Additionally, serotonin is implicated in amygdala inhibition (Rainnie, 1999; Rainnie, Asprodini, & Shinnick-Gallagher, 1991). Anxiety has been found to be inversely related to 5-HT efflux in animal models (Hashimoto, Inoue, & Koyama, 1999). Therefore, examinations of genetic variants that functionally are related to 5-HT activity may be related to stress regulation constructs, such as distress tolerance. The biological activity of 5-HT is regulated by the protein product of the SLC6A4 gene, with the serotonin transporter influencing synaptic availability of 5-HT by sequestering it back into presynaptic neurons. A variable number tandem repeat (VNTR) polymorphism within the SLC6A4 gene, referred to as the 5-HTTLPR polymorphism, has two alleles -- short (s) and long (l). As compared to carriers of the s variant, carriers of the l of the 5-HTTLPR polymorphism transcribe upwards of two times as much mRNA encoding the 5-HT transporter (5-HTT), and when examining protein levels from membrane preparations from lymphoblasts, l homozygotes had 30-40% higher marker binding compared to the membranes from l/s or s/s cells (Lesch et al., 1996). This reduced 5-HTT transcription of the s allele presumably results in less efficient reuptake and increased levels of synaptic serotonin (Lesch, et al., 1996). Converging lines of evidence suggest that the 5-HTTLPR s allele is related to multiple behavioral and neural constructs that share an underlying aspect of negative affect reactivity and regulation, therefore having clear relevance to distress tolerance. For example, in human studies, the s allele has been associated with anxiety and stress related phenotypes. Specifically, the s allele of polymorphism has been associated with greater amygdala responding to fearful faces and other forms of threatening emotional stimuli (Hariri et al., 2002; Munafo, Brown, & Hariri, 2008). In fact, this locus has been found in a meta-analysis to account for up to 10% of the variance in amygdala reactivity to threatening stimuli (Munafo, et al., 2008), underscoring the importance of studying this locus in relation to a behavioral manifestation of tolerance of negative emotion (i.e., distress tolerance). Carriers of the s allele also show decoupling of the amygdala-frontal brain feedback circuit responsible for extinction of fear conditioning (Pezawas et al., 2005). Also relevant to distress tolerance, the s allele has been associated with prolonged cortisol secretion in response to an acute stressor (Gotlib, Joormann, Minor, & Hallmayer, 2008). Taken together, it is possible that stress related increases in 5-HT may be compounded in s’ allele carriers, as they already have elevated synaptic 5-HT, and following, the s’ allele carriers may have poorer distress tolerance.
The COMT enzyme catalyzes the transfer of a methyl group from S-adenosyl-methionine to a hydroxyl group of catecholamines and therefore is involved in the degradation of prefrontal cortex catecholamines (e.g., dopamine, epinephrine, and norepinephrine; Weinshilboum, Otterness, & Szumlanski, 1999). Given the key role catecholamines play in emotion related phenotypes, there is reason to believe that functional variants within the COMT gene may be related to distress tolerance. COMT Val158Met (rs4680) is a functional polymorphism influencing dopaminergic systems. It involves a common valine (Val; high activity) to methionine (Met; low activity) transition that has been associated with a 3-4 fold difference in COMT enzyme activity between homozygotes (Val/Val vs. Met/Met), with heterozygotes showing intermediary enzyme activity (Weinshilboum, et al., 1999).
This polymorphism is a clear candidate for studying in relation to distress tolerance as it has been found to be associated with constructs that are related to distress tolerance (e.g., neural activity to negative stimuli, emotionality during stress); however, there is mixed evidence as to which allele (Met or Val) is associated with these emotion regulation-related phenotypes. Low distress tolerance is related to anxiety disorders (Daughters, et al., 2009), and the Met allele of this polymorphism has been found to be associated with anxiety disorders (Domschke et al., 2004; Enoch, Xu, Ferro, Harris, & Goldman, 2003; McGrath et al., 2004; Olsson et al., 2005; Pooley, Fineberg, & Harrison, 2007; Woo et al., 2004). The Met allele has also been associated with other constructs related to distress tolerance, such as greater pain sensitivity (Diatchenko et al., 2005), and a potentiated startle reflex to aversive stimuli (Montag et al., 2008). Of relevance to the present study of distress tolerance, in a subsample of adolescent girls, homozygous Valallele carriers reported greater maintenance of positive emotions during stress compared to Met158 homozygotes (Waugh, Dearing, Joormann, & Gotlib, 2009). Converging lines of evidence suggest that the Met allele may be related to poorer distress tolerance; however, discrepant findings of this polymorphism have been reported in the literature, and a case could also be made for the Val allele being associated with poorer distress tolerance. For example, the Val carriers have been shown to perform poorer than Met carriers on tasks that are prefrontal cortex dependent, such as the Wisconsin Card Sorting Test (Egan et al., 2001), and Val carriers also display relatively greater activation of the dorsolateral prefrontal cortex (PFC) during the Wisconsin Card Sorting Task performance, suggesting that Val carriers have less efficient PFC function (cf., Tunbridge, Harrison, & Weinberger, 2006), which may also be related to poor emotion regulation.
Taken together, the link between distress intolerance and a variety of important clinical outcomes suggests it may provide an important intermediary phenotype for putative genetic markers having similar clinical associations. In the current study, we address the first step in this line of research by examining whether adolescents who demonstrate diminished persistence during a stressful task (i.e., distress-intolerant youth) are more likely to carry the s allele of the 5-HTTLPR. We also hypothesize a relationship between performance on the task and the COMT Val158Met (rs4680) polymorphism, with distress-intolerant youth being more likely to carry the Met allele, as previous research has found that Met allele youth had lower positive emotions during a stress task than Val carriers (Waugh, et al., 2009).
