Abstract
BACKGROUND & AIMS
The enteric abundance of serotonin (5-HT), its ability to promote proliferation of neural precursors, and reports that 5-HT antagonists affect crypt epithelial proliferation led us to investigate whether 5-HT affects growth and maintenance of the intestinal mucosa in mice.
METHODS
Mice that lack the serotonin re-uptake transporter (SERTKO mice) and wild-type mice were given injections of selective serotonin re-uptake inhibitors (gain-of-function models). We also analyzed mice that lack tryptophan hydroxylase-1 (TPH1KO mice, which lack mucosal but not neuronal 5-HT) and mice deficient in tryptophan hydroxylase-2 (TPH2KO mice, which lack neuronal but not mucosal 5-HT) (loss-of-function models). Wild-type and SERTKO mice were given ketanserin (an antagonist of the 5-HT receptor, 5-HT2A) or scopolamine (an antagonist of the muscarinic receptor). 5-HT2A receptors and choline acetyltransferase were localized by immunocytochemical analysis.
RESULTS
Growth of the mucosa and proliferation of mucosal cells were significantly greater in SERTKO mice and in mice given selective serotonin reuptake inhibitors than in wild-type mice, but were diminished in TPH2KO (but not in TPH1KO) mice. Ketanserin and scopolamine each prevented the ability of SERT knockout or inhibition to increase mucosal growth and proliferation. Cholinergic submucosal neurons reacted with antibodies against 5-HT2A.
CONCLUSIONS
5-HT promotes growth and turnover of the intestinal mucosal epithelium. Surprisingly, these processes appear to be mediated by neuronal, rather than mucosal, 5-HT. The 5-HT2A receptor activates cholinergic neurons, which provide a muscarinic innervation to epithelial effectors.
Keywords: Choline Acetyltransferase, 5-HT2A Receptor, Intestinal Development, Mouse Model
The enteric nervous system (ENS) is unique in its ability to regulate gastrointestinal activity independently of central neural input.1,2 Enteric serotonin (5-HT) levels dwarf those of other organs, including the brain.1 Enteric 5-HT is stored in enterochromaffin (EC) cells3,4 and neurons.5–8 It regulates bone resorption,9,10 intestinal secretion, microcirculation,2 and peristaltic reflexes,11 and is an ENS neurotransmitter.8,12,13 5-HT also promotes development of enteric neurons14,15 and proliferation of central nervous system neuronal precursors,16 and it induces adult stem cells to undergo neurogenesis.17
The high concentration of 5-HT in the gastrointestinal mucosa, 5-HT’s promotion of neural precursor proliferation, 15–17 and prior observations that 5-HT antagonists affect crypt cell proliferation,18,19 raise the possibility that 5-HT regulates intestinal epithelial homeostasis. Epithelial homeostasis is a balance between proliferation20,21 and apoptotic cell loss.22 Epithelial stem cells are located at crypt bases (Lgr5-expressing)23 and the +4 position of intestinal crypts.20,21 Transit divisions amplify numbers of crypt cells, but proliferation stops in villi. Postmitotic cells migrate to villus tips where apoptosis and extrusion occur.24 Increasing stem cell proliferation, incrementing numbers of transit divisions, or decreasing apoptosis would increase epithelial cell numbers. Intestinal crypt cells express 5-HT2A receptors,25 and the ENS innervates intestinal crypts.26,27 5-HT from EC cells or projections of submucosal neurons, which receive a serotonergic innervation, therefore, might alter these activities to regulate epithelial cell number.
Mucosal epithelial innervation has been linked to epithelial homeostasis. Sympathetic nerves, for example, alter crypt cell proliferation because norepinephrine28 and desmethylimipramine29 (potentiates norepinephrine by inhibiting the norepinephrine transporter) accelerate crypt cell proliferation. Sympathetic nerve ablation decreases proliferation.18,30 Sympathetic postganglionic neurons are extrinsic and could enable the central nervous system to influence epithelial homeostasis. Intrinsic ENS components might similarly regulate epithelial homeostasis. 5-HT, similar to norepinephrine, is a putative regulator of epithelial crypt cell proliferation18,31,32; therefore, EC cells or serotonergic neurons are potential intrinsic regulators of epithelial homeostasis.
The current experiments tested the hypothesis that 5-HT regulates epithelial homeostasis and, if so, whether serotonergic effects are EC cell– or neuron-mediated. Gain- and loss-of-function approaches were used. The gain-of-function model was to quantify epithelial turnover and apoptosis in mice lacking the 5-HT re-uptake transporter (serotonin reuptake transporter [SERT]; SERTKO mice). Epithelial cells and enteric serotonergic neurons express SERT,33–35 which takes up 5-HT and inactivates it.36–38 Loss-of-function studies were conducted with mice lacking tryptophan hydroxylase-1 (TPH1KO)14,39,40 or tryptophan hydroxylase-2 (TPH2KO).14,41 Because TPH1 is rate-limiting in 5-HT biosynthesis within EC cells, and TPH2 in neurons,42 EC cells lack 5-HT in TPH1KO mice,39,43 and neurons lack 5-HT in TPH2KO animals.41 Results suggested that 5-HT regulates epithelial proliferation and turnover. Surprisingly, this regulation depended on neuronal rather than mucosal 5-HT. Serotonergic effects were linked to activation of 5-HT2A receptors on cholinergic submucosal neurons.
Materials and Methods
Animals
Wild-type (WT), SERTKO,44 and TPH1KO40 mice were bred on a C57BL/6 background at Taconic Farms (Hudson, NY) and transferred to Columbia University. TPH2KO mice (Lexicon Pharmaceutical Co, The Woodlands, TX) were bred at Columbia on multiple backgrounds (C57BL/6, CD-1, and non-agouti). All mice were housed under pathogen-free conditions on a 12-hour light/dark cycle with food and water ad libitum. Animals were euthanized with CO2 asphyxiation and the ileum was analyzed. Columbia University’s Institutional Animal Care and Use Committee approved all experiments.
Measurement of Villus Height and Crypt Depth
Paraffin sections were stained with H&E. Brightfield microscopy (100×) was used to measure villus height (VH) and crypt depth (CD). Computer-assisted imaging (Volocity 4.0; Perkin– Elmer, Waltham, MA) was used. Villi were selected when the central lacteal was completely visualized. Crypts were analyzed when the crypt–villus junction could be visualized on both sides of the crypt. Twenty villi and crypts were quantified per mouse.
Paneth Cell Counts and Enterocyte Height
Enterocyte height (EH) and Paneth cells were analyzed in 1-µm plastic sections (Epon 812) stained with toluidine blue. EH was measured in the middle of each villus from the basement membrane to the luminal edge of the brush border. Paneth cells (large, toluidine blue–stained, cytoplasmic granules, and triangular cell shape) were counted in crypts with distinct crypt–villus junctions.
Proliferation
A pulse of bromodeoxyuridine (BrdU; 100 mg/kg) was administered intraperitoneally to all animals. After a 1-hour chase, animals were euthanized. BrdU incorporation was analyzed immunocytochemically (BrdU in-Situ Detection Kit; BD Pharmingen, San Diego, CA). The number of BrdU-labeled cells per crypt was counted and divided by the total number of cells per crypt to compute the crypt proliferation index (CPI), which was calculated for at least 15 crypts/mouse.
Apoptosis
Apoptotic cells were analyzed in WT and SERTKO mice. Rabbit antibodies to cleaved caspase-3 (Cell Signaling, Danvers, MA) were used for immunocytochemistry and Western blot analysis. The terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay (In Situ cell death detection kit; Roche Diagnostics, Indianapolis, IN) was performed according to the manufacturer’s instructions (see the Supplementary Materials and Methods section).
