Abstract
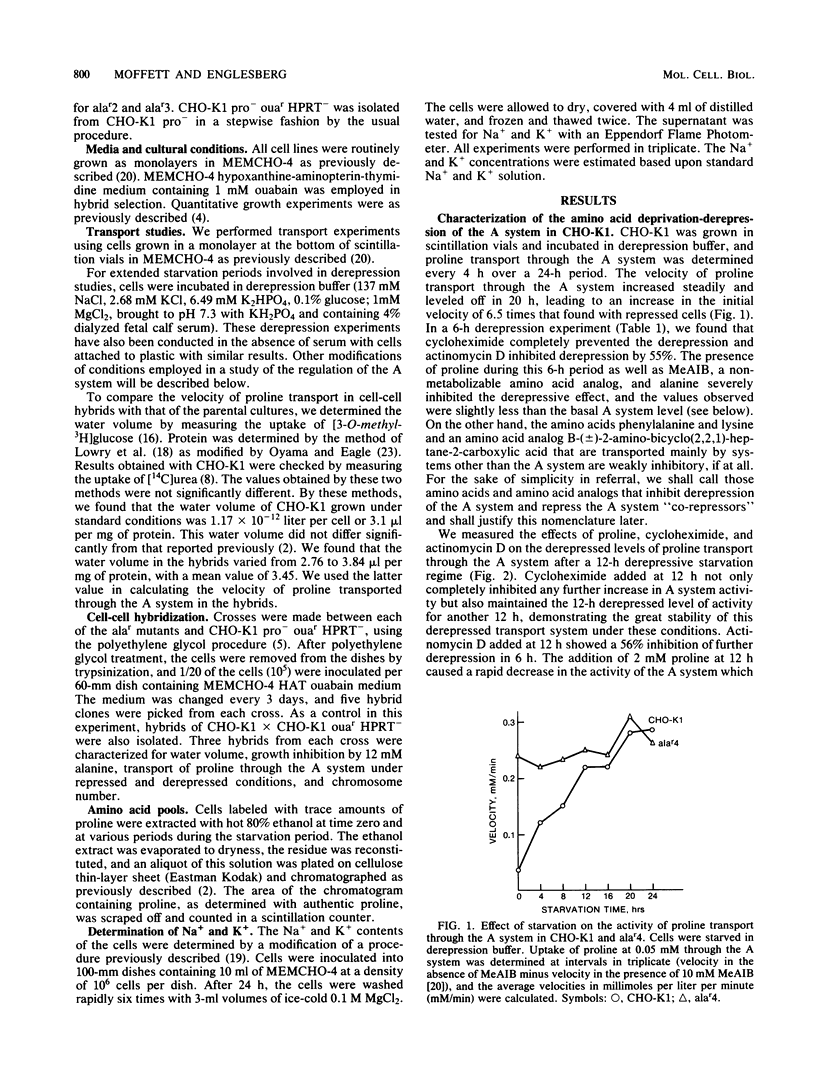
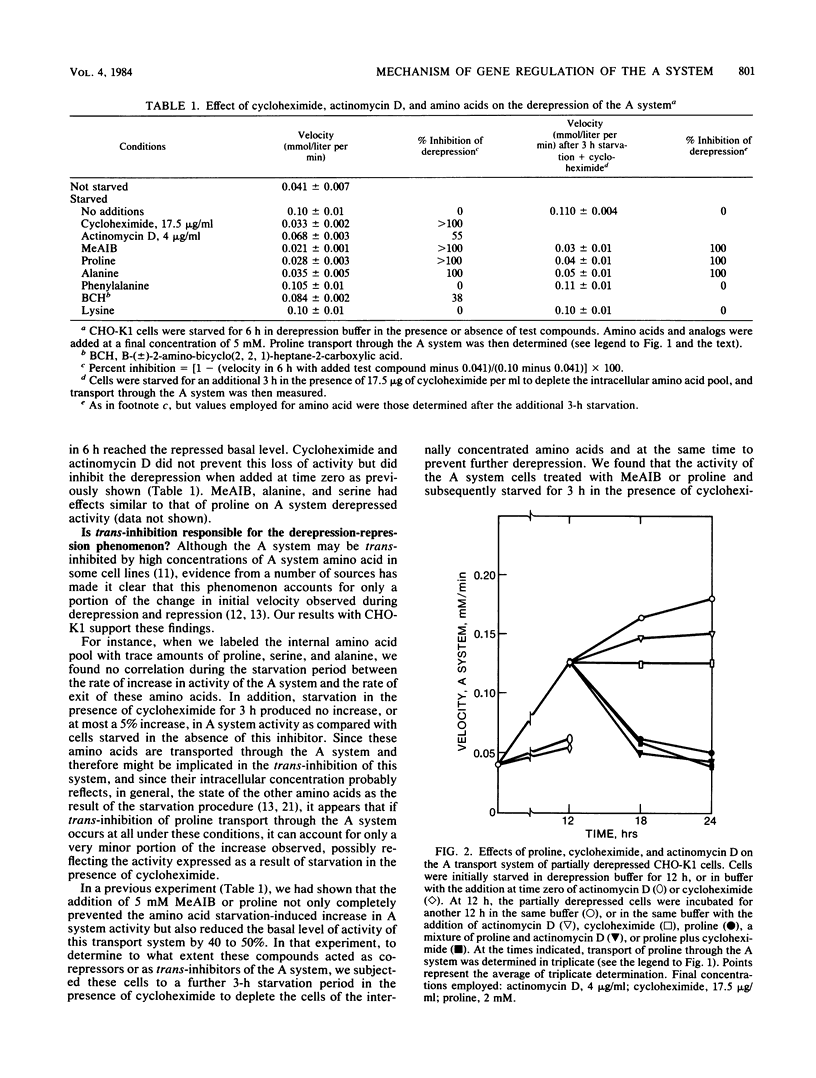
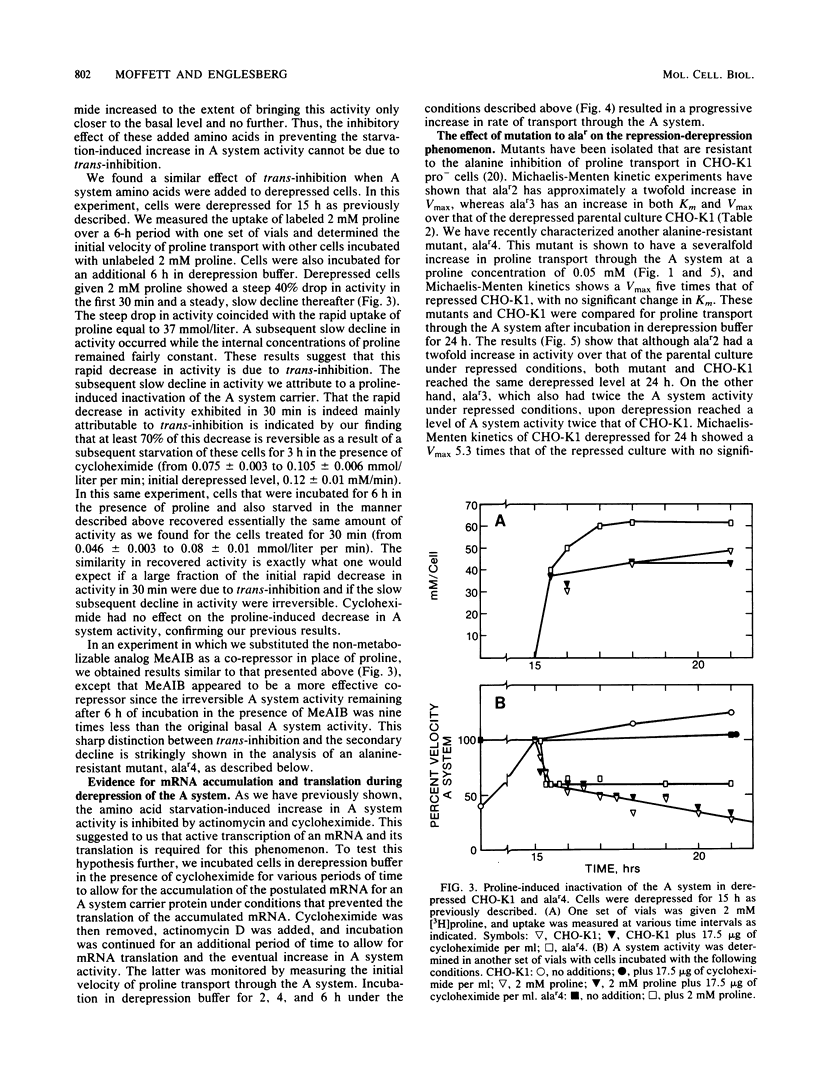
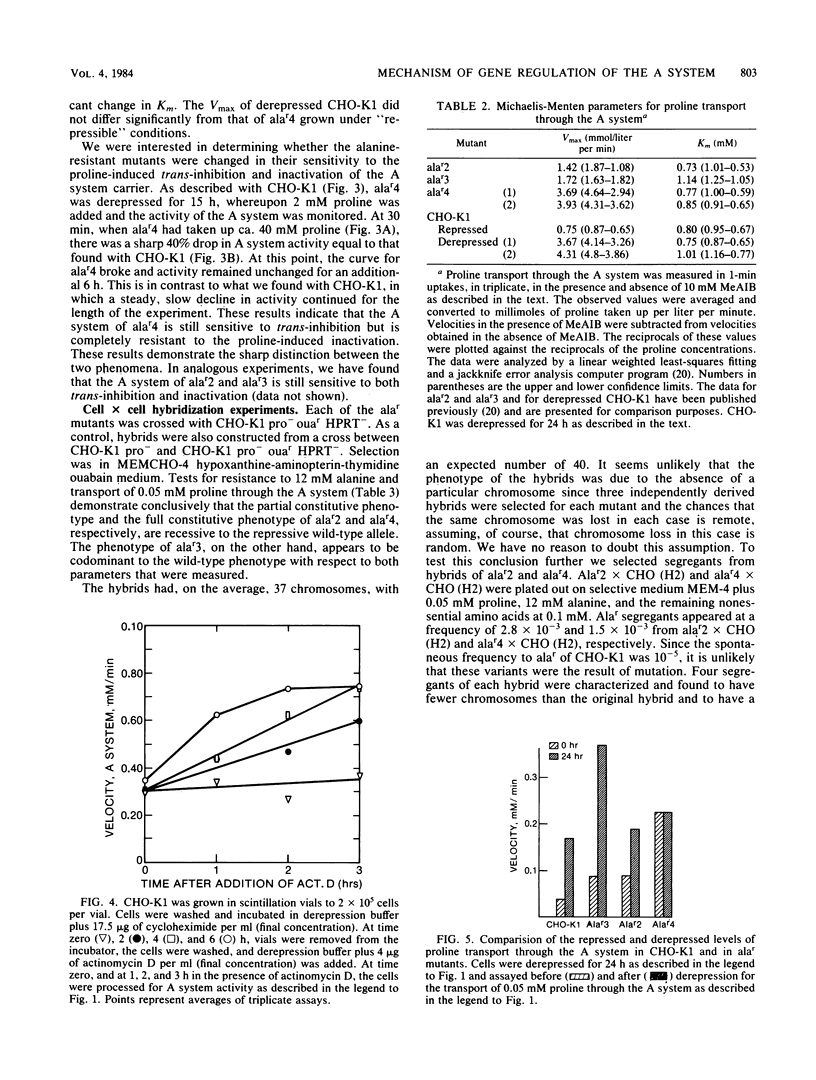
Chinese hamster ovary cells (CHO-K1) starved for 24 h for amino acids show a severalfold increase in velocity of proline transport through the A system (Vmax is five times that of unstarved cells). This increase is inhibited by cycloheximide, actinomycin D, N-methyl-alpha-amino isobutyric acid (MeAIB, a non-metabolizable specific A system amino acid analog), and by other amino acids that are generally transported by the A system. However, transport by the A system is not a prerequisite for this repression, and all compounds that have affinity for the A system do not necessarily act as "co-repressors." The addition of proline, MeAIB, or other amino acids, as described above, to derepressed cells results in a rapid decrease in A system activity. As shown with proline and MeAIB, this decrease in activity is in part due to a rapid trans-inhibition and a slow, irreversible inactivation of the A system. Neither process is inhibited by cycloheximide or actinomycin D. Alanine antagonizes the growth of CHO-K1 pro cells by preventing proline transport, and alanine-resistant mutants (alar) have been isolated (Moffett et al., Somatic Cell Genet. 9:189-213, 1983). alar2 and alar4 are partial and full constitutive mutants for the A system and have two and six times the Vmax for proline uptake by the A system, respectively. The A system in alar4 is also immune to the co-repressor-induced inactivation. Both alar2 and alar4 phenotypes are recessive. Alar3 shows an increase in Vmax and Km for proline