Method
Subjects
This study employed data from a sample of early adolescents (n = 277) ages 9 to 13 at initial enrollment participating in a larger prospective study of behavioral, environmental, and genetic mechanisms of risk for HIV-related risk behaviors in youth. Follow-up assessments were conducted at yearly intervals for 2 consecutive years and are ongoing with additional assessments planned. The current study presents data from the first annual follow-up assessment (Wave 2) of the study. Permission to conduct research was obtained from the University of Maryland Institutional Review Board (IRB). Saliva specimens were collected using the Oragene® DNA Self-Collection Kits as directed by the manufacturer.
Participants included in the present analyses were youth who completed both the baseline and the first annual follow-up assessments (Waves 1 and 2). Participants were excluded from the present analyses if they did not complete Wave 2 of data collection (n = 33) or were missing data on the distress tolerance task (n = 3) or valid genetic data for both polymorphisms of interest (n = 23). Participants lost to attrition included those who could not be located or did not respond to phone or letter inquiries. Excluded participants did not differ significantly on the main study variables (p’s >.05). The resultant sample of 218 youth had an average age of 12.1 years (SD = .90) and was 44.5% female and 51.4% European-American (EA); see Table 1.
Table 1.
Frequencies for Independent Variables by Genotype, (N=218)
| 5-HTTLPR | COMT Val158Met | Total | |||||
|---|---|---|---|---|---|---|---|
| s/s n=28 |
s/l n=99 |
l/l n=91 |
Met/Met N=43 |
Met/Val N=99 |
Val/Val N=76 |
n=218 | |
| Age (M, SD) | 12.2 (.98) | 12.1 (.83) | 12.0 (.96) | 12.2 (.76) | 12.0 (.92) | 12.0 (.96) | 12.1 (.91) |
| Sex | |||||||
| Female (n, %) | 10 (35.7) | 46 (46.5) | 41 (45.1) | 15 (34.9) | 41 (41.4) | 41 (53.9) | 97 (44.5) |
| Male (n, %) | 18 (64.3) | 53 (53.5) | 50 (54.9) | 28 (65.1) | 58 (58.6) | 35 (46.1) | 121 (55.5) |
| Race/Ethnicity | |||||||
| EA (n, %) | 18 (64.3) | 56 (56.6) | 38 (41.8) | 30 (69.8) | 49 (49.5) | 33 (43.4) | 112 (51.4) |
| AA (n, %) | 4 (14.3) | 30 (30.3) | 37 (40.7) | 9 (20.9) | 30 (30.3) | 32 (42.1) | 71 (32.6) |
| Other (n, %) | 6 (21.4) | 13 (13.1) | 16 (17.6) | 4 (9.3) | 20 (20.2) | 11 (14.5) | 35 (16.1) |
| Family Income | |||||||
| 0-48,000 | 5 (17.9) | 19 (19.2) | 26 (28.6) | 5 (11.6) | 26 (26.3) | 19 (25.0) | 50 (22.9) |
| 48,001-85,000 | 4 (14.3) | 26 (26.3) | 22 (24.2) | 11 (25.6) | 20 (20.2) | 21 (27.6) | 52 (23.9) |
| 85,001-120,000 | 5 (17.9) | 24 (24.2) | 22 (24.2) | 11 (25.6) | 24 (24.2) | 16 (21.1) | 51 (23.4) |
| 120,000-highest | 14 (50.0) | 30 (30.3) | 21 (23.1) | 16 (37.2) | 29 (29.3) | 20 (26.3) | 65 (29.8) |
| Emot Abuse | |||||||
| Yes (n, %) | 10 (35.7) | 54 (54.5) | 48 (52.7) | 20 (46.5) | 54 (54.5) | 38 (50.7) | 105 (48.4) |
| No (n, %) | 17 (60.7) | 45 (45.5) | 43 (47.3) | 23 (53.5) | 45 (45.5) | 37 (49.3) | 112 (51.6) |
Note. Due to some missing data not all n’s add up to 218 (valid percentages presented). Abbreviations, EA is European American, AA is African American, Emot Abuse is emotional abuse.
Measures
Demographics
The parent/guardian completed a basic demographics form for personal information, as well as information about the child. The form included age, sex, race, and annual family income. The annual family income variable was collapsed into quartiles (0-48,000, 48,001-85,000, 85,001-120,000, 120,001-highest). Race was analyzed dummy coded variables (EA versus non-EA; AA versus non-AA, “other” versus EA and AA).
Emotional Abuse
Because previous findings related to childhood maltreatment and subsequent symptoms to distress tolerance (Danielson, et al., 2010), the Childhood Trauma Questionnaire-Short Form (CTQ) emotional abuse subscale was used to assess childhood maltreatment experiences (i.e., “while you were growing up”). The emotional abuse subscale contains 5 items rated on a 5-point scale ranging from 1 (never true) to 5 (very often true). Scores on the subscale are stable over time and show convergent and discriminant validity with other trauma measures (Bernstein et al., 1994). The CTQ has good sensitivity and satisfactory specificity when self-reports are compared with trauma ratings form child welfare records and reports of family members and clinicians (Bernstein, Ahluvalia, Pogge, & Handelsman, 1997). Internal consistency of the emotional abuse subscale in the present sample was good (α = .84). This variable was used continuously in regression analyses, however, a median split was used to create a dichotomous variable for interaction analyses.