Selective Serotonin Re-uptake Inhibitors
C57BL/6 mice were treated either with citalopram hydrobromide (Sigma–Aldrich, St. Louis, MO; dissolved in 0.9% saline) or sertraline (Sigma–Aldrich; dissolved in 50% ethanol); treated mice were compared with controls given vehicle. Selective serotonin re-uptake inhibitors (SSRIs) (10 mg/kg/day, n = 5/drug; 25 mg/kg/day, n = 5/drug) and the vehicles (n = 5/for each vehicle) were administered continuously for 3, 7, or 14 days via an osmotic micropump (0.25 µL/h; Alzet, Cupertino, CA) surgically implanted into the peritoneal cavity of ketamine-anesthetized mice.
Antagonists
Five SERTKO and 5 WT mice were treated continuously for 5 days via an osmotic micropump with scopolamine (0.5 mg/kg/day; Sigma-Aldrich) or saline. Five SERTKO and 5 WT mice received daily intraperitoneal injections of ketanserin (1 mg/kg/day) for 5 days. Six control SERTKO and 6 WT mice were treated similarly with saline.
Immunostaining
Primary antibodies to HuC/D (Invitrogen, Carlsbad, CA; biotin-conjugated mouse monoclonal, A21272, 1:20 dilution), 5-HT (Diasorin, Stillwater, MN; ab20080, 1:500 –1:10,000), 5HT2A (Abcam, Cambridge, MA; ab16028, 1:100 dilution), and choline acetyltransferase (ChAT; Millipore, Billerica, MA; goat polyclonal, ab144P, 1:100 dilution) were applied to fixed-frozen sections. Sites of primary antibody binding were detected with species-specific secondary antibodies labeled with Alexa 488 (1: 200 dilution) or Alexa 594 (1:200 dilution). The dichroic mirrors and emission filters were specific and did not permit cross-detection of fluorophors.
Results
SERTKO Mice
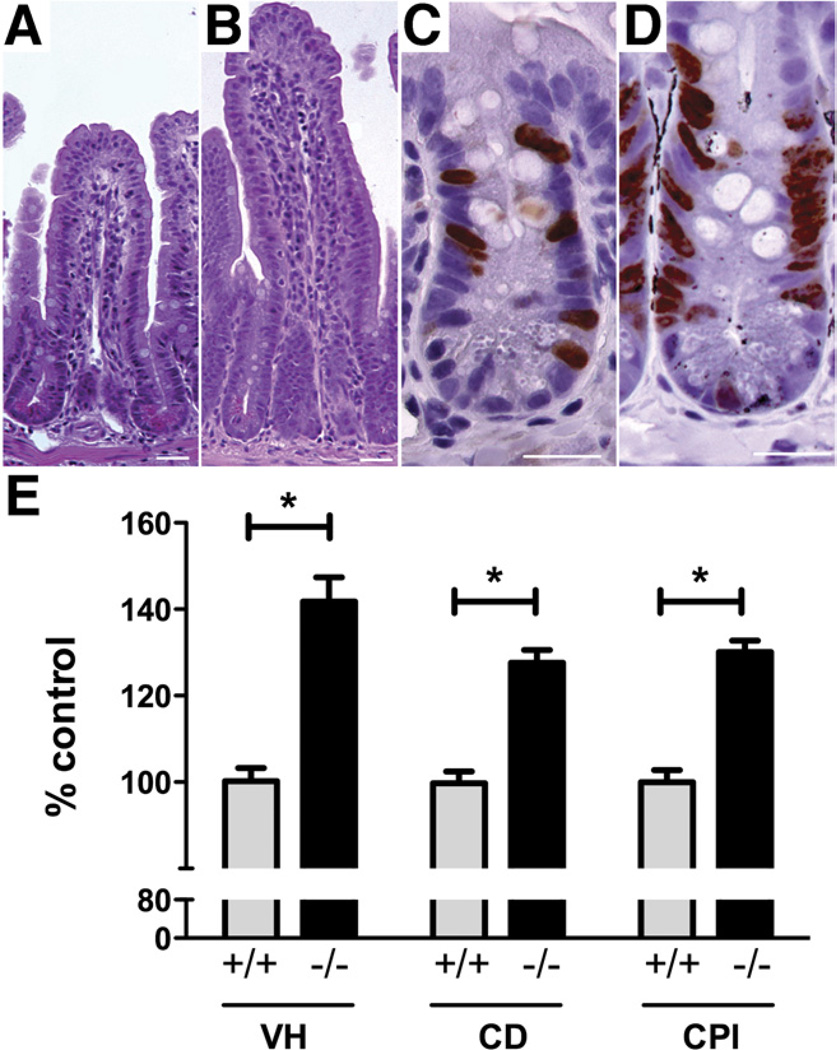
To test the hypothesis that up-regulation of serotonergic signaling affects turnover of intestinal epithelial cells, VH, CD, and CPI were compared in SERTKO and WT mice. Each was significantly greater (P < .0001) in SERTKO than in control animals (Figure 1). For SERTKO mice, the mean values were as follows: VH = 246.7 ± 9.8 µm; CD = 109.7 ± 2.6 µm; and CPI = 40.5% ± 0.8%. For WT, the corresponding values were as follows: VH = 174.3 ± 5.4 µm; CD = 85.8 ± 2.3 µm; and CPI = 31.1% ± 0.9%. These observations are consistent with the idea that 5-HT causes VH and CD to increase by promoting crypt epithelial proliferation.
Figure 1.
VH, CD, and CPI are significantly greater in SERTKO than in WT mice. (A) Mucosal architecture in a section of WT mouse ileum stained with H&E. (B) Mucosal architecture of a SERTKO mouse. (C) BrdU incorporation (brown reaction product) into crypt cells from a WT mouse. (D) BrdU incorporation in a SERTKO mouse. (A–D) Scale bars: 30 µm. (E) VH, CD, and CPI are expressed as the percentage of control (WT). *P < .0001.
