Summary
In fungi, as with all walled organisms, cytokinesis followed by septation marks the end of the cell cycle and is essential for cell division and viability. For yeasts, the septal cross-wall comprises a ring and primary septal plate composed of chitin, and a secondary septum thickened with β(1,3)-glucan. In the human pathogen Candida albicans, chitin synthase enzyme Chs1 builds the primary septum that is surrounded by a chitin ring made by Chs3. Here we show that the lethal phenotype induced by repression of CHS1 was abrogated by stress-induced synthesis of alternative and novel septal types synthesized by other chitin synthase enzymes that have never before been implicated in septation. Chs2 and Chs8 formed a functional salvage septum, even in the absence of both Chs1 and Chs3. A second type of salvage septum formed by Chs2 in combination with Chs3 or Chs8 was proximally offset in the mother-bud neck. Chs3 alone or in combination with Chs8 formed a greatly thickened third type of salvage septum. Therefore, cell wall stress induced alternative forms of septation that rescued cell division in the absence of Chs1, demonstrating that fungi have previously unsuspected redundant strategies to enable septation and cell division to be maintained, even under potentially lethal environmental conditions.
Key words: Chitin, Cytokinesis, Fungal cell wall, Septum, Signal transduction, Stress response
Introduction
In fungi and other walled organisms, successful reproduction via cell division requires accurate partitioning of cellular components via cytokinesis and septation. Interdiction of this process represents a major chemotherapeutic opportunity for the control of pathogens and so an understanding of the regulation of cytokinesis and septation is an important endeavour in microbiology. Relative to our understanding of cell cycle regulation, our knowledge of the vital process of septation in fungi is rudimentary. Candida albicans is the major human fungal pathogen and is responsible for a range of superficial mucosal infections and, in immunocompromised patients, can lead to life-threatening systemic infections (Gow et al., 2012). The cell wall of C. albicans is vital for the viability, shape and interactions of this fungus with its host and the synthesis of the lateral cell wall and the cross-wall/septum is essential in both the yeast and filamentous forms of this pleomorphic fungus (Douglas et al., 1997; Munro and Gow, 2001; Klis et al., 2002; Roncero, 2002; Ruiz-Herrera et al., 2002; Denning, 2003; Klis et al., 2006).
The main structural components of fungal cell walls responsible for maintaining cell wall integrity are chitin and β(1,3)-glucan (Sudoh et al., 2000; Munro and Gow, 2001; Klis et al., 2002; Roncero, 2002; Ruiz-Herrera et al., 2002; Klis et al., 2006; Lenardon et al., 2010). During cell division, yeast cells synthesize a chitin-rich septum which acts as a stabilizing barrier between the mother and daughter cell (Cabib et al., 1989; Cabib, 2004). C. albicans has four chitin synthase enzymes – Chs1, Chs2, Chs3 and Chs8 (Munro and Gow, 2001; Lenardon et al., 2010), of which only Chs1 and Chs3 participate in canonical septum formation (Munro et al., 2001), although all four chitin synthases are present at septation sites prior to cytokinesis (Lenardon et al., 2007). Chs1 synthesizes the primary chitinous septal plate and its action is essential for viability under normal growth conditions in both the yeast and hyphal forms (Munro et al., 2001). Chs3 synthesizes a chitin ring at the incipient bud site which subsequently acts as the cellular navigation point for septum formation. Null mutants lacking Chs3 are viable but have very low cell wall chitin contents (Bulawa et al., 1995). Chs2 and Chs8 account for >90% of in vitro chitin synthase activity (Gow et al., 1994; Munro et al., 1998; Munro et al., 2003), are normally non-essential, but are strongly upregulated in response to cell wall stress such as treatment with echinocandin antifungals (Munro et al., 2007; Walker et al., 2008).
The synthesis of the septum and cell wall must be regulated carefully to ensure that, at cytokinesis, dividing cells do not lyse due to the action of chitinase and glucanase enzymes which partially degrade the completed septum allowing cell separation of the mother and daughter cells to take place (Cabib et al., 1989; Cabib, 2004). Chitin synthesis is therefore critical for septation and growth of C. albicans and is essential for viability of all fungi thus far investigated (Bulawa, 1993; Munro and Gow, 1995; Popolo et al., 1997; Munro et al., 2001). Hence, an antifungal drug that specifically targets the essential septum-synthesizing CaChs1 was generated by Roche (RO-09-3143), but this was only fungicidal in a chs2Δ mutant background (Sudoh et al., 2000).
Much of the molecular detail regarding cytokinesis and septation in yeast has been dissected in studies of Saccharomyces cerevisiae. During vegetative growth, the septins form a ring or collar through which the bud emerges (Gladfelter et al., 2001; Versele and Thorner, 2004) and act as a scaffold for cell division, recruiting key proteins to septation sites (Gladfelter et al., 2001). These include ScMyo1, which is recruited shortly after bud emergence and in turn recruits actin, facilitating the assembly of the contractile actomyosin ring (Bi et al., 1998; Lippincott et al., 2001; Oh and Bi, 2011), and ScChs3, which is tethered to the septin ScCdc10 via interactions with ScBni4, ScChs4 and ScGlc7 (DeMarini et al., 1997; Larson et al., 2008) and lays down a chitin ring at the mother-bud neck prior to cytokinesis (Shaw et al., 1991). At mitotic exit, the septins split into two rings, one on either side of the mother-bud neck (Cid et al., 2001; Lippincott et al., 2001), and ScChs2 (equivalent to the C. albicans Chs1 enzyme) is dephosphorylated by ScCdc14 and transported from the endoplasmic reticulum to the mother bud-neck (Zhang et al., 2006; Chin et al., 2012; Meitinger et al., 2010). As the actomyosin ring contracts, it invaginates the plasma membrane and chitin synthesized by ScChs2 forms the primary septum (Silverman et al., 1988; Schmidt et al., 2002; Meitinger et al., 2010). ScChs2 stabilizes the actomysin ring during contraction (VerPlank and Li, 2005). The β-glucan-rich secondary septum is then synthesized.
ScCHS2 was initially reported as an essential gene as in its absence cells failed to form septa or divide (Silverman et al., 1988). Later studies demonstrated that in a different strain background, Scchs2 mutants could form thickened, aberrant septa that lacked a primary septum and which were synthesised by ScChs3. In addition, mutations in ScMYO1, which encodes myosin I which forms the contractile septal ring, also could form thickened aberrant septa (Schmidt et al., 2002). Therefore, cells deficient in ScCHS2 compensated for the loss of a primary septum by activating ScChs3 to form a salvage septum (Bulawa and Osmond, 1990; Shaw et al., 1991). In contrast, strains lacking ScChs3 had thin septa compared to the wild-type and were able to synthesise a primary septum (Shaw et al., 1991). In S. cerevisiae, CHS1 was the only chitin synthase that was unable to compensate for the loss of the other chitin synthase genes and as a result a Scchs2ΔScchs3Δ double mutant was reported as being synthetically lethal (Shaw et al., 1991).
In C. albicans the septins Cdc3, Cdc10, Cdc11 and Cdc12 appear to be organized and function similarly to those of S. cerevisiae (Gladfelter et al., 2001; Gladfelter and Sudbery, 2008). In C. albicans, Chs1 and Chs3 apparently perform the equivalent septation functions to ScChs2 and ScChs3, although Chs1 also appears to be necessary for the stability of the lateral cell wall of both yeasts cells and hyphae (Munro et al., 2001). In filamentous fungi such as Wangiella dermatitidis and Aspergillus nidulans, disruption of individual chitin synthases had no effect on growth suggesting that mechanisms may exist that can compensate for the loss of individual Chs enzymes. Simultaneous disruption of multiple enzymes, however, was either lethal, or resulted in poor growth and the formation of aberrant septa (Motoyama et al., 1994; Motoyama et al., 1997; Ichinomiya et al., 2005; Zheng et al., 2006).