Distress Tolerance
The Behavioral Indicator of Resiliency to Distress (BIRD; Lejuez, Daughters, Danielson, & Ruggiero, 2006), developed based on the adult computerized distress tolerance task, the Paced Auditory Serial Addition Task – Computerized Version (PASAT-C; Lejuez, Kahler, & Brown, 2003), was used as a behavioral measure of distress tolerance. Ten numbered boxes (1-10) are presented on a computer screen, as is an image of a bird in a cage. Participants are instructed to use the computer mouse to click a green dot that appears above a numbered box before the green dot jumps to another number. If the numbered box where the green dot is located is successfully clicked before the dot moves, the bird then flies out of its cage, the computer makes a pleasant chirping sound, and a point is earned. Alternatively, if the green dot moves before the youth clicks on the numbered box or the wrong numbered box is clicked, a loud and unpleasant noise is made, the bird remains in its cage, and no point is earned. The first level of the BIRD lasts 3 minutes. This level begins with a 5-second latency between dot presentations and changes this latency based on performance (correct answers reduce the latency by 0.5 seconds whereas incorrect answers or non-responses increase the latency 0.5 seconds); from this level an average latency is calculated to index skill level. The second level is more difficult, beginning with the average latency from the previous level for four minutes and then reducing the latency by half for the final minute making the task extremely difficult (i.e., challenge latency). Following a brief rest period, the final level includes the challenge latency for up to 5 minutes. At all points in the final level, the participant has an escape option. Specifically, the participant is informed prior to beginning the final level that they can click the ‘quit game’ button on the computer screen to end the game, but that the magnitude of the prize earned is dependent on task performance.
Throughout the task, the total number of points earned is visible on the upper right hand corner of the screen. Distress tolerance is indicated by persistence on the final level, and is examined as a categorical variable (whether or not they terminated the task); previous studies of the BIRD indicate that approximately 50% of participants quit the task (Danielson, et al., 2010; Daughters, et al., 2009). Participants were told their overall prize would be improved based on their performance but were given no other specific information about the requirements for each prize (Lejuez, et al., 2003). Previous studies using the BIRD reported no relation between quitting the BIRD task and psychological distress, supporting the contention that the BIRD is measuring an ability to tolerate distress, not just the experience of distress (Danielson, et al., 2010; MacPherson, et al., 2010).
To assess change in negative affect during the task, participants completed the positive and negative affect schedule-children (PANAS-C; Laurent et al., 1999) prior to the first level and after the second level of the task. The PANAS-C consists of positive and negative affect subscales on a 10-point scale ranging from ‘not at all’ to ‘extremely’. Participants rated the degree to which they currently felt excited, mad, interested, frustrated, happy, upset, energetic, embarrassed, proud, and nervous. Consistent with previous research (MacPherson, et al., 2010), distress was indexed based on the composite of mad, frustrated, upset, embarrassed, and nervous.
Collection of DNA Samples
Saliva collected via the Oragene protocol was sent to Yale University for DNA isolation and genotypic analyses.
Genotyping
We examined the functional variable number tandem repeat (VNTR) polymorphism in the 5′ flanking regulatory (promoter) region of the gene (SLC6A4) coding for the serotonin transporter protein. This polymorphism (5-HTTLPR) is generally defined by two common alleles that have been categorized according to the number of repeats they have as ‘long’ (l) (16 repeats) and ‘short’ (s) (14 repeats). Other alleles are observed in some populations. The 5-HTTLPR was classified into s and l alleles as was done in a previous report using data from this sample (Sadeh et al., 2010). Polymerase chain reaction and subsequent size fractionation were performed as described elsewhere (Gelernter, Kranzler, & Cubells, 1997). Allele frequencies did not deviate significantly from Hardy-Weinberg equilibrium expectations in the full sample (χ2=.12, ns) or within the EA (χ2=.12, ns), AA (χ2=.43, ns), and “other” groups (χ2=1.28, ns). Genotyping of the COMT Val158Met (rs4680) single nucleotide polymorphism (SNP) was conducted with a fluorogenic 5′ nuclease assay method (“TaqMan”) using the ABI PRISM 7900 Sequence Detection System (ABI, Foster City, CA, USA). This instrument uses probes with two dyes on opposite ends of a target sequence oligonucleotide to recognize SNP polymorphisms. One dye is a reporter dye, the other a quencher. When the probe is intact, the quencher suppresses fluorescence from the reporter; when the quencher and reporter are separated, the reporter emits a fluorescence signal. When the probe hybridizes exactly to its complement, the 5′ exonuclease activity of Taq polymerase cleaves the probe and allows the signal to be detected. Allele frequencies for rs4680 did not deviate significantly from Hardy-Weinberg equilibrium expectations in the full sample (χ2=1.08, ns), or within the EA (χ2=1.73, ns), AA (χ2=.22, ns) and “other” groups (χ2=1.27, ns). For quality control, 8% of the SLC6A4 genotypes, and all COMT rs4680 genotypes, were repeated; no discordant genotypes were found.
Statistical Analyses
Pre-post affect change was examined via a t-test to determine if the BIRD was psychologically stressful. To examine the relation between BIRD performance and genetic makers three sets of analyses were conducted. First, chi-squared analyses were conducted to test whether the polymorphisms were associated with BIRD performance (quitting or persisting on the task). Second, chi-square analyses were conducted to determine if BIRD performance or genotype differed by key variables (e.g., race/ethnicity). Third, a logistic regression analysis was conducted to determine whether any observed association remained significant after adjusting for sex, age, self-reported race, and history of emotional abuse. Next, an allele risk summative count variable to combine the two polymorphisms was created, and another regression was conducted. These regression analyses were also conducted in the subsample of the EA participants. Last, exploratory analyses were conducted to examine possible gene-environment interactions between the two polymorphisms, the genetic risk sum variable, and emotional abuse; these regressions were also conducted in the sub-sample of EA participants.