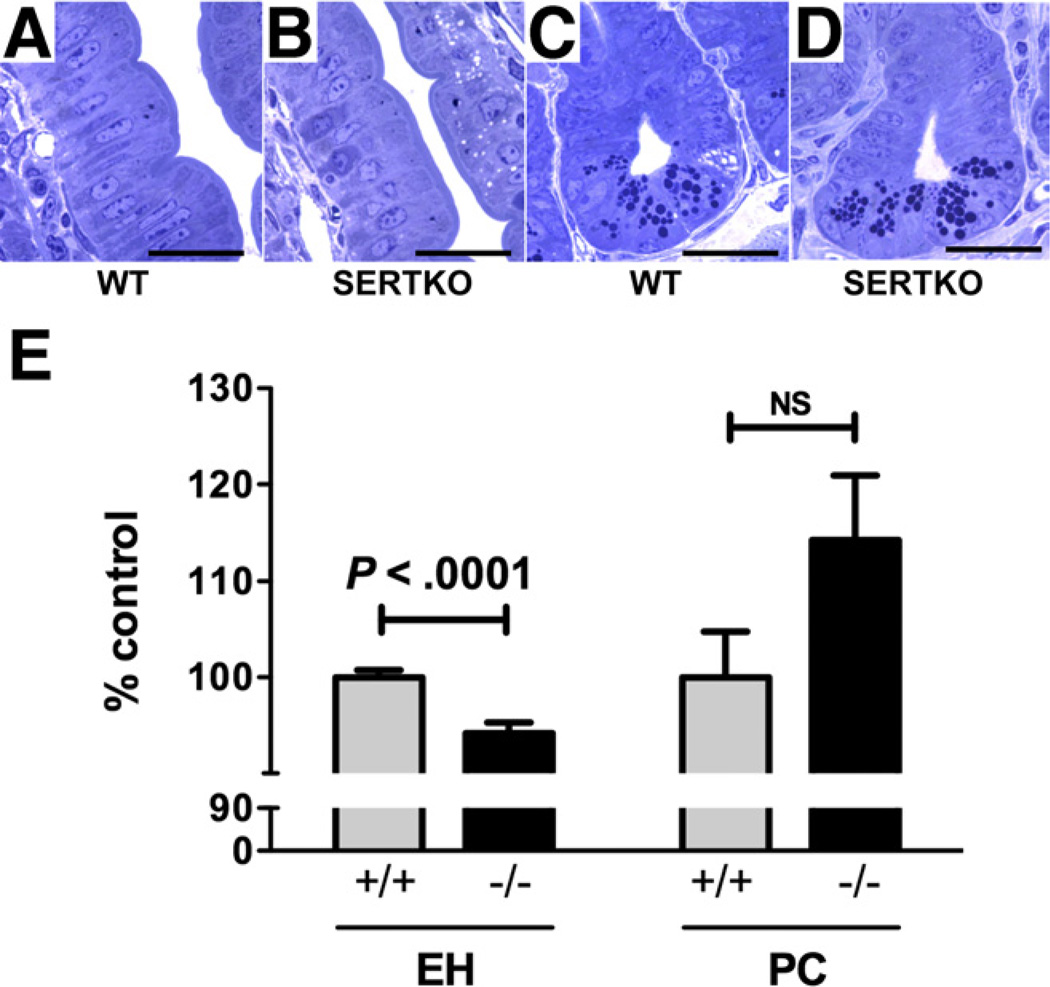
EH was significantly shorter in SERTKO (25.6 ± 0.3 µm) than in WT mice (27.2 ± 0.2 µm; P < .0001) (Figure 2). The number of Paneth cells/crypt in SERTKO mice (5.7 ± 0.3 cells), however, was numerically greater but not significantly different than WT animals (4.9 ± 0.2 cells; P = .0789) (Figure 2).
Figure 2.
Enterocytes are smaller in SERTKO than in WT mice. (A) Toluidine blue–stained semithin sections (0.7 µm). (A) Midvillus, WT. (B) Midvillus, SERTKO. (C) Crypt, WT. (D) Crypt, SERTKO. (A–D) Scale bars: 20µm. (E) EH and Paneth cell (PC) number are quantified and expressed as the percentage of control (WT).
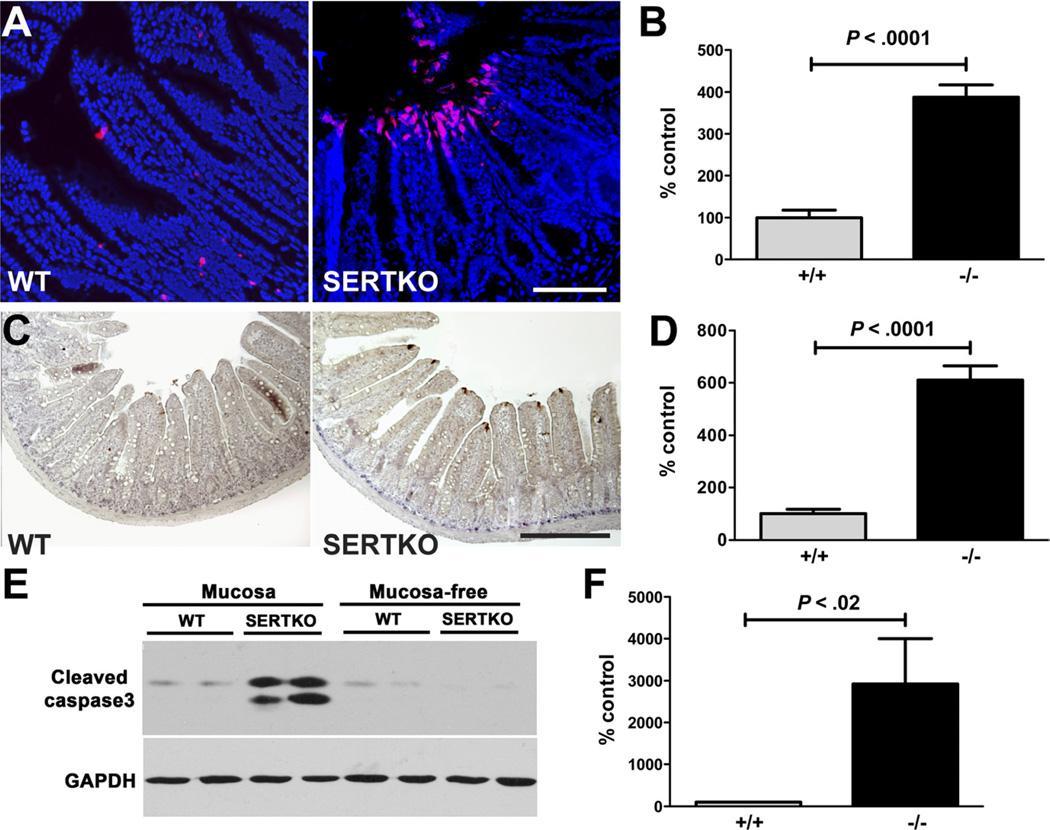
The TUNEL procedure and the detection of cleaved caspase-3 were used to assess apoptosis in SERTKO and WT mice. Immunocytochemistry and immunoblots were used to identify the location and quantity of cleaved caspase-3. Epithelial apoptosis was found predominantly in the villus tip extrusion zone in SERTKO and WT mice (Figure 3A). The number of cells undergoing apoptosis, however, was significantly greater in SERTKO than in WT mice (Figure 3A and B). Immunoblot analyses of isolated preparations of mucosa and the remaining mucosa-free preparations of gut wall confirmed that cleaved caspase-3 was increased significantly in mucosal, but not mucosa-free, preparations of SERTKO intestine (Figure 3C–F). These observations suggest that at equilibrium in SERTKO mice increased apoptosis balances enhanced crypt cell proliferation; however, this balance does not prevent the increase of both VH and CD.
Figure 3.
Apoptosis of enterocytes at villus tips is greater in SERTKO than in WT mice. (A) Apoptotic cells (red) shown with TUNEL in the epithelium of the intestines of WT and SERTKO mice. DNA counterstained with bisbenzimide. Scale bar: 100 µm. (B) TUNEL staining cells quantified and expressed as the percentage of control (WT). (C) Cleaved caspase-3 immunostaining (brown). Scale bar: 200 µm. (D) Numbers of cells displaying cleaved caspase-3 immunostained cells quantified and expressed as the percentage of control (WT). (E) Western blot showing cleaved caspase-3 immunoreactivity in preparations of mucosa and the mucosa-free bowel wall in WT and SERTKO mice. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (F) Cleaved caspase-3 immunoreactivity was quantified in the mucosa of WT and SERTKO mice and expressed as the percentage of control (WT).