Chitin synthesis is regulated by the Ca2+/calcineurin, PKC and HOG signaling pathways (Munro et al., 2007). Treating C. albicans yeast cells with a combination of CaCl2 and Calcofluor White (CFW) activates the Ca2+/calcineurin and PKC pathways and results in stimulation of chitin synthesis (Munro et al., 2007). This stimulation of chitin synthesis protects C. albicans from an otherwise lethal concentration of caspofungin (Walker et al., 2008; Lee et al., 2012), and is capable of restoring viability to cells, even in the absence of Chs1 and Chs3, through formation of septa, which are dependent on the activity of the remaining chitin synthases (Walker et al., 2008). Here we show that the activation of these stress pathways resulted in the synthesis of as many as three morphologically distinct forms of novel salvage septa, all of which were architecturally distinct from the canonical septum formed under non-stressed conditions. These salvage septa restored the capacity for budding in the absence of the Chs1 enzyme and were synthesized by residual combinations of chitin synthase enzymes that have not previously been implicated in septation. These results suggest that fungi have redundant cytokinesis and septation strategies that enable cell division to continue under conditions of cell wall stress.
Results
Redundant septation strategies in the absence of Chs1
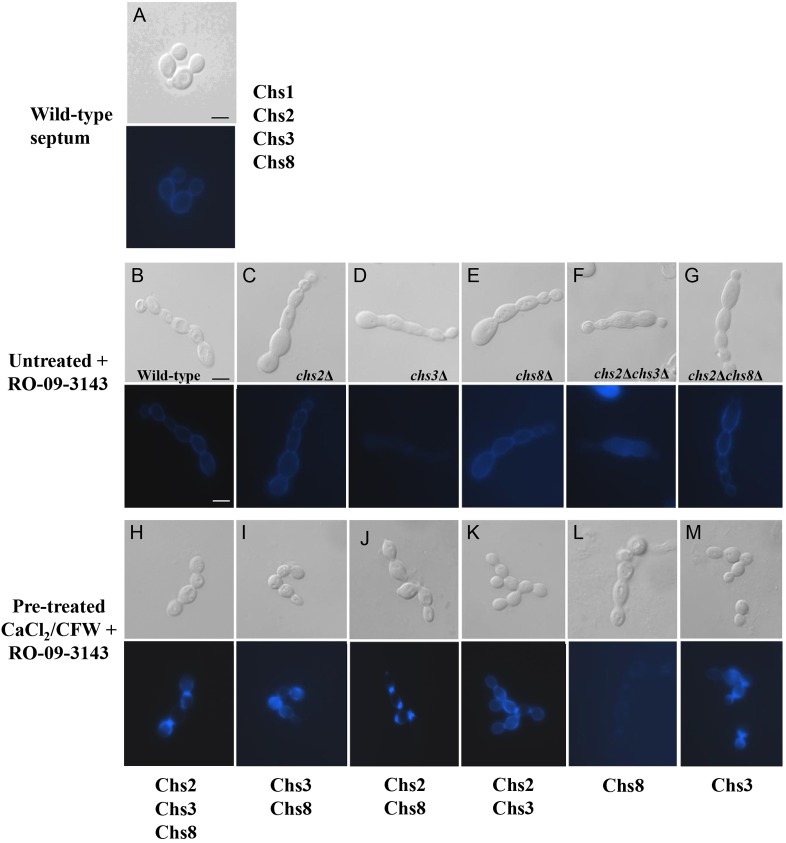
Canonical septation of yeast and hyphal cells of C. albicans involves the activity of two chitin synthases, Chs1 and Chs3, under non-stressed conditions (Fig. 1A), Chs1 is essential for septation and viability (Munro and Gow, 2001). Exposure to RO-09-3143, the Chs1 inhibitor resulted in swollen, septum-less chains of cells that eventually lysed at the growing apex (Fig. 1B–G″) (Sudoh et al., 2000), which phenocopied the effect of the conditional chs1 mutant (Munro et al., 2001). However, if such cells were treated with CaCl2 and CFW, as agonists of the cell wall salvage pathways, novel septa were formed that restored the capacity for cell division (Fig. 1H–K,M). These septa were therefore hypothesized to be fabricated by one or more of the residual three Chs enzymes – Chs2, Chs3 and Chs8.
Fig. 1.
Salvage septa abrogate the chained-cell phenotype that occurs when Chs1 is inhibited. Formation of salvage septa in wild-type and chitin synthase mutants grown in the presence of Chs1 inhibitor RO-09-3143 (10 µM). DIC (top panels) and CFW fluorescent images (bottom panels). (A) Untreated control showing the wild-type septum. (B–M) The chained, septum-less phenotype, as a result of treatment with RO-09-3143 (B–G), was overcome in most cases by pre-growth in YPD with 200 mM CaCl2 and 100 µg/ml CFW (H–M). The active Chs enzymes remaining in each culture are annotated under the lower panels. Scale bars: 2 µm.
To determine which Chs enzymes were involved in the formation of salvage septa, we then examined septum formation in chsΔ mutant strains grown in the presence of the Chs1 inhibitor, RO-09-3143. The chsΔ mutant cells pre-grown either on YPD or YPD with CaCl2 and CFW were exposed to the Chs1 inhibitor for 6 hours. The cells were then examined by fluorescence microscopy after staining with CFW to visualize chitin. The Chs1 inhibitor prevented septum formation resulting in the formation of swollen, septum-less chains of cells (Fig. 1C–G). Pre-growth of the chsΔ mutants in medium containing CaCl2 and CFW for 6 hours prior to addition of the Chs1 inhibitor abrogated chain formation and induced formation of salvage septa which stained brightly with CFW (Fig. 1I–K,M), compared to untreated wild-type septa (Fig. 1A). The exception was the chs2Δchs3Δ mutant, which could not be stimulated by pre-growth with CaCl2 and CFW to synthesize salvage septa in the presence of RO-09-3143 (Fig. 1L). Therefore chitin synthesis by Chs8 alone was not sufficient to form a salvage septum upon stimulation of the cell wall salvage pathways and cell division did not occur.
Morphogenesis of C. albicans salvage septa
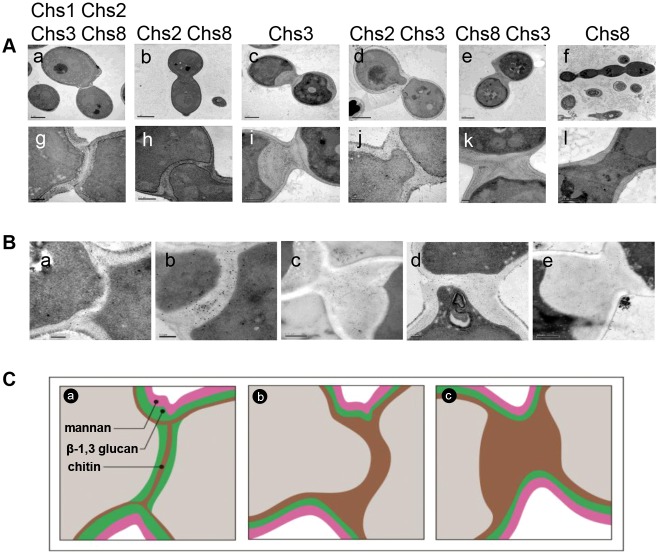
Electron microscopy distinguished four septa types, including three novel salvage septa classes, that were formed using different combinations of Chs enzymes to compensate for repression of CHS1 or inhibition of Chs1 (Fig. 2A–C). In wild-type cells, a trilaminate septum was formed composed of a primary septum synthesized by Chs1 surrounded by a chitin ring synthesized by Chs3 between the two secondary septa composed of β-glucan (Fig. 2Aa,g; Fig. 2Ba,Ca). Treatment of a conditional C. albicans mutant that lacked both Chs1 and Chs3 with CaCl2 and CFW resulted in the formation of a proximally offset salvage septum that appeared unilaminate in transmission electron micrographs (TEMs) (Fig. 2Ab,h,Cb). Cells containing Chs3 alone (chs2Δchs8Δ + Chs1 inhibitor) were capable of forming a thick, amorphous salvage septum, after pre-growth with CaCl2 and CFW (Fig. 2Ac,i). These thickened salvage septa left chitin rich deposits within the cell wall after cell division (Fig. 1I,M; Fig. 2Ac). Similarly, treatment with CaCl2 and CFW stimulated Chs3 in combination with Chs2 or Chs8 to produce different forms of salvage septa (Fig. 2Ad–e,j–k).