Results
Descriptive statistics for independent variables are provided in Table 1. Of those with genotype data for 5-HTTLPR and COMT Val158Met (n=218), 52.3% quit the BIRD task (n=114), and the remaining 47.7% persisted on the task (n=104). The BIRD was psychologically stressful, as indicated by a significant pre-post change in distress (t=6.73, p<.001). However, pre-post change in distress in response to the task was unrelated to whether or not youth quit the BIRD (p >.50), indicating that lower persistence on the task is not simply a measure of increased negative affect. Additionally, genotypes for both polymorphisms were not related to changes in negative affect pre- to post-task (p’s >.6). Self-reported race/ethnicity was not associated with BIRD performance in the EA vs. all others (χ2[1, n=218]=.02, p=.88), AA vs. all others (χ2[1, n=218]=.69, p=.41), or “other” vs. EA and AA group (χ2[1, n=218]=.72, p=.39), nor was age (χ2[4, n=218]=1.77, p=.78), nor was sex (χ2[1, n=218]=.12, p=.73). Emotional abuse was marginally, but not significantly, associated with BIRD performance (χ2[1, n=218]=2.0, p=.10).
As can be seen by the frequencies reported in Table 1, genotype frequencies for the 5-HTTLPR (χ 2[2, n=218]=6.31, p<.05) and COMT (rs4680) (χ 2[2, n=218]=7.89, p<.05) genotype distribution differed between EA and non-EAs. Additionally, the frequencies for 5-HTTLPR (χ 2[2, n=218]=7.21, p<.05) and COMT (4680) differed between AA vs. non-AAs (χ 2[2, n=218]=6.03, p<.05). Therefore correction based on self-reported racial/ethnic status (two dummy coded variables: EA versus non-EA, AA versus non-AA) was used to ensure that population stratification was not a potential cause of false positive findings. Of note, frequencies for these genotypes did not differ between “other” participants and EA and AA participants.
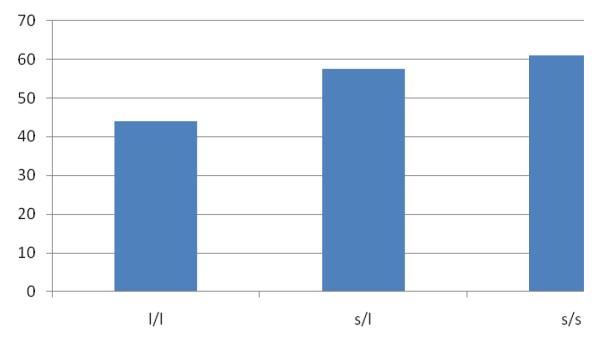
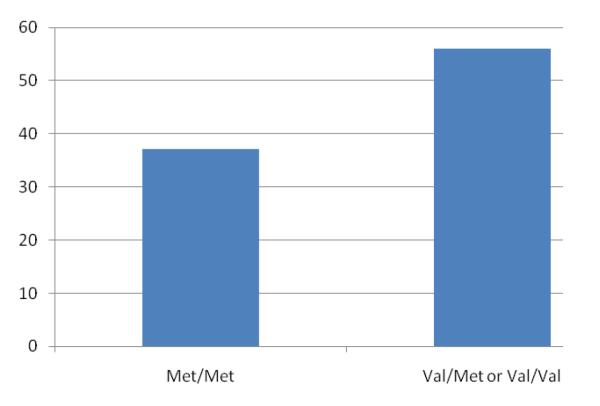
The chi-squared linear-by-linear association test revealed a significant association between the 5-HTTLPR polymorphism and BIRD performance (χ2[1, n=218]=3.92, p<.05; see Figure 1). The additive model of COMT Val158Met was not significant (χ2[1, n=218]=1.59, p=.21); however, when a Val dominance model was examined (consistent with previous studies; Barnett, Jones, Robbins, & Muller, 2007; Dumontheil et al., In press; Hersrud & Stoltenberg, 2009; Reuter & Hennig, 2005), there was a significant relationship between the Val158Met polymorphism and BIRD performance (χ2[1, n=218]=4.86, p<.05; see Figure 2). To test whether the associations between the polymorphisms and BIRD performance remained significant after controlling for sex, age, racial/ethnic status, and emotional abuse history, a logistic regression analysis was conducted. As shown in Table 2, the s allele of the 5-HTTLPR (additive model) remained significantly associated with increased likelihood of quitting the BIRD task (OR=3.86) after controlling for key variables. The relationship between the COMT Val158Met polymorphism and BIRD performance also remained significant after accounting for demographic variables and emotional abuse history (Table 2). The Val allele was associated with 5.08 fold higher likelihood of quitting the task. The epistatic interaction (including the two variants studied) was also examined, and it did not reach significance (p=.06). The analysis was conducted again in the subsample of EA participants, and the findings were in the same direction as they were in the full sample (Table 2), however, neither the 5-HTTLPR (OR=2.14, p=.29) or the COMT Val158Met (OR=2.14, p=.29), perhaps due to decreased power.
Figure 1.
Percent of participants who quit the BIRD by 5-HTTLPR genotype.
Figure 2.
Percent of participants who quit the BIRD by COMT Val158Met genotype.
Table 2.