SERT Inhibition
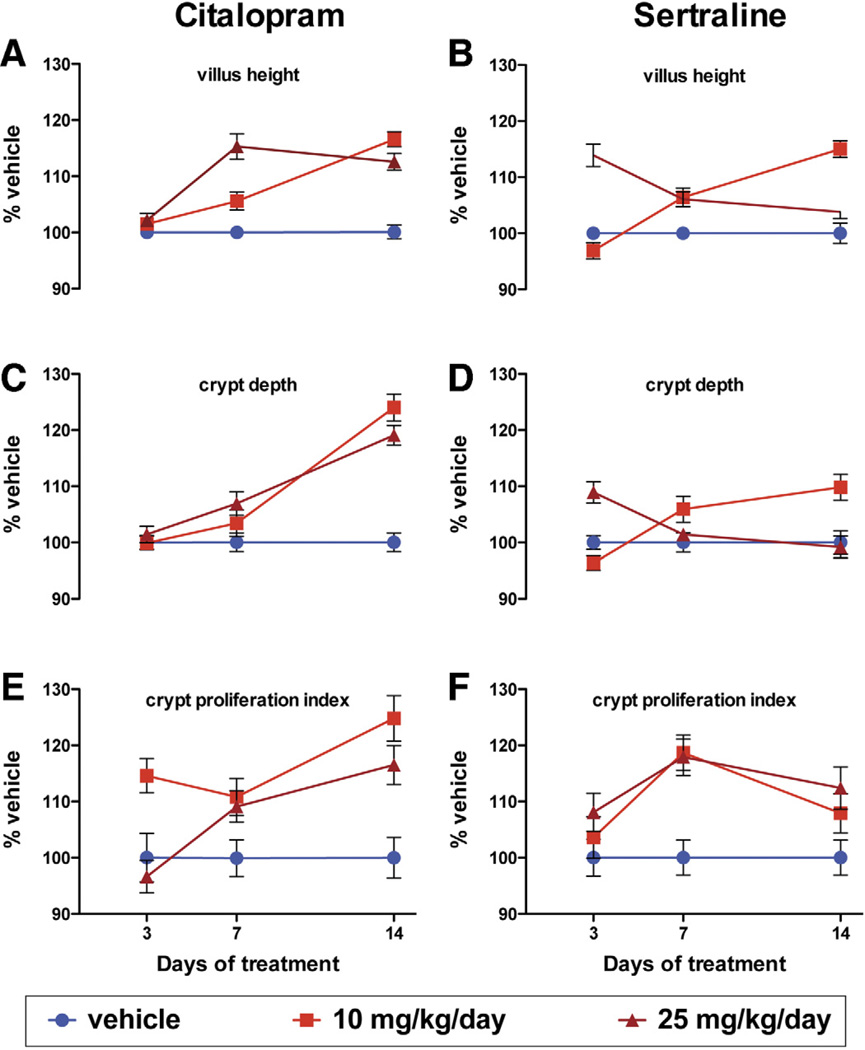
Two SSRIs, citalopram and sertraline, were investigated to determine whether acute SERT inhibition mimics the effects of SERTKO on VH, CD, and CPI (Figure 4). Each parameter was measured at 3, 7, and 14 days of exposure to the SSRIs or corresponding vehicle. Measurements in mice receiving SSRIs were normalized to those in vehicle-treated animals. Two-way analysis of variance was used to analyze the difference between SSRI- and vehicle-treated tissue as well as the effect of duration of treatment. Both citalopram and sertraline increased VH, CD, and the CPI; however, the degree to which effects were seen was found to depend significantly on time and dose. The time needed for evidence of the first effect on most parameters was less for sertraline than for citalopram; moreover, for both sertraline and citalopram, evidence of desensitization was seen as efficacy waned by 14 days at the higher dose of each compound. The observation that SSRIs exert effects on VH, CD, and the CPI that are similar to those seen in SERTKO mice suggests that these effects are consequences of SERT deficiency and probably caused by 5-HT potentiation.
Figure 4.
VH, CD, and CPI in WT mice that received vehicle (saline or 50% ETOH), citalopram (10 or 25 mg/kg/day), or sertraline (10 or 25 mg/kg/day) for 3, 7, or 14 days.
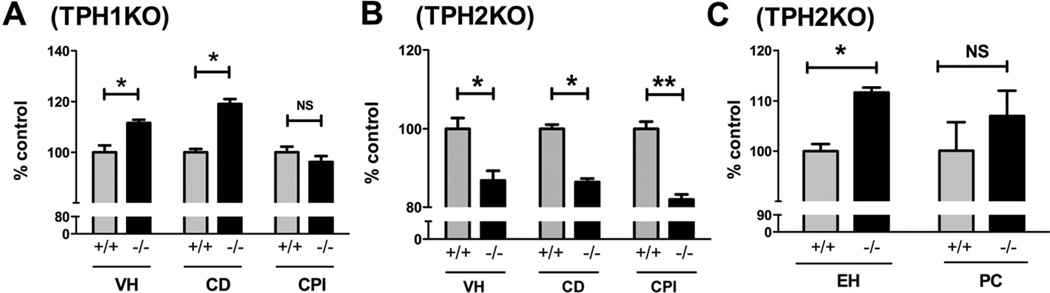
Deletion of TPH
Studies were performed to determine whether neurons or EC cells are the responsible source of 5-HT that putatively regulates VH, CD, and CPI (Supplementary Figure 1; compare Supplementary Figure 1A and B [WT] with Supplementary Figure 1C and D [TPH1KO] and Supplementary Figure 1E and F [TPH2KO]). TPH1KO mice were examined to test the hypothesis that 5-HT from EC cells modifies the proliferation of the intestinal epithelium (Figure 5A). Surprisingly, VH (194.5 ± 2.2 µm) and CD (74.2 ± 1.2 µm) each were found to be significantly greater (P < .0001) in TPH1KO than in WT mice (VH = 174.3 ± 4.7 µm; CD = 62.3 ± 0.8 µm). The CPI, however, in TPH1KO mice was not significantly different from that measured in WT (34.3% ± 0. 8% vs 33.0% ± 0.8%; P = .2431); neither the height (WT = 27.3 ± 0.3 µm; TPH1KO = 27.8 ± 0.4 µm; P = .3718) nor the width (WT = 5.6 ± 0.1 µm; TPH1KO = 5.5 ± 0.2 µm; P = .6297) of enterocytes differed between TPH1KO and WT mice. These observations are inconsistent with the idea that EC cell–derived 5-HT stimulates proliferation of epithelial cells.
Figure 5.
(A and B) VH, CD, and CPI have been quantified and expressed as the percentage of control (WT). (A) VH and CD, but not CPI, are significantly greater in TPH1KO than in WT mice. *P < . 0001, NS = 0. 2431. (B) VH, CD, and CPI are significantly lower in TPH2KO than in WT mice. (B) *P < . 0001, **P < . 01. (C) The height of enterocytes but not the numbers of Paneth cells in TPH2KO was greater than in WT mice. *P < . 05.
Experiments then were performed to determine whether serotonergic neurons are the source of the 5-HT that effects intestinal epithelial proliferation. This hypothesis was tested in TPH2KO mice (Figure 5B), which lack neuronal but not EC cell 5-HT (Supplementary Figure 1E and F). VH, CD, and CPI were each significantly lower (P < .0001) in TPH2KO mice than in WT littermates. For TPH2KO mice, mean values were as follows: VH = 172 ± 1.9 µm; CD = 61.5 ± 1.1 µm; and CPI = 27.2% ± 1.3%. For WT, the corresponding values were as follows: VH = 191.1 ± 2.6 µm; CD = 67.9 ± 1.0 µm; and CPI = 33.1% ± 1.8%. In contrast to SERTKO mice, in which EH was less than that of WT animals, EH in TPH2KO mice (30.7 ± 0.3 µm) was greater than that of WT littermates (27.5 ± 0.4 µm; P < .0001) (Figure 5C); however, numbers of Paneth cells did not differ between TPH2KO and WT mice (Figure 5C). These observations support the idea that endogenous neuronal, rather than EC cell, 5-HT increases VH, CD, and CPI.