Fig. 2.
Multiple types of salvage septa are formed under stress. (A) TEMs of the wild-type septum (Aa,g) and salvage septa (Ab–f,h–l) shown at lower magnification (upper panels) and higher magnification (lower panels). The chsΔ mutants were treated with the Chs1 inhibitor RO-09-3143 (10 µM) after pre-treatment with 0.2 M CaCl2 and 100 µg/ml CFW to stimulate chitin synthesis in the mutants. Salvage septa shown are for the following mutants: chs3Δ (Ab,h), chs2Δchs8Δ (Ac,i), chs8Δ (Ad,j), chs2Δ (Ae,k) and chs2Δchs3Δ (Af,l). The residual active Chs enzymes are shown above each pair of micrographs. TEM images are representative of three independent experiments. (B) Salvage septa are chitin-rich. WGA-colloidal gold-stained TEM sections showing chitin in wild-type septum (Ba), Chs2/Chs8 (chs3Δ + RO-09-3143) salvage septum (Bb), Chs3 (chs2Δ8Δ + RO-09-3143) salvage septum (Bc), Chs2/Chs3 (chs8Δ + RO-09-3143) salvage septum (Bd) and Chs8/Chs3 (chs2Δ + RO-09-3143) salvage septum (Be). (C) Hypothetical model showing the deployment of Chs enzymes in the synthesis of different septal types. The wild-type septum of C. albicans (Ca) and salvage septa (Cb,c) are illustrated: (Cb) The proximally offset septum formed by Chs2/Chs8 and Chs2/Chs3. These two septa were distinguishable by their morphogenesis since only Chs2/Chs8 septa formed by invagination from one side of the cell. (Cc) The thickened septum formed by Chs3 alone and by Chs3/Chs8 together. Scale bars: 0.2 µm (Ag,i–k; Ba,b,d), 0.5 µm (Ah,l; Bc), 1 µm (Aa,c–e), 2 µm (Ab,f).
Staining of TEM sections with wheat germ agglutinin (WGA) conjugated to colloidal gold revealed the salvage septa contained chitin (Fig. 2Bb). Controls for non-specific binding of colloidal gold using Goat anti-Mouse (Fab′)2 conjugated to colloidal gold were negative (not shown). Therefore, the salvage septum formed in the absence of both Chs1 and Chs3 contained chitin that was synthesized by Chs2 and/or Chs8. These septa were proximally offset 0.57±0.16 µm (n = 30) towards the daughter cell. Likewise, the thick salvage septum synthesized in cells containing Chs3 alone or in combination with Chs8 were chitin rich (Fig. 2Bc,e,Cc). The Chs2/Chs3 salvage septa were also offset 0.32±0.09 µm (n = 30) towards the daughter cell and were chitin rich (Fig. 2Bd).
Shadow cast TEM was used to confirm the nature of the chitin present in salvage septa that was synthesized by different Chs enzymes. In C. albicans, the septum of wild-type cells is comprised predominantly of long microfibrils (Gow et al., 1980; Gow and Gooday, 1982; Lenardon et al., 2007) (supplementary material Fig. S1Aa). Chs8 is required for the formation of long microfibrils, whereas Chs3 is required for the synthesis of the short chitin rodlets predominantly seen in the cell walls of wild-type cells and septa of chs8Δ cells (Lenardon et al., 2007). Examination of the chitin in the salvage septa reflected the presence or absence of the particular chitin synthase enzyme required for synthesis of the longer or shorter microfibrils. The salvage septum generated by Chs2/Chs8 was comprised primarily of long microfibrils (supplementary material Fig. S1Ab), whilst the Chs3 septum was formed solely of short chitin rodlets (supplementary material Fig. S1Ac). The septum synthesized by Chs2/Chs3 was predominantly composed of shorter rodlets with some evidence of a small proportion of longer microfibrils interwoven with the rodlets (supplementary material Fig. S1Ad).
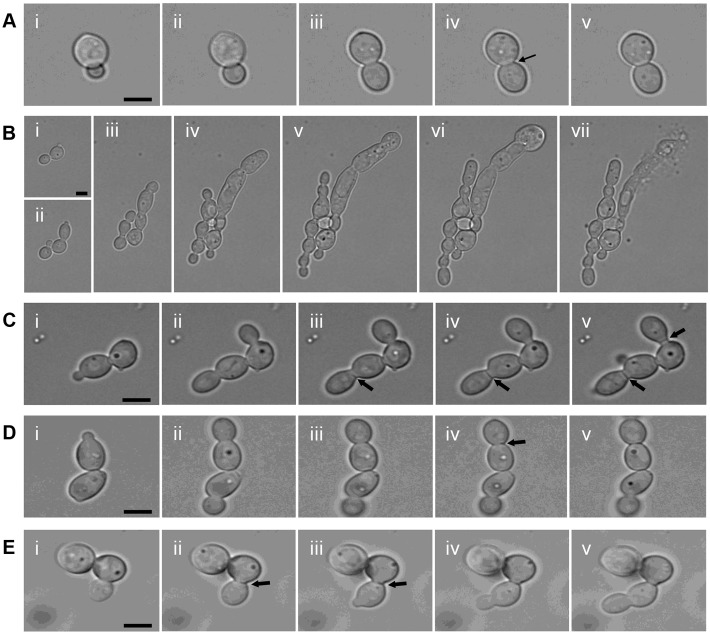
Time lapse imaging revealed that the Chs2/Chs8 synthesized unilaminar septa often formed by extending across the cell from one side (Fig. 3Ciii-v; Fig. 4Ab; supplementary material Movie 1), rather than by centripetal invagination as in wild-type cells (Fig. 3A; Fig. 4Aa). The Chs2/Chs3 salvage septa were also proximally offset but in this case invagination was centripetal (Fig. 3D; Fig. 4Ac; supplementary material Movie 2). No obvious secondary septa were observed in the Chs2/Chs8 and Chs2/Chs3 septa.
Fig. 3.
Dynamics of salvage septum formation. (A,B) Selected time-lapse images illustrating septum formation (arrow) in wild-type cells (Ai–v), and the formation of the septum-less chains of cells undergoing lysis in the conditional chs1chs3Δ mutant under repressing conditions (Bi–vii). (C–E) CaCl2 and CFW was added to cultures to induce cell wall stress responses. The formation of the Chs2/Chs8 (chs3Δ + RO-09-3143) salvage septum is shown in Ci–v, and the Chs2/Chs3 (chs8Δ + RO-09-3143) salvage septum in Di–v. The Chs3 (chs2Δ8Δ + RO-09-3143) salvage septum formation is also shown Ei–v. Arrows indicate positions of septum formation. Scale bars: 2 µm (A,C–E) and 5 µm (B).
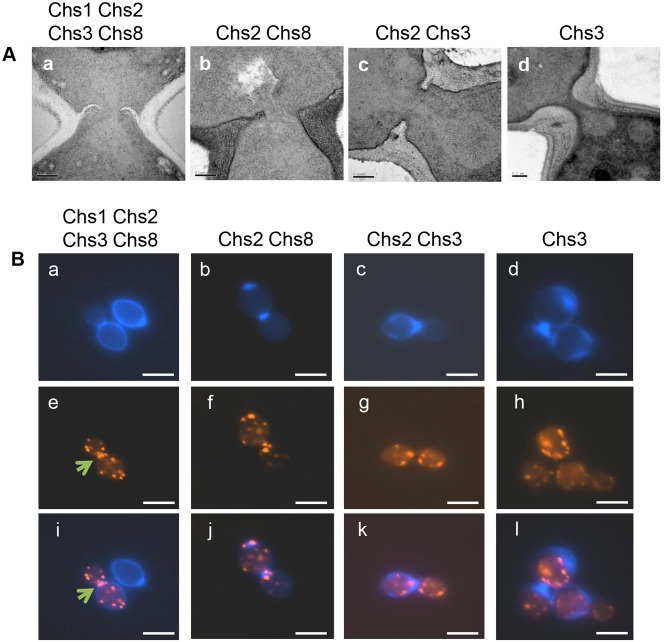
Fig. 4.