Final Logistic Regression Analysis of the Association between 5-HTTLPR and COMT genotypes and BIRD Performance in the Full Sample
| BIRD Quit vs. No-Quit |
|||
|---|---|---|---|
| Adjusted Odds | |||
| Variable | Ratio | 95% CI | p |
| Age | .92 | .66-1.30 | .65 |
| Male Sex (vs. female) | .95 | .54-1.67 | .87 |
| European American (vs. non-European American) | 1.55 | .70-3.45 | .28 |
| African American (vs. non-African American) | 1.73 | .75-3.99 | .20 |
| Emotional Abuse Subscale Total | 1.02 | .94-1.01 | .72 |
| 5-HTTLPR (s allele is risk) | 3.86 | 1.37-10.88 | .01 |
| COMT Val158Met (Val/- vs. Met/Met) | 5.08 | 1.54-16.72 | .007 |
| 5-HTTLPR × COMT Val158Met | .34 | .11-1.06 | .06 |
A summary variable was created to combine these two polymorphisms into a summative risk allele count, and this was used in a regression analysis. As shown in Table 3, each risk allele the child had was associated with a 1.75 fold increased likelihood of quitting the task. This analysis was also conducted within the subsample of EA participants, resulting in the same pattern of findings from the full sample (genetic sum score OR=1.76, p=.02).
Table 3.
Final Regression Analysis of the Association of the Genetic Sum Score and BIRD Performance in the Full Sample
| BIRD Quit vs. No-Quit |
|||
|---|---|---|---|
| Adjusted Odds | |||
| Variable | Ratio | 95% CI | p |
| Age | .91 | .65-1.28 | .58 |
| Male Sex (vs. female) | .91 | .52-1.58 | .74 |
| European American (vs. non-European American) | 1.34 | .61-2.92 | .47 |
| African American (vs. non-African American) | 1.69 | .73-3.90 | .22 |
| Emotional Abuse Subscale Total | 1.01 | .94-1.09 | .77 |
| Genetic Sum Score | 1.75 | 1.22-2.51 | .003 |
Exploratory analyses were conducted to examine if the relationship of the polymorphisms to performance on the BIRD is moderated by a history of childhood emotional abuse. Three regressions were conducted in the full sample, and repeated in the EA subsample. The first examined the interaction between the 5-HTTLPR and emotional abuse, and this interaction was significant in the full sample (OR=2.26, p=.02), suggesting that youth with the s/s genotype who have a history of emotional abuse were the most likely to quit the task. In the EA-only subsample this finding was not significant (p=.06). The relationship between the COMT Val158Met (rs4680) polymorphism was not moderated by emotional abuse in the full sample or EA only sub-sample (ps>.41), however, the effect of the genetic risk allele count variable that combines the two polymorphisms was moderated by emotional abuse in the full sample (OR=1.54, p=.04), but not in the EA-only subsample.
Discussion
Within the behavioral genetics literature there has been limited success in the identification of specific genes that predict psychiatric phenotypes, and investigations of potential intermediary phenotypes, such as the present study, may help in this line of research. The present study extends the literature by employing a behavioral task that reliably produced psychological stress, as indicated as pre-post changes in negative affect, to assess a potential intermediary phenotype (“distress tolerance”) and examining the association between distress tolerance and well-characterized genetic polymorphisms, the 5-HTTLPR s/l variants of SLC6A4 and the COMT Val158Met (rs4680) SNP. A genetic sum score suggests that each risk allele of these two polymorphisms is associated with a higher likelihood of quitting the task. This analysis was also significant in the EA-only subsample. Exploratory analyses revealed a significant moderation effect of an environmental variable, childhood emotional abuse, on the relationship between both the 5-HTTLPR and the genetic sum score, on likelihood of quitting the BIRD.
Results of the current study indicate that children who carry the s allele of the serotonin transporter polymorphism are more likely to quit the task compared to those who do not have an s allele. The s allele has been associated with less efficient 5-HT reuptake in previous research, and it is possible that stress related influx of 5-HT may be compounded in those with the s allele. Given the higher levels of synaptic 5-HT associated with the s allele, heightened sensitivity to the environment may result, and in high stress situations this heightened sensitivity may be particularly maladaptive (Koenen et al., 2009). Consistently, the s allele has been linked to lower emotional resilience in young adults (Stein, Campbell-Sills, & Gelernter, 2009). Given that 5-HT is involved in amygdala inhibition (Rainnie, 1999; Rainnie, et al., 1991), under stressful conditions, such as performing the BIRD task, s carriers who have less efficient 5-HT neurotransmission may have altered neural responses, and therefore the possibility of more negative emotion, during stressful situations like BIRD performance. This interpretation is consistent with research suggesting that s allele carriers show greater neural reactivity in the amygdala in response to negative or threatening stimuli (Hairiri et al., 2002; Munafo, et al., 2008). Indeed, this is an empirical question in need of future study. Additionally, our results associating the s allele with poorer distress tolerance are also consistent with previous findings of the s allele being related to prolonged cortisol secretion following a stressor (Gotlib, et al., 2008). Additionally, our results are consistent with studies showing a relationship between this polymorphism and other forms of emotion regulation difficulties (e.g., disinhibition variables including impulsivity, sensation-seeking, and risk-taking; Kreek, Nielsen, RButelman, & LaForge, 2005). Notably, exploratory analyses revealed that for youth who experienced emotional abuse and were s’ allele carriers the risk of quitting was higher. This gene-by-environment (GxE) interaction is consistent with recent meta analyses suggesting that the 5-HTTLPR and traumatic life events interact to produce risk for depression (Sen et al., 2011); however, this is a current subject of much debate.