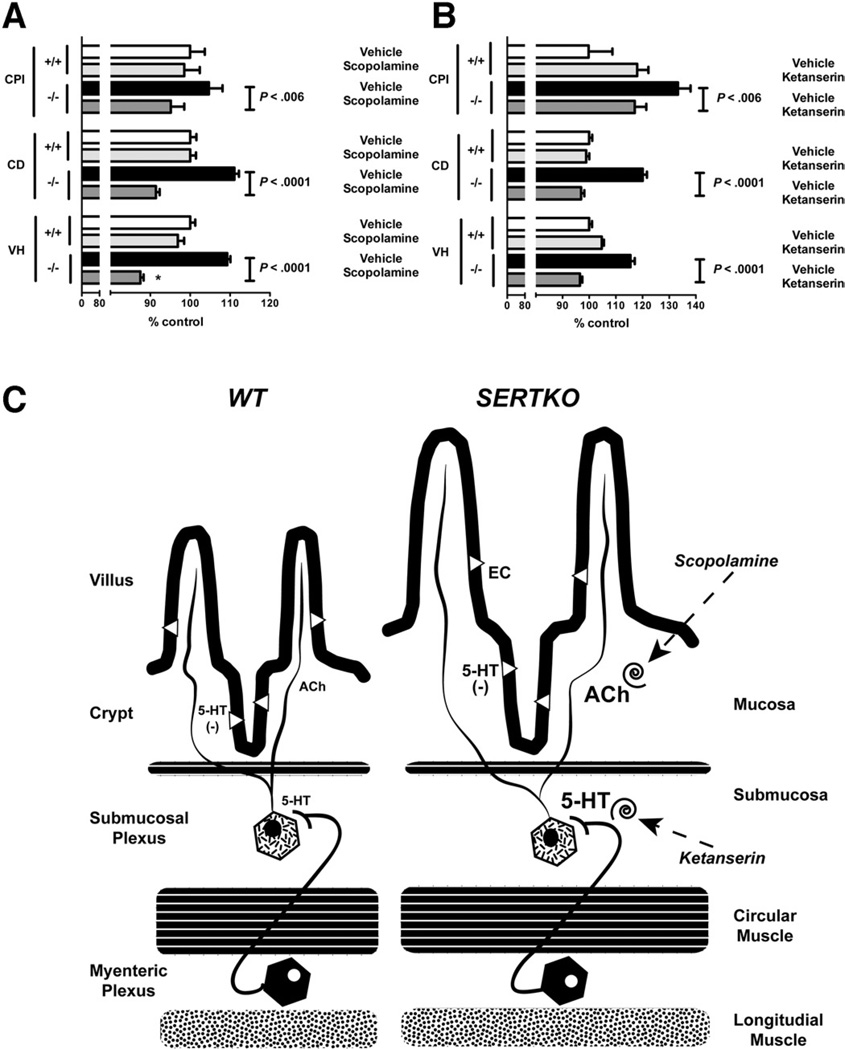
Scopolamine
Submucosal secretomotor neurons innervate the mucosal epithelium2; therefore, it is plausible that these neurons regulate intestinal epithelial proliferation as well as secretion. Enteric serotonergic neurons, however, are interneurons, and the intestinal mucosa has not been reported to receive a serotonergic innervation45; moreover, we did not detect 5-HT immunoreactivity in mucosal nerves in WT mice (Supplementary Figure 1A and B). Enteric serotonergic neurons nevertheless might regulate intestinal epithelial turnover indirectly because they synapse on submucosal neurons, innervating the proliferating mucosa.7,45 Many of the submucosal neurons that innervate the epithelium are cholinergic and stimulate secretion via muscarinic receptors.7,26,46 We thus tested the hypothesis that 5-HT activates cholinergic neurons, which stimulate muscarinic receptors to promote epithelial turnover. We used scopolamine, a general muscarinic antagonist, to determine whether muscarinic blockade suppresses the SERTKO-associated increases in VH, CD, CPI (Figure 6A), and apoptosis. As a control, WT mice also were treated with scopolamine. VH, CD, the CPI (Figure 6A), and apoptosis (Supplementary Figure 2) were all significantly lower in scopolamine-treated SERTKO mice (VH and CD, P < .0001; CPI, P < .05) than in SERTKO animals given saline. For scopolamine-treated SERTKO mice, the mean values were as follows: VH = 174.0 ± 1.6 µm; CD = 64.6 ± 0.7 µm; and CPI = 32.2% ± 1.1%. For SERTKO animals given saline, the corresponding values were as follows: VH = 217.4 ± 1.6 µm; CD = 78.5 ± 0.8 µm; and CPI = 35.4% ± 1.2%. Scopolamine, however, did not affect any parameter when it was administered to WT control mice.
Figure 6.
(A) VH, CD, and CPI are all significantly shorter in SERTKO mice treated with scopolamine than with vehicle. VH, CD, and CPI were quantified and expressed as the percentage of control (WT + vehicle). (B) VH, CD, and the CPI are all significantly shorter in SERTKO mice treated with ketanserin than with vehicle. (C) A hypothetical explanation of the mucosal changes seen in SERTKO mice. The deletion of SERT amplifies the effects of 5-HT that neurons secrete. Serotonergic stimulation of 5-HT2A–receptor– expressing cholinergic neurons in submucosal ganglia causes release of ACh in the mucosa, which stimulates epithelial growth. Blocking muscarinic receptors with scopolamine or 5-HT2A receptors with ketanserin thus prevent SERTKO-associated mucosal growth.
Ketanserin
Intestinal crypt cells and submucosal neurons express 5-HT2A receptors.25 We used ketanserin, a relatively selective 5-HT2A antagonist, to test the idea that a receptor in the 5-HT2 family mediates the increased VH, CD, CPI (Figure 6B), and apoptosis (Supplementary Figure 2) that is seen in SERTKO animals. The actions of ketanserin (and saline controls) were compared in SERTKO and WT mice. VH was significantly shorter in ketanserin-treated SERTKO mice (184.6 ± 1.7 µm) than in SERTKO animals given saline (220.8 ± 3.0 µm; P < .0001). The same was true of CD, which also was significantly lower in ketanserin-treated SERTKO mice (78.0 ± 1.0 µm) than in SERTKO animals treated with saline (96.6 ± 1.2 µm; P < .0001). Similarly, the CPI was significantly lower in ketanserin-treated SERTKO mice (27.7% ± 1.0%) than in saline-treated SERTKO animals (31.6% ± 1.1%; P = .0212). Ketanserin almost eliminated apoptosis (Supplementary Figure 2). Although ketanserin did not affect CD when it was administered to WT mice, it increased VH and CPI (Figure 6B). Methysergide, a nonselective 5-HT1/2 inhibitor, also has been reported to increase jejunal CPI.18 Interestingly, VH in ketanserin-treated SERTKO mice was significantly less than that in ketanserin-treated WT mice (P < .0001); furthermore, although ketanserin inhibited the SERTKO-associated increase in CPI, the CPI in ketanserin-treated SERTKO animals was reduced only to the level seen in ketanserin-treated WT mice. These observations suggest that despite its ability to inhibit SERTKO-enhanced mucosal growth, ketanserin itself enhances intestinal crypt epithelial cell proliferation and VH in WT mice.