Salvage septum formation deploys non-canonical mechanisms. (A) TEMs of early stages of salvage septa formation in the wild-type septum (Aa), Chs2/Chs8 (chs3Δ + RO-09-3143) salvage septum (Ab), Chs2/Chs3 (chs8Δ + RO-09-3143) salvage septum (Ac) and Chs3 (chs2Δ8Δ + RO-09-3143) salvage septum (Ad). (B) Chitin and actin localization in cells forming salvage septa. An actomyosin ring typical of wild-type septa (Be,i, arrows) is not seen in any of the salvage septa (Ba,e,i). Chs2/Chs8 (chs3Δ + RO-09-3143) salvage septum (Bb,f,j), Chs2/Chs3 (chs8Δ + RO-09-3143) salvage septum (Bc,g,k) and Chs3 (chs2Δ8Δ + RO-09-3143) salvage septum (Bd,h,l). CFW-staining of chitin (Ba–d), rhodamine-phalloidin staining of actin (Be–h), and overlay of CFW and rhodamine-phalloidin images (Bi–l). Scale bars:1 µm (B), 0.2 µm (A).
The greatly thickened chitin-rich salvage septa made by Chs3 and Chs3/Chs8 formed at the bud-mother cell neck (Fig. 4Ad). These again apparently lacked secondary septa and the total chitin content per cell in these cells was over double that of normal wild-type yeast cells (supplementary material Fig. S1B). Therefore, in addition to the canonical Chs1/Chs3 septum, C. albicans can synthesize three salvage septa types using different permutations of chitin synthases: (i) Chs2/Chs8; (ii) Chs2/Chs3 or (iii) Chs3 (alone) or Chs3/Chs8 (Fig. 2C).
We next examined the distribution of actin in septating cells. Unlike in wild-type cells (Fig. 4Ba,e,i), no contractile ring of actin could be resolved in cells generating salvage septa (Fig. 4Bb–d,f–h,j–l). In cells containing Chs2/Chs8 salvage septa, a bright spot of actin was observed predominantly to one side of the bud neck (Fig. 4Bb,f,j). In the Chs2/Chs3 salvage septa, that exhibited centripetal invagination actin appeared as a central punctuate patch in the septal region (Fig. 4Bc,g,k). Regions with focussed deposits of chitin (for example in the Chs3 salvage septum) exhibited punctuate actin plaque staining along the periphery of the chitin-rich zones (Fig. 4Bd,h,l), perhaps indicating extensive endocytosis and membrane turnover in these regions.
Altered localization of Chs3-YFP in the presence of chitin synthase inhibitors
The salvage septa that were formed were frequently abnormally positioned in the neck region of buds (above). Therefore, we used time lapse video-microscopy to determine whether inhibition of different classes of chitin synthase affected the localization of Chs3-YFP. RO-09-3143 was used to inhibit Chs1, and nikkomycin Z to preferentially inhibit the class I enzymes, Chs2 and Chs8 (Gaughran et al., 1994; Munro and Gow, 1995; Munro et al., 1998).
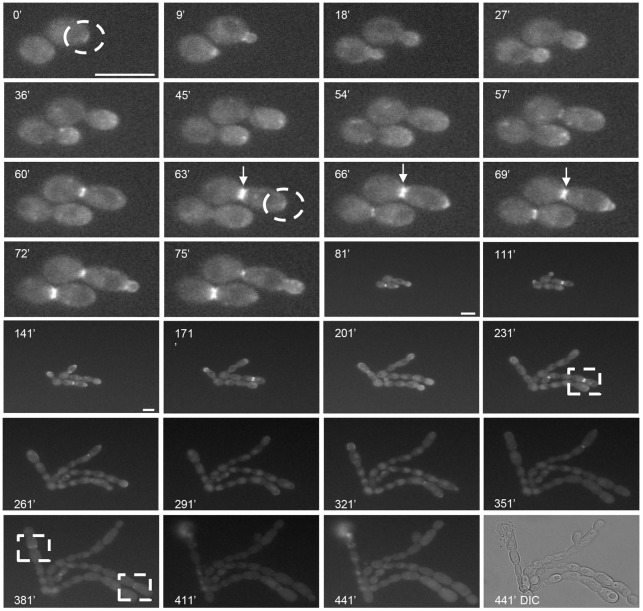
Chs3-YFP normally localizes to the tip of growing buds and is then observed as a single ring at the site of septum formation prior to cytokinesis (Lenardon et al., 2007; Lenardon et al., 2010). After a short lag-phase, live cells expressing Chs3-YFP formed long chains of cells in the presence of the Chs1 inhibitor. New buds emerged in a polarized fashion and growth stasis occurred 7 hours after the emergence of the first bud [Fig. 5, 441 minutes; supplementary material Movie 3 (DIC) and Movie 4 (YFP)]. Instead of being localized at the very tips of emerging buds, Chs3-YFP was observed as a crescent on the budding mother cell wall (Fig. 5, 0 minutes, 63 minutes). During the first few cell cycles, Chs3-YFP formed a double ring at the site where septa formation normally occurred (Fig. 5, 63–69 minutes), and in later cell cycles, Chs3-YFP formed a large ring that did not close (Fig. 5, 231 minutes, 381 minutes). Therefore, Chs3 was repositioned when Chs1 was inhibited and functional septa could not be formed.
Fig. 5.
Chs3-YFP is mislocalized when Chs1 is inhibited. Selected frames from a time-lapse movie (supplementary material Movie 4) showing the localization of Chs3-YFP in cells grown in the presence of the Chs1 inhibitor RO-09-3143 (10 µM). Dashed circles indicate Chs3-YFP localized as a crescent on the budding mother cell wall. Arrows indicate Chs3-YFP in double ring conformation at sites where septum formation would normally occur. Dashed squares indicate Chs3-YFP forming a large ring that did not close. The last frame shows the DIC image at 441 minutes (supplementary material Movie 3). Scale bars: 10 µm.
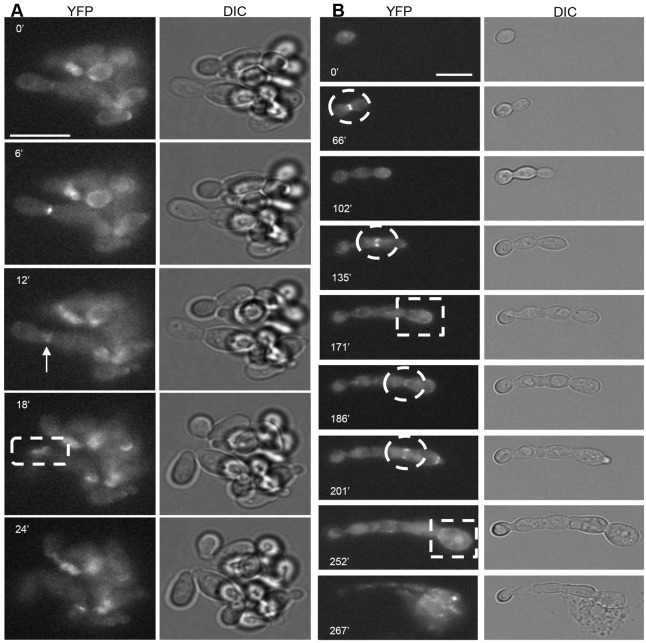
Cells growing in the presence of nikkomycin Z budded in an unusual manner [Fig. 6A; supplementary material Movie 5 (DIC) and Movie 6 (YFP)]. Chs3-YFP formed a double ring in the centre of an elongated bud neck (Fig. 6A, 12 minutes). Buds could be observed during cytokinesis where one Chs3-YFP ring was visible at the poles of the cells (Fig. 6A, 18 minutes). Cells did not lyse but continued to grow in this fashion for 8 hours or more, even in the presence of nikkomycin Z, indicating that Chs2 and Chs8 were required to correctly position Chs3 as septa form, but that functional septa were formed despite the altered localization of Chs3.
Fig. 6.