transport through the A system, and this phenotype is codominant. All three mutants have a pleiotropic effect, producing increases in activity of the ASC and P systems of amino acid transport. This increase is not due to an increase in the Na+ gradient. The ASC and P phenotypes behave similarly to the A system in hybrids. A model has been proposed incorporating these results.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass R., Hedegaard H. B., Dillehay L., Moffett J., Englesberg E. The A, ASC, and L systems for the transport of amino acids in Chinese hamster ovary cells (CHO-K1). J Biol Chem. 1981 Oct 25;256(20):10259–10266. [PubMed] [Google Scholar]
- Curriden S., Englesberg E. Inhibition of growth of proline-requiring Chinese hamster ovary cells (CHO-k1) resulting from antagonism by a system amino acids. J Cell Physiol. 1981 Feb;106(2):245–252. doi: 10.1002/jcp.1041060210. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., O'Malley K. A., Wheeler T. B. Polyethylene glycol-induced mammalian cell hybridization: effect of polyethylene glycol molecular weight and concentration. Somatic Cell Genet. 1976 May;2(3):271–280. doi: 10.1007/BF01538965. [DOI] [PubMed] [Google Scholar]
- Englesberg E., Bass R., Heiser W. Inhibition of the growth of mammalian cells in cuture by amino acids and the isolation and characterization of L-phenylalanine transport. Somatic Cell Genet. 1976 Sep;2(5):411–428. doi: 10.1007/BF01542722. [DOI] [PubMed] [Google Scholar]