Identification of 5HT2A Receptors on Submucosal Muscarinic Neurons
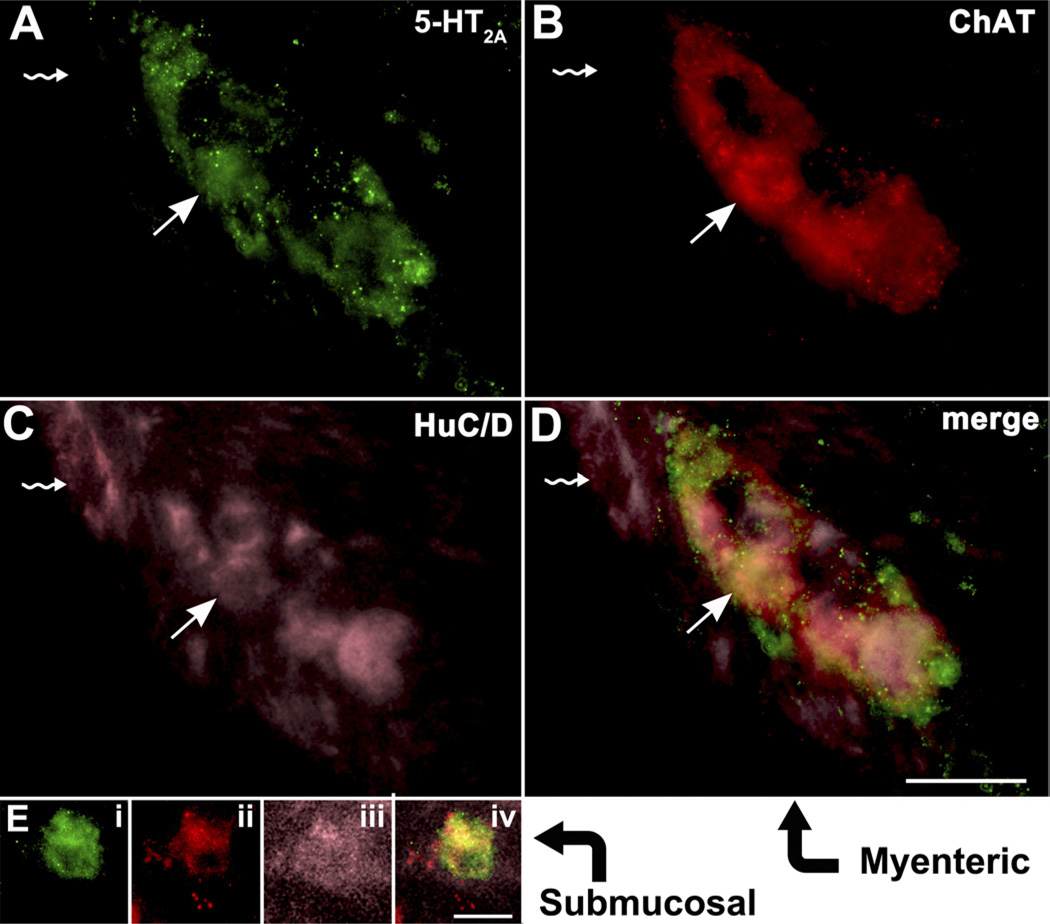
The observations that scopolamine and ketanserin each block the SERTKO-induced enhancement of VH, CD, and CPI are compatible with the idea that enteric neuronal 5-HT stimulates mucosa-innervating cholinergic neurons via 5-HT2A receptors (Figure 6C). We thus determined whether enteric cholinergic neurons express 5-HT2A receptors. Immunocytochemical identification markers were mouse monoclonal antibodies to HuC/D for neurons, rabbit polyclonal antibodies for 5-HT2A receptors, and goat antibodies to ChAT for cholinergic cells. Triply labeled 5-HT2A–immunoreactive enteric neurons were found in both submucosal and myenteric ganglia (Figure 7). Interestingly, noncholinergic neurons, identified by their lack of ChAT immunoreactivity, did not contain 5-HT2A immunoreactivity. Almost no cholinergic neurons were seen that were not 5-HT2A immunoreactive. These observations support the idea that enteric cholinergic neurons express 5-HT2A receptors.
Figure 7.
Cholinergic neurons in both the myenteric and submucosal plexuses express 5-HT2A immunoreactivity. (A–D) Myenteric ganglion. (A) 5-HT2A immunoreactivity. (B) ChAT immunoreactivity. (C) HuC/D immunoreactivity. (D) Merged image. The arrows depict a neuron that is triply labeled by all 3 markers. The arrows with a rippled stem depict a neuron that is not cholinergic and expresses neither 5-HT2A nor ChAT. (Ei–Eiv) Neuron of the submucosal plexus (neurons tend to occur singly in sections through the murine submucosal plexus). Ei, 5-HT2A immunoreactivity; Eii, ChAT immunoreactivity; Eiii, HuC/D immunoreactivity; and Eiv, merged image. Scale bars: 20 µm.
Discussion
The hypothesis that intestinal epithelial homeostasis is subject to serotonergic regulation was tested (Figure 6C). Deletion of SERT provided a gain-of-function model in which 5-HT–mediated responses are amplified as a result of deficient inactivation of released 5-HT.36,47,48 The observations that VH, CD, and CPI all were greater in SERTKO than in WT mice support the idea that 5-HT regulates epithelial proliferation and turnover. Two loss-of-function models were used to determine which of the 2 enteric 5-HT pools is most important in this process. Selective depletion of mucosal 5-HT through the genetic deletion of TPH1 (Supplementary Figure 1; compare Supplementary Figure 1A and B [WT] with Supplementary Figure 1C and D [TPH1KO] and Supplementary Figure 1E and F [TPH2KO]), which is responsible for 5-HT biosynthesis in EC and mast cells,9,10,40,43 failed to change VH, CD, or CPI from those of WT animals. In contrast, selective depletion of neuronal 5-HT through the deletion of TPH2, which is responsible for 5-HT biosynthesis in neurons14,42,49 (Supplementary Figure 1), caused VH, CD, and CPI all to be significantly less than those measured in WT control animals. These observations confirmed that the intestinal epithelium is under serotonergic regulation and further suggested that neuronal 5-HT provides a drive that increases the size of villi by enhancing crypt epithelial cell proliferation. EC cell– derived 5-HT probably opposes this effect; thus, both VH and CD were increased over WT in TPH1KO mice. In SERTKO mice, in which released 5-HT is present in excess, the neuronal effect of 5-HT appears to be dominant, leading to larger villi and deeper crypts than in WT mice. It is not yet clear why VH and CD are greater in TPH1KO mice than in WT controls while the CPI is unaffected. EC cell– derived 5-HT does not increase the width of crypt cells or enterocytes, which would lead to an increase in VH and CD without a corresponding increase in proliferation or cell number. Alternatively, if EC cell– derived 5-HT was to promote cell death, the absence of 5-HT in TPH1KO mice would be associated with increases from WT in VH and CD without a corresponding change in CPI.
EH was significantly smaller in SERTKO mice than in WT controls. It is possible that the rapid turnover of enterocytes in SERTKO animals might not allow the cells sufficient time for optimal growth. This possibility is supported by the mirror effect of increased EH seen in TPH2KO mice, which lack the serotonergic drive to enhance enterocyte turnover associated with the deletion of SERT.
Exactly why apoptosis increases along with the proliferation of enterocytes in SERTKO animals is unclear. Conceivably, the cholinergic neurons that stimulate proliferation also shorten the lifespan of enterocytes. Alternatively, apoptosis may be independent of innervation but increase as a response to the enhanced proliferation of transit-amplifying cells. The maintenance of enterocytes, for example, might be compromised by crowding of cells on villi as a result of excessive cell division.