Chs3-YFP is mislocalized in the presence of chitin synthase inhibitors. (A) The localization of Chs3-YFP (left; supplementary material Movie 6) and corresponding DIC images (right; supplementary material Movie 5) of cells grown in the presence of nikkomycin Z (10 µM). The arrow indicates Chs3-YFP forming a double ring in the centre of an elongated mother-bud neck. The dashed squares indicate Chs3-YFP rings visible at each pole of the split cells. (B) The localization of Chs3-YFP (left; supplementary material Movie 8) and corresponding DIC images (right; supplementary material Movie 7) of cells grown in the presence of RO-09-3143 (10 µM) and nikkomycin Z (10 µM). Dashed circles indicate Chs3-YFP localized to sites of unclosed septa. Dashed squares indicate Chs3-YFP localized to the entire cell surface. In all panels, the time (minutes) since the emergence of the first bud is indicated on each frame. Scale bars: 10 µm.
Cells growing in the presence of both the Chs1 inhibitor and nikkomycin Z were unable to lay down septa and formed short chains of cells that ballooned and lysed after 4 hours [Fig. 6B; supplementary material Movie 7 (DIC) and Movie 8 (YFP)]. As in the presence of the Chs1 inhibitor alone, Chs3-YFP was observed a as crescent as the bud emerged (Fig. 6B, 201 minutes) and formed a ring at the site of unclosed early septa (Fig. 6B, 66 minutes, 135 minutes, 186–201 minutes). Although Chs3-YFP became localized to the entire surface in later cell cycles (Fig. 6B, 171 minutes, 252 minutes), the integrity of the wall was not maintained and cell lysis was frequent (Fig. 6B, 267 minutes). Therefore, the normal localization of Chs3-YFP was again altered and functional septa were not formed when Chs1, Chs2 and Chs8 were all inhibited simultaneously.
Salvage septa restores the capacity for cell division
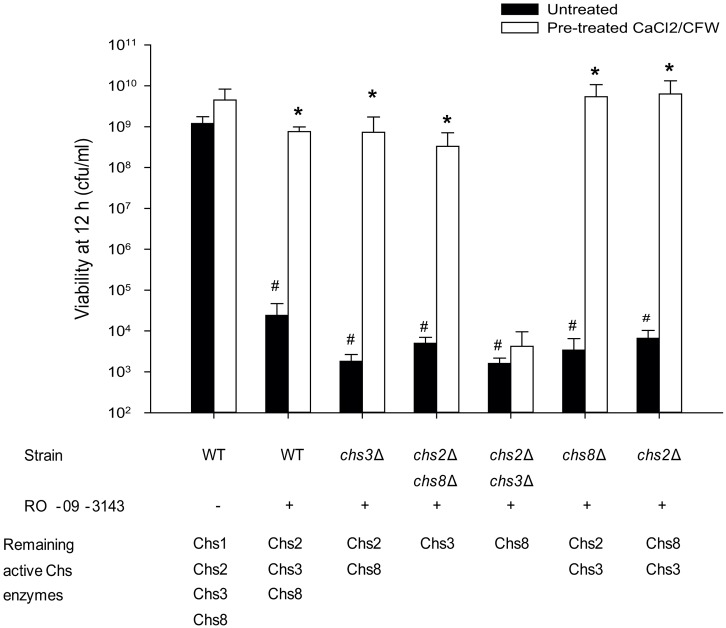
Finally we determined whether the various salvage septa observed were able to restore the capacity for growth. The untreated and pre-treated wild-type and chs mutants were grown in YPD in the presence of the Chs1 inhibitor and after 12 hours samples were removed, washed and dilutions plated on YPD plates and incubated overnight. There was a significant reduction in the number of viable cells after 12 hours in the presence of the Chs1 inhibitor in all genetic backgrounds (Fig. 7). Pre-treatment of the wild-type or chs mutant strains with CaCl2 and CFW had no effect on growth or viability (Fig. 7). Therefore, cells pre-treated with CaCl2 and CFW synthesized salvage septa that maintained their viability and the ability to undergo cell division when exposed to Chs1 inhibitor (Fig. 7). In contrast, CaCl2 and CFW pre-treatment could not rescue the chs2Δchs3Δ mutant strain from the effects of the Chs1 inhibitor implying that Chs8 was not sufficient on its own to rescue growth when Chs1 function was compromised (Fig. 7).
Fig. 7.
Stimulation of chitin synthesis with CaCl2 and CFW induces multiple forms of salvage septa that restore the capacity for growth in the presence of the Chs1 inhibitor RO-09-3143. Wild-type (WT) and chsΔ mutants were pre-incubated with 0.2 M CaCl2 and 100 µg/ml CFW for 24 hours prior to the addition of 10 µM RO-09-3143. The wild type was also grown without addition of RO-09-3143, showing that pre-treatment with CaCl2 and CFW had no effect on growth. Asterisks indicate significant differences (t-test, P<0.05) compared with untreated controls in the same genetic background. Hash symbol indicates significant difference to wild-type cells in the same growth conditions. Error bars indicate s.d. (n = 3).
Discussion
In wild-type cells, the primary septum is synthesized by Chs1 and is built upon a chitin ring synthesized by Chs3 that is scaffolded by a protein complex that assembles on the septin rings. The Chs1 chitin synthase has been considered to be essential for septation and cell division under most growth conditions (Munro and Gow, 2001). Here we show that activation of cell wall salvage pathways can induce the formation of a number of alternative types of salvage septa that restores the capacity for cell division even in the absence of Chs1. The salvage septum can be fabricated from chitin synthases, including Chs2 and Chs8 that have been shown to localize to septation sites (Lenardon et al., 2007) but have thus far not been functionally implicated in this process.
The salvage septa synthesized by Chs2/Chs8 in live cells was unusual in so far as: (a) it was located on the daughter side of the mother bud neck and not at the narrowest part of the bud-neck; (b) actin was abnormally localized at the site of septation and did not form a normal actomyosin ring and (c) septation proceeded from one side of the mother-bud neck to the other rather than by centripetal invagination. This suggests that Chs2/Chs8 do not associate with a normal actomyosin ring in the absence of Chs1 or Chs3. In S. cerevisiae a similar asymmetrical septum is formed in mutants involved in the mitotic-exit network that coordinates mitotic exit and cytokinesis by controlling the localization of components of septum formation including ScChs2 (Meitinger et al., 2010).
The Chs2/Chs3 salvage septum was similar in morphology to the Chs2/Chs8 septum but differed in exhibiting centripetal invagination and the actomyosin ring appeared to be positioned centrally in the mother bud neck. In the absence of Chs1, Chs2 and Chs8 and upon stimulation of chitin synthesis, Chs3 alone synthesized a thick chitinous plug with actin localized to the periphery of the plug. Therefore salvage septum formation involves departures from canonical mechanisms that normally couple chitin synthesis to actomyosin ring function.
Salvage septa of a similar appearance to those synthesized in C. albicans by Chs3 (alone or with Chs8) after treatment with CaCl2 and CFW were observed in Scchs2Δ, Scchs1Δchs2Δ and Scmyo1Δ mutants (Bulawa and Osmond, 1990; Shaw et al., 1991; Schmidt et al., 2002). The PKC cell wall integrity pathway is activated in both Scchs2Δ and Scmyo1Δ mutants (Rodríguez-Quiñones and Rodríguez-Medina, 2009), and chitin synthesized by ScChs3 is necessary for the functionality of this salvage septum (Schmidt et al., 2002; Cabib and Schmidt, 2003). However, Scmyo1Δ mutants have also been shown to induce alternative mechanisms which are capable of restoring growth and cytokinesis. This abrogation of cell division correlated with an increased copy number of specific regulatory genes as a result of induced aneuploidy (Rancati et al., 2008). The salvage septa synthesized by ScChs3 also formed in an aberrant manner and appeared to be the result of increased and unregulated deposition of chitin at the bud neck, which is sufficient to close and cauterise the channel between the mother and daughter cell (Shaw et al., 1991; Schmidt et al., 2002; Cabib, 2004).