Carriers of the Val allele of COMT (rs4680) were also more likely to quit the BIRD compared to those without a Val allele in the dominant model. As this polymorphism is functional and associated with more rapid catabolism of synaptic dopamine, Met carriers may have enhanced dopamine signaling compared to Val carriers (Tunbridge, Bannerman, Sharp, & Harrison, 2004). The Val allele has been associated with cognitive inefficiency during various tasks involving cognitive control. For example, the Val allele has been found to be related to poorer performance on tasks that are prefrontal cortex dependent, such as the Wisconsin Card Sorting Test (Egan, et al., 2001), and Val carriers also display relatively greater activation of the dorsolateral prefrontal cortex (PFC) during the Wisconsin Card Sorting Task performance, suggesting that Val carriers have less efficient PFC function (cf., Tunbridge, et al., 2006), perhaps explaining why such individuals were more likely to quit the BIRD task. Also consistent with our findings, recent studies suggest that healthy female Val/Val carriers exhibit greater neural activation in the left amygdala and right temporal pole in response to fearful faces (Kempton et al., 2009), and Val/Val carriers of both sexes display increased immediate reward bias (Boettiger et al., 2007). It is possible that the immediate reinforcement value of quitting the task outweighed the delayed reward to be earned after persisting on the task.
Notably, findings regarding the COMT Val158Met (rs4680) polymorphism are mixed and may be sex dependent (e.g., Coman et al., 2010), and may also be affected by epigenetic processes, such as methylation (Ursini et al., 2011). With regard to population genetics, both alleles are maintained at a high level, and following, each allele may have environmentally specific benefits. Multiple studies have reported the Met allele to be related to greater amygdala and limbic activation in response to emotional stimuli (e.g., Drabant et al., 2006; Hariri, et al., 2002; Smolka et al., 2005), suggesting potential difficulties in emotion regulation. The Met allele has also been associated with greater prefrontal activity during reward anticipation (Yacubian et al., 2007), which is related to BIRD performance in that persistence on the task increases the likelihood of a higher reward. In our data, the Met allele, in a dominate model, was associated with more preservative responses, which is consistent with the association between perseverative errors and this allele on the Wisconsin Card Sorting Test (Egan, et al., 2001), and, although in the context of measurement of distress tolerance this is often perceived positively, it will be informative to examine other measurement forms of distress tolerance. Therefore, although our findings suggest an important role for this polymorphism with respect to distress tolerance, the findings should be interpreted within the context of arguably inconsistent results in the literature, and suggest the importance of further studies including those that examine this variant under a variety of stress conditions.
To best understand the practical utility of the current findings, it is important to consider the potential clinical implications that stem from a youth’s performance on the BIRD. Studies examining the relationship between distress tolerance (i.e., BIRD performance) and externalizing and internalizing problems among youth suggest have provided mixed results, such that low distress tolerance is associated with early alcohol use, risk behavior, internalizing problems, delinquent behavior, and PTSD symptoms only among specific populations (Daughters, et al., 2009) (Danielson, et al., 2010; Daughters, et al., 2009). Specifically, low distress tolerance confers increased risk for alcohol use among Caucasians, internalizing symptoms among females, delinquent behavior among African Americans, and PTSD symptoms among trauma exposed girls. No significant associations were found between distress tolerance and alcohol use among ethnic minority youth, nor were significant associations found between distress tolerance and PTSD avoidance symptoms among trauma-exposed boys (Danielson, et al., 2010). Thus, low distress tolerance may be a key risk factor for the development of internalizing and externalizing psychopathology, the specific manifestation of which is dependent on additional factors. Although continued research is needed to determine how distress tolerance manifests across individuals, our knowledge to date indicates that distress tolerance is a specific target for prevention and early intervention efforts. Additionally, data from the cohort studies in this paper suggest that distress tolerance was related to risk taking behaviors under the context of high risk taking propensity (MacPherson, et al., 2010). The current study extends these findings by potentially further identifying specific features (genotypes) and thus subgroups who may have difficulty tolerating negative affect—and potentially clarifying why previous studies have not yielded consistent findings among distress tolerance problems and negative outcomes among all youth. For example, perhaps youth with the ss genotype have lower distress tolerance and may benefit from bolstering of related coping skills—so as to ultimately reduce risk for substance use problems and/or PTSD following trauma exposure. Future research is needed to test this and other hypotheses.
Limitations and Future Directions
An important caveat about the measurement of distress tolerance in the current study is important to note; specifically the use of a behavioral task versus self-report. Recent findings have indicated that there are inconsistencies in the measurement of this construct with respect to self-report versus behavioral tasks (Levro, Zvolensky, & Bernstein, 2010; Marshall-Berenz, Vujanovic, Bonn-Miller, Bernstein, & Zvolensky, 2010; McHugh et al., 2011) and accordingly speculations that these assessment methods capture different aspects of distress intolerance. It has been suggested that self-report measurement more directly taps perceived sensitivity to distress whereas behavioral measurement assess the inability to persist in a goal-directed behavior when distressed (McHugh et al., 2011). This work comparing methods of measurement has been conducted exclusively with adults thus it is difficult to say with much certainty how these findings apply to youth and consequently whether the generalizability of our findings is limited. Future work would benefit from examining both how these types of measurement relate in youth as well as if the relationships with the genes outlined in the current study vary by method of measurement.