Secretomotor neurons innervate the enteric epithelium. 2 The epithelium, however, does not receive a serotonergic innervation7 (Supplementary Figure 1). Enteric serotonergic neurons are located in the myenteric plexus, function largely as descending interneurons, and project not only to other myenteric neurons, but also to neurons in submucosal ganglia, which express many 5-HT–receptor subtypes.50 The idea that neuronal 5-HT drives proliferation in the intestinal epithelium thus suggests that 5-HT may stimulate the secretomotor or intrinsic primary afferent neurons, which provide the epithelium with its intrinsic innervation.51–53 Scopolamine, a muscarinic antagonist, was used to test the hypothesis that serotonergic neurons stimulate cholinergic neurons to drive epithelial proliferation54 (Figure 6C). Muscarinic antagonism was chosen because muscarinic receptors mediate the secretomotor effects of acetylcholine (ACh) on crypt epithelium. 26,54 The ability of scopolamine to abolish the SERTKO-associated increases in VH, CD, CPI, and apoptosis supports the idea that effects of neuronal 5-HT are indirect and caused by serotonergic activation of cholinergic neurons. The possibility that transmitters other than ACh also might participate in mediating effects of 5-HT is not eliminated, but ACh fully accounts for the mucosal changes in SERTKO mice. Curiously, scopolamine did not affect VH, CD, or CPI when given to WT mice, which suggests that the cholinergic drive to epithelial proliferation does not occur in the absence of the serotonergic provocation associated with the deletion of SERT. The overcorrection (below the values in scopolamine-treated WT mice) of VH and CD, but not CPI, that occurred when scopolamine was given to SERTKO mice is compatible with the idea that blockade of the proliferative drive from neurons unmasks an opposing inhibition of proliferation and/or promotion of apoptosis by EC cell– derived 5-HT. The increases in VH and CD observed in TPH1KO mice also are consistent with, and could be explained by, these actions of EC cell– derived 5-HT.
The SERTKO-induced increase in the size of villi cannot be explained entirely as an epithelial phenomenon. CPI increases as a result of enhanced cholinergic stimulation in SERTKO mice, but the resulting increase in VH also must involve an accompanying increase in the volume of the mesenchyme-derived components of villi. Again, it is unclear whether coordination between epithelial and connective tissues in SERTKO mice involves an effect of cholinergic neuronal activity on both tissues or a secondary effect of epithelial growth on the underlying connective tissue. An effect of nerves on epithelial cells, for example, might be transmitted to the connective tissue by signaling molecules, such as indian or sonic hedgehog. 55,56 Alternatively, it is equally conceivable that cholinergic nerves affect connective tissue cells, which transmit signals via bone morphogenetic proteins or Wnt1 to the epithelium.57
The indirect effects of 5-HT on epithelial proliferation and turnover are likely to be mediated by a member of the 5-HT2 family of receptors because ketanserin blocks them.58 Ketanserin has an affinity that is greatest for the 5-HT2A receptor, but the difference between its affinity for 5-HT2A is not so different from that for 5-HT2B and 5-HT2C that it reliably can distinguish between them.59 5-HT2A receptors are present at the bases of intestinal crypts and are abundant on intestinal smooth muscle and neurons of both enteric plexuses,25 especially submucosal neurons. In contrast, 5-HT2B receptors have been associated primarily with interstitial cells of Cajal60 and neurons of the myenteric plexus61; moreover, this receptor is highly abundant during development but down-regulated in adult life.15 5-HT2C receptors are not highly expressed in the bowel.25 The location of 5-HT2A receptors on neurons, which are likely to be the targets of the TPH2-dependent 5-HT that drives mucosal proliferation, as well as the ability of ketanserin to block SERTKO-associated epithelial proliferation, are consistent with the idea that the effects of neuronal 5-HT on mucosal homeostasis are 5-HT2A–mediated (Figure 6C). Co-localization of 5-HT2A receptors on cholinergic neurons in the submucosal ganglia further supports this mechanism.
Ketanserin interference with SERTKO-driven changes in VH, CD, and CPI suggests that these effects require the ongoing enhancement of serotonergic signaling and are not a developmental effect of SERTKO. This idea is supported further by the ability of acute administration of SSRIs to mimic SERTKO mice in their effects on VH, CD, and CPI.
The susceptibility of mucosal homeostasis to pharmacologic modulation by drugs that affect 5-HT2A receptors or SERT provides a potentially valuable means of taking therapeutic advantage of the serotonergic regulation of epithelial turnover. Drugs that affect neuronal 5-HT also may be beneficial in altering the environment in which gastrointestinal carcinomas develop.31,62
Supplementary Material
Acknowledgments
Erica Gross, Michael Gershon, and Robert Cowles were responsible for the study concept and design, analysis and interpretation of data, statistical analysis, and drafting the manuscript; Erica Gross, Zoya Gertsberg, and Kara Margolis were responsible for the acquisition of data; and Zoya Gertsberg and Kara Margolis were responsible for administrative, technical, or material support.
Funding
Supported by National Institutes of Health grants (NS12969 and NS15547 to M.D.G.), and by the Charles Edison Fund (R.A.C.).
Abbreviations used in this paper
- ACh
acetylcholine
- BrdU
bromodeoxyuridine
- CD
crypt depth
- ChAT
choline acetyltransferase
- CPI
crypt proliferation index
- EC
enterochromaffin
- EH
enterocyte height
- ENS
enteric nervous system
- 5-HT
serotonin
- SERT
serotonin re-uptake transporter
- SERTKO mice
mice that lack the serotonin re-uptake transporter
- SSRI
selective serotonin re-uptake inhibitor
- TPH1KO mice
mice that lack tryptophan hydroxylase-1
- TPH2KO mice
mice that lack neuronal but not mucosal 5-HT
- TUNEL
terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
- VH
villus height
- WT
wild type
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2012.05.007.
Conflicts of interest
These authors disclose the following: Kara Margolis and Michael Gershon received research support from Lexicon Genetics, and Michael Gershon is also a consultant for The Dannon Company, Inc. The remaining authors disclose no conflicts.