- Ertsey R., Englesberg E. Recessive 2-(methylamino)-isobutyrate (MeAIB)-resistant mutant of Chinese hamster ovary cells (CHO-K1) with increased transport through ASC system. Somat Cell Mol Genet. 1984 Mar;10(2):171–182. doi: 10.1007/BF01534906. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Pardee A. B. Transport of amino acids by confluent and nonconfluent 3T3 and polyoma virus-transformed 3T3 cells growing on glass cover slips. J Biol Chem. 1969 May 25;244(10):2675–2681. [PubMed] [Google Scholar]
- Franchi-Gazzola R., Gazzola G. C., Ronchi P., Saibene V., Guidotti G. G. Regulation of amino acid transport in chick embryo heart cells. II. Adaptive control sites for the "A mediation". Biochim Biophys Acta. 1973 Jan 26;291(2):545–556. doi: 10.1016/0005-2736(73)90506-3. [DOI] [PubMed] [Google Scholar]
- Gazzola G. C., Dall'Asta V., Guidotti G. G. Adaptive regulation of amino acid transport in cultured human fibroblasts. Sites and mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3191–3198. [PubMed] [Google Scholar]
- Gazzola G. C., Franchi R., Saibene V., Ronchi P., Guidotti G. G. Regulation of amino acid transport in chick embryo heart cells. I. Adaptive system of mediation for neutral amino acids. Biochim Biophys Acta. 1972 May 9;266(2):407–421. doi: 10.1016/0005-2736(72)90097-1. [DOI] [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Heaton J. H., Gelehrter T. D. Derepression of amino acid transport by amino acid starvation in rat hepatoma cells. J Biol Chem. 1977 May 10;252(9):2900–2907. [PubMed] [Google Scholar]
- Kelley D. S., Potter V. R. Regulation of amino acid transport systems by amino acid depletion and supplementation in monolayer cultures of rat hepatocytes. J Biol Chem. 1978 Dec 25;253(24):9009–9017. [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lubin M. Control of growth by intracellular potassium and sodium concentrations is relaxed in transformed 3T3 cells. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1060–1067. doi: 10.1016/0006-291x(80)91483-7. [DOI] [PubMed] [Google Scholar]