Observing the localization of Chs3-YFP in the presence of chitin synthase inhibitors provided further insights into the mechanisms of septum formation. In the presence of the Chs1 inhibitor, Chs3-YFP formed a crescent, probably part of a larger ring as the bud emerged instead of being tightly localized to the tip of the emerging bud. Later in the cell cycle, it was observed as an open, double ring at the site of septum formation. This localization mirrors that of the septin collar and split rings. However, in this case, cytokinesis did not occur. In the presence of nikkomycin Z, a primary septum was formed and Chs3-YFP was visible as two large, abnormal open rings on either side of an elongated mother-bud neck that were visible on both the mother and daughter cells after cell separation. This indicates that the inhibition of Chs2 or Chs8 affects the normal localization of Chs3 during septum formation or that nikkomycin Z causes mislocalization of Chs3-YFP directly. Nikkomycin Z can inhibit class IV chitin synthases (ScChs3, Chs3), albeit with a Ki six times higher than for the class I chitin synthases (ScChs1, Chs2 and Chs8) (Gaughran et al., 1994).
The Chs1 inhibitor RO-09-3143 is fungistatic on its own, but is fungicidal in a chs2Δ mutant background (Sudoh et al., 2000). We show here that this inhibitor is also not lethal in the chs2Δ mutant if chitin synthesis was activated by pre-treatment with CaCl2 and CFW. However, combinations of RO-09-3143 and nikkomycin Z were lethal and nikkomycin Z prevented CaCl2/CFW abrogation of growth and viability in the absence of Chs1. Therefore although redundancy exists within the repertoire of C. albicans chitin synthases that can be used for septation, chitin synthesis was ultimately essential for viability of C. albicans under all conditions tested.
In conclusion, we show that priming cells to activate chitin synthesis can compensate for the loss of the Chs1 through formation of novel forms of salvage septa which are capable of restoring viability and cell division in C. albicans. This demonstrates remarkable compensation in the way in which the essential process of septum formation is regulated in this fungus and suggests that fungi may have evolved redundant mechanisms to enable cell division to occur under conditions of severe cell wall stress.
Materials and Methods
Strains, media and growth conditions
C. albicans strains used in this study are listed in supplementary material Table S1. Strains were maintained on solid YPD medium [1% (w/v) yeast extract, 2% (w/v) mycological peptone, 2% (w/v) glucose, 2% (w/v) agar] and yeast cell cultures were grown at 30°C in YPD with shaking at 200 rpm. The MRP1p-CHS1/chs1Δ conditional mutant was maintained in SMal medium [1% (w/v) yeast extract, 2% (w/v) mycological peptone, 2% (w/v) maltose] that induces CHS1 expression and grown in YPD to repress expression of CHS1 (Munro et al., 2001).
Growth and viability in the presence of chitin synthase inhibitors
Cells were grown in YPD supplemented with the chitin synthase inhibitors nikkomycin Z which preferentially inhibits the class I enzymes Chs2 and Chs8 (Munro and Gow, 1995) and RO-09-3143 which inhibits Chs1 (Sudoh et al., 2000). Nikkomycin Z (Sigma-Aldrich, Dorset, UK) at 10 µM was dissolved in sterile water and RO-09-3143 at 10 µM in DMSO. RO-09-3143 was synthesized and provided by D. van Aalten (University of Dundee, Dundee, UK). In some experiments the inoculum was pre-treated by growing in YPD containing 0.2 M CaCl2 and 100 µg/ml CFW (Sigma-Aldrich, Dorset, UK) to induce the cell wall salvage pathways (Munro et al., 2007) and then washed before exposing to RO-09-3143. Cultures were incubated for 6 hours at 30°C with shaking at 200 rpm to maintain hypha-free cell preparations. The viability of cell cultures was measured following serial dilution of cultures spotted onto YPD plates which were incubated at 30°C for 24 hours.
Fluorescence microscopy
Samples were fixed in 10% (v/v) neutral buffered formalin (Sigma-Aldrich, Dorset, UK) and examined by differential interference contrast (DIC) microscopy. Cells were stained with 25 µg/ml CFW to visualize chitin. For actin staining with rhodamine-phalloidin, cells were fixed with 4% formaldehyde for 1 hour at room temperature. Cells were washed twice in PBS and resuspended in 500 µl of PBS. After addition of 6.6 µM rhodamine-phalloidin (Invitrogen, Paisley, UK), cells were incubated in the dark for 1 hour and washed five times with PBS before viewing. All samples were examined by DIC and fluorescence microscopy using a Zeiss Axioplan 2 microscope. Images were recorded digitally using the Openlab system (Openlab v 4.04, Improvision, Coventry, UK) and a Hamamatsu C4742- 95 digital camera (Hamamatsu Photonics, Hamamatsu, Hertfordshire, UK). CFW fluorescence was quantified for individual yeast cells using region of interest measurements (Walker et al., 2008). Mean fluorescence intensities were then calculated for at least 35 individual cells. In some experiments the exposure time for a series of fluorescence images was fixed so the intensity of fluorescence relative to a control of known chitin content was used as standard.
Electron microscopy
Yeast cultures were harvested by centrifugation and the pellets were fixed in 2.5% (v/v) gluteraldehyde in 0.1 M sodium phosphate buffer (pH 7.3) for 24 hours at 4°C. Samples were encapsulated in 3% (w/v) low melting point agarose prior to processing in Spurr's resin following a 24 hours processing schedule on a Lynx tissue processor (secondary 1% OsO4 fixation, 1% uranyl acetate as contrasting agents, ethanol dehydration and infiltration with acetone/Spurr resin). Additional infiltration was provided under vacuum at 60°C before embedding in TAAB embedding capsules and polymerizing at 60°C for 48 hours. Survey sections of 0.5 µm thickness were stained with toluidine blue to identify areas of optimal cell density. Ultrathin sections (60 nm) were then prepared using a Diatome diamond knife on a Leica UC6 ultramicrotome, and stained with uranyl acetate and lead citrate for examination with a Philips CM10 transmission microscope (FEI UK Ltd, Cambridge, UK) and imaging with a Gatan Bioscan 792 (Abingdon, UK). Shadow cast electron microscopy of septal regions was performed as described in Lenardon et al. (Lenardon et al., 2007).
Lectin colloidal gold staining of chitin
To establish if septa contained chitin, TEM thin sections were stained with WGA (Hilenski et al., 1986; Tronchin et al., 1981; Munro et al., 2001). Unstained ultrathin sections were mounted on 300 mesh nickel grids (Agar Scientific Ltd, Essex, UK) and labeled with 10 nm WGA-colloidal gold particles (British Biocell International Ltd, Cardiff, UK). All incubation steps were performed at room temperature by placing the grids into drops of reagent on dental wax. The grids were immersed for 1 hour in WGA-gold which had been diluted 1:5 with Tris-buffered saline (TBS). To test for non-specific binding of colloidal gold, grids were incubated with a 1:10 dilution of Goat anti-Mouse F(ab′)2 conjugated to colloidal gold (British Biocell International Ltd, Cardiff, UK). All grids were transferred through 10 drops of TBS and then jet-rinsed in TBS followed by washing in six drops of dH2O and finally jet-rinsed in dH2O. The thin sections were post-stained for 10 minutes with 5% (w/v) aqueous uranyl acetate and with lead citrate for 4 minutes (Reynolds, 1963).
Time-lapse photography
To visualize the formation of salvage septa, chs mutant strains were pre-treated with 0.2 M CaCl2 and 100 µg/ml CFW for 6 hours at 30°C. Cells were collected and resuspended in 20 ml of sterile dH2O. Cultures were diluted 1:250 and 3 µl was spotted onto a cavity slide containing 2% (w/v) agarose in YPD + 10 µM RO-09-3143 (Veses and Gow, 2008). A coverslip was applied immediately and cells were visualized by DIC microscopy using a DeltaVision RT microscope (Applied Precision, Leeds, UK) with a CoolSNAP camera (Photometrics, London, UK) in a constant temperature hood surrounding the microscope which was maintained at 30°C. Pictures were taken every 10 seconds for 2 hours to visualize the formation of salvage septa. To visualize the phenotype of the MRP1p-CHS1/chs1 conditional mutant, cells were grown overnight in YPMal then collected, washed and resuspended in 10 ml of water. The culture was diluted 1:500 and 3 µl was spotted onto a concave slide containing 2% (w/v) agarose in YPD in which the presence of glucose and the absence of maltose represses expression of CaCHS1. Pictures were taken every 2 minutes for 6 hours at 30°C to visualize the chaining and lysis of the conditional Cachs1 mutant.