References
- 1.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Furness JB. The enteric nervous system. Malden, MA: Blackwell Publishing; 2006. [Google Scholar]
- 3.Erspamer V. Pharmacology of indolealklyamines. Pharmacol Rev. 1954;6:425–487. [PubMed] [Google Scholar]
- 4.Vialli M. Histology of the enterochromaffin cell system. In: Erspamer V, editor. Handbook of experimental pharmacology: 5-hydroxytryptamine and related indolealkylamines. Volume 19. New York: Springer-Verlag; 1966. pp. 1–65. [Google Scholar]
- 5.Gershon MD, Drakontides AB, Ross LL. Serotonin: synthesis and release from the myenteric plexus of the mouse intestine. Science. 1965;149:197–199. doi: 10.1126/science.149.3680.197. [DOI] [PubMed] [Google Scholar]
- 6.Dreyfus CF, Bornstein MB, Gershon MD. Synthesis of serotonin by neurons of the myenteric plexus in situ and in organotypic tissue culture. Brain Res. 1977;128:125–139. doi: 10.1016/0006-8993(77)90240-2. [DOI] [PubMed] [Google Scholar]
- 7.Furness JB, Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea pig small intestine. Neuroscience. 1982;7:341–350. doi: 10.1016/0306-4522(82)90271-8. [DOI] [PubMed] [Google Scholar]
- 8.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol. 2009;587:567–586. doi: 10.1113/jphysiol.2008.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav VK, Balaji S, Suresh PS, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bülbring E, Lin RCY, Schofield G. An investigation of the peristaltic reflex in relation to anatomical observations. Q J Exp Physiol. 1958;43:26–37. doi: 10.1113/expphysiol.1958.sp001305. [DOI] [PubMed] [Google Scholar]
- 12.Wade PR, Tamir H, Kirchgessner AL, et al. Analysis of the role of 5-HT in the enteric nervous system using anti-idiotypic antibodies to 5-HT receptors. Am J Physiol. 1994;266:G403–G416. doi: 10.1152/ajpgi.1994.266.3.G403. [DOI] [PubMed] [Google Scholar]
- 13.Gershon MD. Enteric serotonergic neurones … finally! J Physiol. 2009;587:507. doi: 10.1113/jphysiol.2008.167676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT(2B) receptor in the development of enteric neurons. J Neurosci. 2000;20:294–305. doi: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banasr M, Hery M, Brezun JM, et al. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu MT, Kuan YH, Wang J, et al. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tutton PJM. The influence of serotonin on crypt cell proliferation in the jejunum of rat. Virchows Arch B Cell Pathol. 1974;16:79–87. doi: 10.1007/BF02894066. [DOI] [PubMed] [Google Scholar]
- 19.Tutton PJM, Barkla DH. Neural control of colonic cell proliferation. Cancer. 1980;45:1172–1177. doi: 10.1002/1097-0142(19800315)45:5+<1172::aid-cncr2820451322>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Potten CS, Gandara R, Mahida YR, et al. The stem cells of small intestinal crypts: where are they? Cell Prolif. 2009;42:731–750. doi: 10.1111/j.1365-2184.2009.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 22.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 23.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Zou Z, Liu D, et al. Active deformation of apoptotic intestinal epithelial cells with adhesion-restricted polarity contributes to apoptotic clearance. Lab Invest. 2011;91:462–471. doi: 10.1038/labinvest.2010.182. [DOI] [PubMed] [Google Scholar]
- 25.Fiorica-Howells E, Hen R, Gingrich J, et al. 5-HT(2A) receptors: location and functional analysis in intestines of wild-type and 5-HT(2A) knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G877–G893. doi: 10.1152/ajpgi.00435.2001. [DOI] [PubMed] [Google Scholar]
- 26.Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci. 2000;915:77–80. doi: 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- 27.Neal KB, Bornstein JC. Mapping 5-HT inputs to enteric neurons of the guinea-pig small intestine. Neuroscience. 2007;145:556–567. doi: 10.1016/j.neuroscience.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Tutton PJ, Helme RD. Proceedings: the role of catecholamines in the regulation of crypt cell proliferation. I. Adrenergic stimulation and blockade. J Anat. 1973;116:467–468. [PubMed] [Google Scholar]
- 29.Tutton PJ, Barkla DH. Effect of an inhibitor of noradrenaline uptake, desipramine, on cell proliferation in the intestinal crypt epithelium. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57:349–352. doi: 10.1007/BF02899100. [DOI] [PubMed] [Google Scholar]
- 30.Helme RD, Tutton PJ, Wong WC. Proceedings: the role of catecholamines in the regulation of crypt cell proliferation. II. Sympathectomy. J Anat. 1973;116:468. [PubMed] [Google Scholar]
- 31.Tutton PJ, Barkla DH. The influence of serotonin on the mitotic rate in the colonic crypt epithelium and in colonic adenocarcinoma in rats. Clin Exp Pharmacol Physiol. 1978;5:91–94. doi: 10.1111/j.1440-1681.1978.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 32.Tutton PJ, Barkla DH. Serotonin receptors influencing cell proliferation in the jejunal crypt epithelium and in colonic adenocarcinomas. Anticancer Res. 1986;6:1123–1126. [PubMed] [Google Scholar]
- 33.Chen J-X, Pan H, Rothman TP, et al. Guinea pig 5-HT transporter: cloning, expression, distribution and function in intestinal sensory reception. Am J Physiol. 1998;275:G433–G448. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 34.Wade PR, Chen J, Jaffe B, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wade PR, Chen J, Jaffe B, et al. Localization of a serotonin transporter in the mucosa of rat small intestine. Neurosci Abst. 1993;19:495. [Google Scholar]
- 36.Bian X, Patel B, Dai X, et al. High mucosal serotonin availability in neonatal guinea pig ileum is associated with low serotonin transporter expression. Gastroenterology. 2007;132:2438–2447. doi: 10.1053/j.gastro.2007.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel BA, Bian X, Quaiserova-Mocko V, et al. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst. 2007;132:41–47. doi: 10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- 38.Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2010;153:47–57. doi: 10.1016/j.autneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Cote F, Fligny C, Bayard E, et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cote F, Thevenot E, Fligny C, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutknecht L, Kriegebaum C, Waider J, et al. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur Neuropsychopharmacol. 2009;19:266–282. doi: 10.1016/j.euroneuro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 43.Ghia JE, Li N, Wang H, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 44.Bengel D, Murphy DL, Andrews AM, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxy-methamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 45.Meedeniya AC, Brookes SJ, Hennig GW, et al. The projections of 5-hydroxytryptamine-accumulating neurones in the myenteric plexus of the small intestine of the guinea-pig. Cell Tissue Res. 1998;291:375–384. doi: 10.1007/s004410051007. [DOI] [PubMed] [Google Scholar]
- 46.Cooke HJ. Neural and humoral regulation of small intestinal electrolyte transport. In: Johnson LR, editor. Physiology of the gastrointestinal tract. Volume 2. New York: Raven Press; 1987. pp. 1307–1350. [Google Scholar]
- 47.Chen JJ, Zhishan L, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high affinity serotonin transporter (SERT): abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertrand PP, Hu X, Mach J, et al. Serotonin (5-HT) release and uptake measured by real-time electrochemical techniques in the rat ileum. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1228–G1236. doi: 10.1152/ajpgi.90375.2008. [DOI] [PubMed] [Google Scholar]
- 49.Gutknecht L, Waider J, Kraft S, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm. 2008;115:1127–1132. doi: 10.1007/s00702-008-0096-6. [DOI] [PubMed] [Google Scholar]
- 50.Frieling T, Cooke HJ, Wood JD. Serotonin receptors on submucous neurons in guinea pig colon. Am J Physiol. 1991;261:G1017–G1023. doi: 10.1152/ajpgi.1991.261.6.G1017. [DOI] [PubMed] [Google Scholar]
- 51.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci. 2000;20:3295–3309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertrand PP, Kunze WAA, Furness JB, et al. The terminals of myenteric intrinsic primary afferent neurons of the guinea pig ileum are excited by 5-HT acting at 5-HT3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 53.Furness JB, Kunze WA, Bertrand PP, et al. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 54.Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog Histochem Cytochem. 2010;44:173–202. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 55.van Dop WA, Uhmann A, Wijgerde M, et al. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway. Gastroenterology. 2009;136:2195–2203. e1–e7. doi: 10.1053/j.gastro.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 56.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 57.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Julius D, Huang KN, Livelli TJ, et al. The HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci U S A. 1990;87:928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glennon RA, Metwally K, Dukat M, et al. Ketanserin and spiperone as templates for novel serotonin 5-HT(2A) antagonists. Curr Top Med Chem. 2002;2:539–558. doi: 10.2174/1568026023393787. [DOI] [PubMed] [Google Scholar]
- 60.Wouters MM, Gibbons SJ, Roeder JL, et al. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133:897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Bassil AK, Taylor CM, Bolton VJ, et al. Inhibition of colonic motility and defecation by RS-127445 suggests an involvement of the 5-HT2B receptor in rodent large bowel physiology. Br J Pharmacol. 2009;158:252–258. doi: 10.1111/j.1476-5381.2009.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drozdov I, Kidd M, Gustafsson BI, et al. Autoregulatory effects of serotonin on proliferation and signaling pathways in lung and small intestine neuroendocrine tumor cell lines. Cancer. 2009;115:4934–4945. doi: 10.1002/cncr.24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.