Abstract
The humoral and cellular immune responses in the genital mucosa likely play an important role in the prevention of sexually transmitted infections, including infection with human immunodeficiency virus type 1 (HIV-1). Here we show that vaginal infection of progesterone-treated BALB/c mice with a recombinant influenza virus bearing the immunodominant P18IIIB cytotoxic T-lymphocyte (CTL) epitope of the gp160 envelope protein from an HIV-1 IIIB isolate (P18IIIB; RIQRGPGRAFVTIGK) can induce a specific immune response in regional mucosal lymph nodes, as well as in a systemic site (the spleen). A single inoculation of mice with the recombinant influenza virus induced long-lasting (at least 5 months) antigen-specific CTL memory detectable as a rapid recall of effector CTLs upon vaginal infection with recombinant vaccinia virus expressing HIV-1 IIIB envelope gene products. Long-term antigen-specific CTL memory was also induced and maintained in distant mucosal tissues when mice were intranasally immunized with the recombinant influenza virus. These results indicate that mucosal immunization and, in particular, local vaginal immunization with recombinant influenza virus can provide strong, durable immune responses in the female genital tract of mice.
Mucosal surfaces represent the primary portal of entry into animals for a variety of pathogens, including human immunodeficiency virus type 1 (HIV-1). Due to functionally distinct compartmentalization of the immune system, the systemic routes of immunization are usually of limited value for the prevention of some mucosa-contracted infectious diseases, while mucosal immunization is capable of inducing both mucosal and systemic immunity (18, 26, 46). Thus, induction of strong mucosal immunity is important for the development of effective vaccines. In particular, immunization targeting local mucosal surfaces or the regional lymph nodes to elicit both humoral and cellular specific immune responses may present a strategy for preventing or controlling HIV-1 replication as well as that of other mucosally transmitted pathogens (2, 29, 30).
Recombinant influenza viruses engineered to express foreign antigens have successfully induced a vigorous immune response in mice immunized by the intranasal route (11, 17, 31, 40, 47). In particular, the ability of influenza virus to infect dendritic cells and promote their phenotypic conversion to mature and effective antigen-presenting cells is thought to play the most significant role in the induction of immunity to foreign antigens delivered by recombinant virus (6, 12, 39).
Previous studies showed that progesterone pretreatment overcomes the age-dependent resistance of adult mice to vaginal herpes simplex virus type 2 (HSV-2) infection, making them a suitable model for long-term studies of immunity (42, 43). Progesterone treatment was also reported to increase the susceptibility of rhesus macaques to genital infection by simian immunodeficiency virus (33) and of mice to infection by Chlamydia trachomatis (49). Here, we evaluate whether an influenza virus can replicate in the mouse vaginal tract and induce mucosal immunity to an HIV-1 epitope. To this end, we generated a recombinant influenza A virus (Flu/P18IIIB), expressing the P18IIIB cytotoxic T-lymphocyte (CTL) epitope derived from the V3 loop of HIV-1 IIIB envelope protein (residues 315 to 329, RIQRGPGRAFVTIGK) (H-2Dd) (45) in the neuraminidase stalk of A/WSN/33 (WSN) virus, by reverse genetics (10, 15). Flu/P18IIIB virus was attenuated in BALB/c mice; its dose required to kill 50% of infected mice (MLD50) was 106 PFU upon intranasal inoculation, whereas the MLD50 of the wild-type WSN virus was 102.5 PFU.
Intravaginal infection of mice with influenza A virus.
Groups of female BALB/c mice (Charles River, Calco, Italy), 6 to 8 weeks old, were subcutaneously injected with 3 mg of progesterone (Depo-Provera; Pharmacia & Upjohn), and 5 days later they were vaginally infected with influenza viruses (3 × 105 PFU/10 μl). Titers of virus in the vaginal washes were determined with MDCK cells.
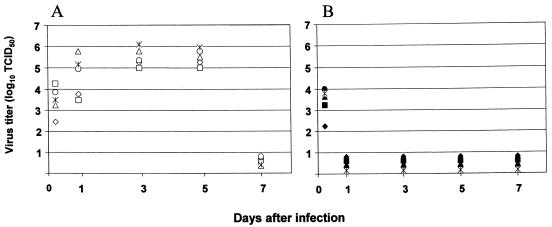
Viral replication was evident with the highest titers of virus present in vaginal washes on days 3 to 5 (Fig. 1A). By contrast, the virus did not efficiently replicate in untreated control mice (Fig. 1B). On day 7, virus was not detected in any of the vaginal wash samples. Viral replication patterns similar to those seen with Flu/P18IIIB virus were obtained with the wild-type A/WSN/33, A/PR/8/34 (PR8), and X-31 viruses (data not shown). These data suggest the presence of a protease responsible for cleavage of PR8 and X-31 viral hemagglutinin in vaginal tissues.
FIG. 1.
Titers of virus in vaginal wash samples of mice vaginally infected with Flu/P18IIIB virus. Progesterone-treated mice (A) and untreated mice (B) were vaginally infected with 3 × 105 PFU of Flu/P18IIIB virus, and vaginal wash samples were analyzed for virus after 5 h and 1, 3, 5, and 7 days postinfection using MDCK cells. Results are shown for individual mice.