Although this study represents an important extension to the literature, it is not without its limitations. First, ancestral informative markers were not available in this study, and therefore we had to rely on controlling for population stratification was based on self-reported racial/ethnic status. Additionally, when analyzing the EA-only sample the independent polymorphism analyses were only trends, not significant, however, the combined polygenetic risk score analysis was significant in this subsample. Second, the sample size is relatively small for a genetic association study. Third, we focused on single polymorphisms. Future studies should utilize fine mapping of candidate genes to capture the majority of the variation in regions of interest. Additionally, the 5-HTTLPR data only captured the VNTR two alleles (i.e., s and l). Within the L allele there is an A-to-G single nucleotide polymorphism rs25531 (Nakamura, Ueno, Sano, & Tanabe, 2000) that affects the transcriptional activity of the gene, with LG having functional activity comparable to the s allele (Hu et al., 2005). This should be examined in future studies. Additionally, the COMT rs4680 polymorphism was examined in a dominate model given the small sample and low power. Although we believe the BIRD is a promising behavioral measure of distress tolerance, it still needs to be subject to increased research and psychometric validation. Lastly, the present study is only using one wave of data from a longitudinal study. As additional waves of data become available from this project, we will be able to further examine the use of the genetic predictors of performance on this behavioral task of distress tolerance as potential indicators of a latent vulnerability towards future engagement in risk behaviors among youth.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. he manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ananda B. Amstadter, Virginia Commonwealth University
Stacey B. Daughters, University of Maryland--College Park
Laura MacPherson, University of Maryland--College Park.
Elizabeth K. Reynolds, University of Maryland--College Park and Brown Medical School
Carla Kmett Danielson, Medical University of South Carolina.
Frances Wang, Arizona State University.
Marc N. Potenza, Yale University
Joel Gelernter, Yale University School of Medicine.
C. W. Lejuez, University of Maryland--College Park
References
- Anestis MD, Selby EA, Fink EL, Joiner TE. The multifaceted role of distress tolerance in dysregulated eating behaviors. International Journal of Eating Disorders. 2007;40(8):718–726. doi: 10.1002/eat.20471. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulationed: An affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: A meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Molecular Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric populatio. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’Esposito M. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158Val/Val genotype. The Journal of Neuroscience. 2007;27(52):14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111(1):180–185. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse. Clinical Psychology Review. 2005;25:713–733. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman IL, Gnirke MH, Middleton FA, Antshel KM, Fremont W, A.M. H. The effects of gender and catechol O-methyltransferase (COMT) Val108/158Met polymorphism on emotion regulation in velo-cardio-facial syndrome (22q11.2 deletion syndrome): An fMRI study. Neuroimage. 2010;53(3):1043–1050. doi: 10.1016/j.neuroimage.2010.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson CK, Ruggiero KJ, Daughters SB, Lejuez CW. Distress tolerance, risk-taking propensity, and PTSD symptoms in trauma-exposed youth: Pilot study. The Behavior Therapist. 2010;33:28–34. [Google Scholar]
- Daughters SB, Lejuez CW, Bornovalova MA, Kahler CW, Strong DR, Brown RA. Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. Journal of Abnormal Psychology. 2005;114(4):729–734. doi: 10.1037/0021-843X.114.4.729. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Reynolds EK, MacPherson L, Kahler CW, Danielson CK, Zvolensky M. Distress tolerance and early adolescent externalizing and internalizing symptoms: The moderating role of gender and ethnicity. Behaviour Research and Therapy. 2009;47(3):198–205. doi: 10.1016/j.brat.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Sargeant MN, Bornovalova MA, Gratz KL, Lejuez CW. The relationship between distress tolerance and antisocial personality disorder among male inner-city treatment seeking substance users. Journal of Personality Disorders. 2008;22(5):509–524. doi: 10.1521/pedi.2008.22.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Human Molecular Genetics. 2005;14(1):135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Domschke K, Freitag CM, Kuhlenbäumer G, Schirmacher A, Sand P, Nyhuis P. Association of the functional V158M catechol-O-methyl-transferase polymorphism with panic disorder in women. International Journal of Neuropsychopharmacology. 2004;7(2):183–188. doi: 10.1017/S146114570400416X. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS. Catechol O-methyltransferase Val158Met genotype and neural mechanisms related to affective arousal and regulation. Archives of General Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Roggeman C, Ziermans T, Peyrard-Janvid M, Matsson H, Kere J. Influence of the COMT genotype on working memory and brain activity changes during development. Biological Psychiatry. doi: 10.1016/j.biopsych.2011.02.027. In press. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: A role for a functional catechol-O-methyltransferase polymorphism. Psychiatric Genetics. 2003;13(1):33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African-and European-American and Japanese populations and in alcohol-dependent subjects. Human genetics. 1997;101:243. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Rosenthal MZ, Tull MT, Gunderson JG, Lejuez CW. An experimental investigation of emotion dysregulation in borderline personality disorder. Journal of Abnormal Psychology. 2006;115:850–855. doi: 10.1037/0021-843X.115.4.850. [DOI] [PubMed] [Google Scholar]
- Hairiri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D. Serotonin transporter genetic variation and the reponse of the human amygdala. Science. 2002;297:400–402. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Inoue T, Koyama T. Effects of conditioned fear stress on serotonin neurotransmission and freezing behavior in rats. European Journal of Pharmacology. 1999;378:23–30. doi: 10.1016/s0014-2999(99)00441-0. [DOI] [PubMed] [Google Scholar]