- Moffett J., Curriden S., Ertsey R., Mendiaz E., Englesberg E. Alanine-resistant mutants of Chinese hamster ovary cells, CHO-K1, producing increases in velocity of proline transport through the A, ASC, and P systems. Somatic Cell Genet. 1983 Mar;9(2):189–213. doi: 10.1007/BF01543177. [DOI] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Oxender D. L., Lee M., Cecchini G. Regulation of amino acid transport activity and growth rate of animal cells in culture. J Biol Chem. 1977 Apr 25;252(8):2680–2683. [PubMed] [Google Scholar]
- Oxender D. L., Lee M., Moore P. A., Cecchini G. Neutral amino acid transport systems of tissue culture cells. J Biol Chem. 1977 Apr 25;252(8):2675–2679. [PubMed] [Google Scholar]
- Schimke R. T., Alt F. W., Kellems R. E., Kaufman R. J., Bertino J. R. Amplification of dihydrofolate reductase genes in methotrexate-resistant cultured mouse cells. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):649–657. doi: 10.1101/sqb.1978.042.01.067. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Jayme D. W., Kilberg M. S., Oxender D. L. Neutral amino acid transport systems in Chinese hamster ovary cells. J Biol Chem. 1981 Jun 10;256(11):5422–5427. [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Taub M., Englesberg E. 5-fluorotryptophan resistant mutants affecting the A and L transport systems in the mouse L cell line A9. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):477–486. doi: 10.1002/jcp.1040970323. [DOI] [PubMed] [Google Scholar]