Time-lapse fluorescence microscopy
To assess the localization of Chs3-YFP in cells growing in the presence of chitin synthase inhibitors, time-lapse movies of C. albicans strain NGY477 expressing Chs3-YFP from its native chromosomal locus as described previously (Veses and Gow, 2008). Cells from an overnight culture were washed in PBS and inoculated on the surface of an agar pad filling the cavity of a glass cavity slide (Agar Scientific, Essex, UK), covered with a coverslip and sealed using a mixture of lanoline, Vaseline and paraffin wax (1:1:1). The agar pad was made from rich medium (SC) containing 0.67% yeast nitrogen base with ammonium sulphate, 0.2% complete amino acid mix, 2% glucose and 1.2% purified agar. Chitin synthase inhibitors were added to the agar pad at a final concentration of 10 µM. Slides were incubated in the environmental chamber in a DeltaVision RT microscope at 30°C. DIC and YFP-fluorescent images were taken with a CoolSNAP camera every 3 minutes.
Supplementary Material
Acknowledgments
We thank Daan van Aalten (University of Dundee, Dundee, UK) for synthesizing the Chs1 inhibitor, Gillian Milne for help with EM and Gordon Stables for graphics support.
Footnotes
Author contributions
L.A.W., M.D.L., C.A.M. and N.A.R.G. conceived and designed the experiments. L.A.W., M.D.L. and K.P. performed the experiments. L.A.W., M.D.L., K.P., C.A.M. and N.A.R.G. analysed the data. L.A.W., M.D.L., C.A.M. and N.A.R.G. wrote the paper.
Funding
We acknowledge grant funding from the Gilead Sciences, the Wellcome Trust [grant numbers 086827 and 080088] and the European Commission (Ariadne Marie Curie Training Network). C.A.M. and M.D.L. are recipients of Medical Research Council (MRC) New Investigator Awards [grant numbers G0400284 and MR/J008203/1]. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.118885/-/DC1
References
- Bi E., Maddox P., Lew D. J., Salmon E. D., McMillan J. N., Yeh E., Pringle J. R. (1998). Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 142, 1301–1312 10.1083/jcb.142.5.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa C. E. (1993). Genetics and molecular biology of chitin synthesis in fungi. Annu. Rev. Microbiol. 47, 505–534 10.1146/annurev.mi.47.100193.002445 [DOI] [PubMed] [Google Scholar]
- Bulawa C. E., Osmond B. C. (1990). Chitin synthase I and chitin synthase II are not required for chitin synthesis in vivo in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 87, 7424–7428 10.1073/pnas.87.19.7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa C. E., Miller D. W., Henry L. K., Becker J. M. (1995). Attenuated virulence of chitin-deficient mutants of Candida albicans. Proc. Natl. Acad. Sci. USA 92, 10570–10574 10.1073/pnas.92.23.10570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E. (2004). The septation apparatus, a chitin-requiring machine in budding yeast. Arch. Biochem. Biophys. 426, 201–207 10.1016/j.abb.2004.02.030 [DOI] [PubMed] [Google Scholar]
- Cabib E., Schmidt M. (2003). Chitin synthase III activity, but not the chitin ring, is required for remedial septa formation in budding yeast. FEMS Microbiol. Lett. 224, 299–305 10.1016/S0378-1097(03)00477-4 [DOI] [PubMed] [Google Scholar]
- Cabib E., Sburlati A., Bowers B., Silverman S. J. (1989). Chitin synthase 1, an auxiliary enzyme for chitin synthesis in Saccharomyces cerevisiae. J. Cell Biol. 108, 1665–1672 10.1083/jcb.108.5.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin C. F., Bennett A. M., Ma W. K., Hall M. C., Yeong F. M. (2012). Dependence of Chs2 ER export on dephosphorylation by cytoplasmic Cdc14 ensures that septum formation follows mitosis. Mol. Biol. Cell 23, 45–58 10.1091/mbc.E11-05-0434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid V. J., Adamiková L., Sánchez M., Molina M., Nombela C. (2001). Cell cycle control of septin ring dynamics in the budding yeast. Microbiology 147, 1437–1450 [DOI] [PubMed] [Google Scholar]
- DeMarini D. J., Adams A. E., Fares H., De Virgilio C., Valle G., Chuang J. S., Pringle J. R. (1997). A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 139, 75–93 10.1083/jcb.139.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. W. (2003). Echinocandin antifungal drugs. Lancet 362, 1142–1151 10.1016/S0140-6736(03)14472-8 [DOI] [PubMed] [Google Scholar]
- Douglas C. M., D'Ippolito J. A., Shei G. J., Meinz M., Onishi J., Marrinan J. A., Li W., Abruzzo G. K., Flattery A., Bartizal K. et al. (1997). Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-D-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41, 2471–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi W. A., Irwin M. Y. (1993). Isogenic strain construction and gene mapping in Candida albicans. Genetics 134, 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughran J. P., Lai M. H., Kirsch D. R., Silverman S. J. (1994). Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isozyme Chs3 in vitro and in vivo. J. Bacteriol. 176, 5857–5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A. S., Sudbery P. E. (2008). Septins in four model fungal systems: diversity in form and function. The Septins Hall P A, Russell S E H, Pringle J R, ed125–146West Sussex: Wiley-Blackwell [Google Scholar]
- Gladfelter A. S., Pringle J. R., Lew D. J. (2001). The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 4, 681–689 10.1016/S1369-5274(01)00269-7 [DOI] [PubMed] [Google Scholar]
- Gow N. A. R., Gooday G. W. (1982). Growth kinetics and morphology of colonies of the filamentous form of Candida albicans. J. Gen. Microbiol. 128, 2187–2194 [DOI] [PubMed] [Google Scholar]
- Gow N. A. R., Gooday G. W., Newsam R. J., Gull K. (1980). Ultrastructure of the septum in Candida albicans. Microbiology 4, 357–359 [Google Scholar]
- Gow N. A. R., Robbins P. W., Lester J. W., Brown A. J. P., Fonzi W. A., Chapman T., Kinsman O. S. (1994). A hyphal-specific chitin synthase gene (CHS2) is not essential for growth, dimorphism, or virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 91, 6216–6220 10.1073/pnas.91.13.6216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N. A. R., van de Veerdonk F. L., Brown A. J. P., Netea M. G. (2012). Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10, 112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilenski L. L., Naider F., Becker J. M. (1986). Polyoxin D inhibits colloidal gold-wheat germ agglutinin labelling of chitin in dimorphic forms of Candida albicans. J. Gen. Microbiol. 132, 1441–1451 [DOI] [PubMed] [Google Scholar]
- Ichinomiya M., Yamada E., Yamashita S., Ohta A., Horiuchi H. (2005). Class I and class II chitin synthases are involved in septum formation in the filamentous fungus Aspergillus nidulans. Eukaryot. Cell 4, 1125–1136 10.1128/EC.4.6.1125-1136.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis F. M., Mol P., Hellingwerf K., Brul S. (2002). Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26, 239–256 10.1111/j.1574-6976.2002.tb00613.x [DOI] [PubMed] [Google Scholar]
- Klis F. M., Boorsma A., De Groot P. W. (2006). Cell wall construction in Saccharomyces cerevisiae. Yeast 23, 185–202 10.1002/yea.1349 [DOI] [PubMed] [Google Scholar]