- Hersrud SL, Stoltenberg SF. Epistatic interaction between COMT and DAT1 genes on eating behavior: A pilot study. Eating Behavior. 2009;10:131–133. doi: 10.1016/j.eatbeh.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism Clinical and Experimental Research. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Haldane M, Jogia J, Christodoulou T, Powell J, Collier D. The effects of gender and COMT Val158Met polymorphism on fearful facial affect recognition: a fMRI study. The International Journal of Neuropsychopharmacology. 2009;12(3):371–381. doi: 10.1017/S1461145708009395. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Aiello A, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. American Journal of Epidemiology. 2009;169(6):704–711. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, RButelman ER, LaForge KS. Genetic influences on impuslivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience. 2005;8(11):1450–1458. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE, Rudolph KD, Potter KI, Lambert S. A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment. 1999;11:326–338. [Google Scholar]
- Lejuez CW, Daughters SB, Danielson CW, Ruggiero K. The behavioral indicator of resiliency to distress (BIRD) 2006 Unpublished manual. [Google Scholar]
- Lejuez CW, Kahler CW, Brown RA. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor: Implications for behavioral assessment. The Behavior Therapist. 2003;26:290–293. [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levro TM, Zvolensky MJ, Bernstein A. Distress tolerance and psychopathological symptoms and disorders: A review of the empirical literature among adults. Psychological Bulletin. 2010;136:576–600. doi: 10.1037/a0019712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Reynolds EK, Cassidy J, Daughters S, Mayes L, Wang F. Positive and negative reinforcement underlying risk behavior in early adolescents. Prevention Science. 2010;11:331–342. doi: 10.1007/s11121-010-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall-Berenz EC, Vujanovic AA, Bonn-Miller MO, Bernstein A, Zvolensky MJ. Multimethod study of distress tolerance and PTSD symptom severity in a trauma-exposed community sample. Journal of Traumatic Stress. 2010;23:623–630. doi: 10.1002/jts.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M, Kawachi I, Ascherio A, Colditz GA, Hunter DJ, De Vivo I. Association between catechol-O-methyltransferase and phobic anxiety. Am J Psychiatry. 2004;161(9):1703–1705. doi: 10.1176/appi.ajp.161.9.1703. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Daughters SB, Lejuez CW, Murray HW, Hearon BA, Gorka SM. Shared variance among self-report and behavioral measures of distress intolerance. Cognitive Therapy and Research. 2011;35:266–275. doi: 10.1007/s10608-010-9295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J. COMT genetic variation affects fear processing: Psychophysiological evidence. Behavioral Neuroscience. 2008;122(4):901–909. doi: 10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Molecular Psychiatry. 2000;5(1):32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Nock MK, Mendes WB. Physiological arousal, distress tolerance, and social problem-solving deficits among adolescent self-injurers. Journal of Consulting and Clinical Psychology. 2008;76(1):28–38. doi: 10.1037/0022-006X.76.1.28. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Anney RJL, Lotfi-Miri M, Byrnes GB, Williamson R, Patton GC. Association between the COMT Val15 8 Met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatric Genetics. 2005;15(2):109–115. doi: 10.1097/00041444-200506000-00007. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pooley EC, Fineberg N, Harrison PJ. The met15 8 allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: Case-control study and meta-analysis. Molecular Psychiatry. 2007;12(6):556–561. doi: 10.1038/sj.mp.4001951. [DOI] [PubMed] [Google Scholar]
- Quinn EP, Brandon TH, Copeland AL. Is task persistence related to smoking and substance abuse? The application of learned industriousness theory to addictive behaviors. Experimental & Clinical Psychopharmacology. 1996;4(2):186–190. [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82(1):69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol. 1991;66(3):999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- Reuter M, Hennig J. Association of the functional catechol-O-methyltransferase val158met polymorphism with the personality trait of extraversion. NeuroReport. 2005;16:1135–1138. doi: 10.1097/00001756-200507130-00020. [DOI] [PubMed] [Google Scholar]
- Sadeh N, Javdani S, Jackson J, Reynolds EK, Potenza MN, Gelernter J. Serotonin transporter gene associations with psychopathic traits in youth vary as a function of socioeconomic resources. Journal of Abnormal Psychology. 2010;119:604–609. doi: 10.1037/a0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K. Catechol-O-Methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. The Journal of Neuroscience. 2005;24(5):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Campbell-Sills L, Gelernter J. Genetic variation in 5HTTLPR is associated with emotional resilience. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2009;150B(7):900–906. doi: 10.1002/ajmg.b.30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. Journal of Neuorscience. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-Methyltransferase, cognition, and psychosis: Val158Met and beyond. Biological Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Ursini G, Bollati V, Fazio L, Porcelli A, Iacovelli L, Catalani A. Stress-related methylation of the catechol-O-methyltransferase val158 allele predicts human prefrontal cognition and activity. The Journal of Neuroscience. 2011;31(18):6692–6698. doi: 10.1523/JNEUROSCI.6631-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Dearing KF, Joormann J, Gotlib IH. Association between the catechol-O-methyltransferase Val158Met polymorphism and self-perceived social acceptance in adolescent girls. Journal of Child and Adolescent Psychopharmacology. 2009;19(4):395–401. doi: 10.1089/cap.2008.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics:: Catechol O-Methyltransferase, Thiopurine Methyltransferase, and Histamine N-Methyltransferase. Annual Review of Pharmacology and Toxicology. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Woo J, Yoon K, Choi Y, Oh K, Lee Y, Yu B. The association between panic disorder and the L/L genotype of catechol-O methyltransferase. Journal of Psychiatric Research. 2004;38(4):365–370. doi: 10.1016/j.jpsychires.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Glascher J, Kalisch R, Leuenberger B. Gene–gene interaction associated with neural reward sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(19):8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]