- Larson J. R., Bharucha J. P., Ceaser S., Salamon J., Richardson C. J., Rivera S. M., Tatchell K. (2008). Protein phosphatase type 1 directs chitin synthesis at the bud neck in Saccharomyces cerevisiae. Mol. Biol. Cell 19, 3040–3051 10.1091/mbc.E08-02-0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Maccallum D. M., Jacobsen M. D., Walker L. A., Odds F. C., Gow N. A. R., Munro C. A. (2012). Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob. Agents Chemother. 56, 208–217 10.1128/AAC.00683-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardon M. D., Whitton R. K., Munro C. A., Marshall D., Gow N. A. R. (2007). Individual chitin synthase enzymes synthesize microfibrils of differing structure at specific locations in the Candida albicans cell wall. Mol. Microbiol. 66, 1164–1173 10.1111/j.1365-2958.2007.05990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardon M. D., Munro C. A., Gow N. A. R. (2010). Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 13, 416–423 10.1016/j.mib.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J., Shannon K. B., Shou W., Deshaies R. J., Li R. (2001). The Tem1 small GTPase controls actomyosin and septin dynamics during cytokinesis. J. Cell Sci. 114, 1379–1386 [DOI] [PubMed] [Google Scholar]
- Meitinger F., Petrova B., Lombardi I. M., Bertazzi D. T., Hub B., Zentgraf H., Pereira G. (2010). Targeted localization of Inn1, Cyk3 and Chs2 by the mitotic-exit network regulates cytokinesis in budding yeast. J. Cell Sci. 123, 1851–1861 10.1242/jcs.063891 [DOI] [PubMed] [Google Scholar]
- Mio T., Yabe T., Sudoh M., Satoh Y., Nakajima T., Arisawa M., Yamada-Okabe H. (1996). Role of three chitin synthase genes in the growth of Candida albicans. J. Bacteriol. 178, 2416–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama T., Kojima N., Horiuchi H., Ohta A., Takagi M. (1994). Isolation of a chitin synthase gene (chsC) of Aspergillus nidulans. Biosci. Biotechnol. Biochem. 58, 2254–2257 10.1271/bbb.58.2254 [DOI] [PubMed] [Google Scholar]
- Motoyama T., Fujiwara M., Kojima N., Horiuchi H., Ohta A., Takagi M. (1997). The Aspergillus nidulans genes chsA and chsD encode chitin synthases which have redundant functions in conidia formation [corrected and republished article originally appeared in Mol Gen Genet 1996 Jun; 251(4):442-50]. Mol. Gen. Genet. 253, 520–528 10.1007/s004380050353 [DOI] [PubMed] [Google Scholar]
- Munro C. A., Gow N. A. R. (1995). Chitin biosynthesis as a target for antifungals. Antifungal Agents: Discovery and Mode of Action Dixon G K, Copping L G, Hollomon D W, ed161–171Oxford: Bios Scientific [Google Scholar]
- Munro C. A., Gow N. A. R. (2001). Chitin synthesis in human pathogenic fungi. Med. Mycol. 39 Suppl. 1, 41–53 [PubMed] [Google Scholar]
- Munro C. A., Schofield D. A., Gooday G. W., Gow N. A. R. (1998). Regulation of chitin synthesis during dimorphic growth of Candida albicans. Microbiology 144, 391–401 10.1099/00221287-144-2-391 [DOI] [PubMed] [Google Scholar]
- Munro C. A., Winter K., Buchan A., Henry K., Becker J. M., Brown A. J. P., Bulawa C. E., Gow N. A. R. (2001). Chs1 of Candida albicans is an essential chitin synthase required for synthesis of the septum and for cell integrity. Mol. Microbiol. 39, 1414–1426 10.1046/j.1365-2958.2001.02347.x [DOI] [PubMed] [Google Scholar]
- Munro C. A., Whitton R. K., Hughes H. B., Rella M., Selvaggini S., Gow N. A. R. (2003). CHS8-a fourth chitin synthase gene of Candida albicans contributes to in vitro chitin synthase activity, but is dispensable for growth. Fungal Genet. Biol. 40, 146–158 10.1016/S1087-1845(03)00083-5 [DOI] [PubMed] [Google Scholar]
- Munro C. A., Selvaggini S., de Bruijn I., Walker L. A., Lenardon M. D., Gerssen B., Milne S., Brown A. J. P., Gow N. A. R. (2007). The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 63, 1399–1413 10.1111/j.1365-2958.2007.05588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y., Bi E. (2011). Septin structure and function in yeast and beyond. Trends Cell Biol. 21, 141–148 10.1016/j.tcb.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popolo L., Gilardelli D., Bonfante P., Vai M. (1997). Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1delta mutant of Saccharomyces cerevisiae. J. Bacteriol. 179, 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancati G., Pavelka N., Fleharty B., Noll A., Trimble R., Walton K., Perera A., Staehling-Hampton K., Seidel C. W., Li R. (2008). Aneuploidy and polyploidy underlie rapid adaptive evolution of yeast cells deprived of a conserved cytokinesis motor. Cell 135, 879–893 10.1016/j.cell.2008.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds E. S. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 10.1083/jcb.17.1.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Quiñones J. F., Rodríguez-Medina J. R. (2009). Differential gene expression signatures for cell wall integrity found in chitin synthase II (chs2Delta) and myosin II (myo1Delta) deficient cytokinesis mutants of Saccharomyces cerevisiae. BMC Res. Notes 2, 87 10.1186/1756-0500-2-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero C. (2002). The genetic complexity of chitin synthesis in fungi. Curr. Genet. 41, 367–378 10.1007/s00294-002-0318-7 [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J., González-Prieto J. M., Ruiz-Medrano R. (2002). Evolution and phylogenetic relationships of chitin synthases from yeasts and fungi. FEMS Yeast Res. 1, 247–256 10.1111/j.1567-1364.2002.tb00042.x [DOI] [PubMed] [Google Scholar]
- Schmidt M., Bowers B., Varma A., Roh D. H., Cabib E. (2002). In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. J. Cell Sci. 115, 293–302 [DOI] [PubMed] [Google Scholar]
- Shaw J. A., Mol P. C., Bowers B., Silverman S. J., Valdivieso M. H., Durán A., Cabib E. (1991). The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 114, 111–123 10.1083/jcb.114.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S. J., Sburlati A., Slater M. L., Cabib E. (1988). Chitin synthase 2 is essential for septum formation and cell division in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 85, 4735–4739 10.1073/pnas.85.13.4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudoh M., Yamazaki T., Masubuchi K., Taniguchi M., Shimma N., Arisawa M., Yamada-Okabe H. (2000). Identification of a novel inhibitor specific to the fungal chitin synthase. Inhibition of chitin synthase 1 arrests the cell growth, but inhibition of chitin synthase 1 and 2 is lethal in the pathogenic fungus Candida albicans. J. Biol. Chem. 275, 32901–32905 10.1074/jbc.M003634200 [DOI] [PubMed] [Google Scholar]
- Tronchin G., Poulain D., Herbaut J., Biguet J. (1981). Localization of chitin in the cell wall of Candida albicans by means of wheat germ agglutinin. Fluorescence and ultrastructural studies. Eur. J. Cell Biol. 26, 121–128 [PubMed] [Google Scholar]
- VerPlank L., Li R. (2005). Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol. Biol. Cell 16, 2529–2543 10.1091/mbc.E04-12-1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versele M., Thorner J. (2004). Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J. Cell Biol. 164, 701–715 10.1083/jcb.200312070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veses V., Gow N. A. R. (2008). Vacuolar dynamics during the morphogenetic transition in Candida albicans. FEMS Yeast Res. 8, 1339–1348 10.1111/j.1567-1364.2008.00447.x [DOI] [PubMed] [Google Scholar]
- Walker L. A., Munro C. A., de Bruijn I., Lenardon M. D., McKinnon A., Gow N. A. R. (2008). Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 4, e1000040 10.1371/journal.ppat.1000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Kashimshetty R., Ng K. E., Tan H. B., Yeong F. M. (2006). Exit from mitosis triggers Chs2p transport from the endoplasmic reticulum to mother-daughter neck via the secretory pathway in budding yeast. J. Cell Biol. 174, 207–220 10.1083/jcb.200604094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Mendoza L., Wang Z., Liu H., Park C., Kauffman S., Becker J. M., Szaniszlo P. J. (2006). WdChs1p, a class II chitin synthase, is more responsible than WdChs2p (Class I) for normal yeast reproductive growth in the polymorphic, pathogenic fungus Wangiella (Exophiala) dermatitidis. Arch. Microbiol. 185, 316–329 10.1007/s00203-006-0101-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.