To assess virus replication in vaginal mucosa, we inoculated groups of mice with log dilutions of the A/WSN/33 virus inoculum (2 × 104 to 2 × 107 PFU). In the group vaginally infected with influenza virus at 2 × 104 PFU, low or undetectable levels of virus were present in vaginal washes collected during the early phases of infection (5 h and 1 day postinfection). Nonetheless, the influenza virus titers peaked on days 3 to 5, with titers comparable to levels obtained with the higher inoculum, and the virus was cleared by day 7 (data not shown). Notably, the vaginal inoculation of mice with 50 MLD50 of WSN virus (as determined by intranasal inoculation) was well tolerated, producing no obvious signs of illness (e.g., distress, weight loss, or ruffled fur).
Induction of mucosal and systemic cellular immune responses by vaginal immunization with Flu/P18IIIB virus.
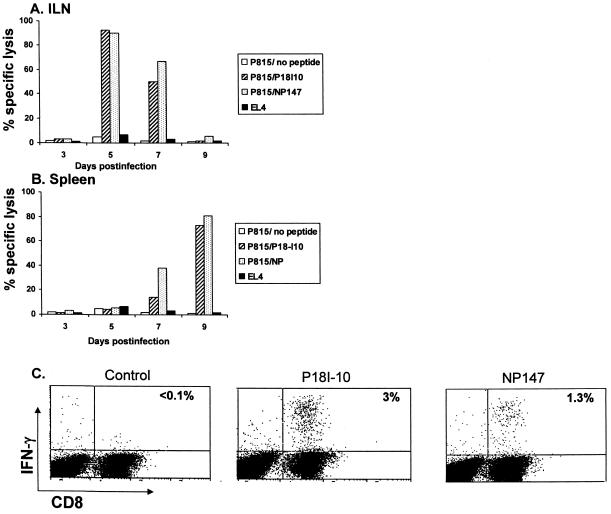
We next investigated whether vaginal infection with influenza virus would elicit CTLs specific for the minimal P18IIIB-I10 epitope (RGPGRAFVTI) and the immunodominant epitope NP147 (TYQRTRALV) (8) (H-2Kd) of influenza virus nucleoprotein (NP) in the spleen and iliac lymph nodes (ILNs) draining the genitorectal mucosa. ILN-derived lymphocytes of mice vaginally infected with Flu/P18IIIB virus, cultured for 3 days in the presence of 10 U of recombinant interleukin 2/ml and without antigen stimulation, demonstrated a major histocompatibility complex-restricted antigen-specific CTL response that peaked around day 5 postinfection, with 92% and 90% mean levels of specific lysis determined for the respective HIV P18IIIB- and NP-derived epitopes at the effector-to-target (E:T) ratio of 50:1 (Fig. 2A). Similarly, the splenocytes isolated at the same time points and restimulated in vitro with specific peptides for 6 days showed strong CTL activity against both P18IIIB- and NP-derived epitopes, although the response on day 9 postinfection was greater (Fig. 2B).
FIG. 2.
Induction of specific CTL responses by vaginal infection with Flu/P18IIIB virus. Groups of six mice were vaginally infected with 3 × 105 PFU of Flu/P18IIIB virus, and specific CTL activity was determined in ILNs (A) and spleens (B) at different time points. The ILNs were cultured without antigen stimulation for 3 days before study with a 51Cr release assay (A). Spleen cells were stimulated for 6 days in vitro with irradiated peptide-loaded stimulators and then examined for CTL activity against peptide-coated target cells (B). E:T ratios were 50:1. Lysis of unpulsed P815 target cells and allogeneic EL4 target cells are shown. The data are mean percentages of specific lysis for triplicate samples; standard deviations were <5%. (C) The presence of CD8+-T-lymphocyte effectors in ILNs isolated at day 5 postinfection was also evaluated by intracellular staining for IFN-γ following peptide-specific stimulation. Percentages of IFN-γ-positive CD8+ T cells are indicated in the upper right corner of each panel. Representative data from three experiments are shown.
We also used intracellular cytokine staining for gamma interferon (IFN-γ) to identify and quantify CTLs that were functionally reactive to the P18-I10 peptide. CD8+ T cells (gated for CD8) from the ILNs, isolated 5 days postimmunization and cultured in vitro for 3 days without exogenous antigen stimulation, were examined by flow-cytometric analysis for IFN-γ production following in vitro peptide-specific stimulation for 5 h in the presence of brefeldin A (37). Approximately 3 and 1.3% of CD8- and IFN-γ-positive cells were specific for P18-I10 and NP147, respectively (Fig. 2C). Background IFN-γ staining for immunized mice that were not stimulated with peptide was low, similar to that for the isotype control immunoglobulin (data not shown). Reactivity was not detected using irrelevant peptides that bind to the same major histocompatibility complex molecule in preliminary experiments.
CTL memory responses in vaginally immunized mice.
To determine whether systemic memory CTL responses were induced by vaginal immunization, we harvested splenocytes from Flu/P18IIIB-infected mice at 5 months after the single immunization, restimulated them in vitro, and tested for peptide-specific CTL activity. CTL effector cells specific for both the P18-I10 and the NP147 epitopes were detected and produced 41% and 64% specific lysis, respectively, against peptide-pulsed target cells when tested at an E:T ratio of 50:1 (data not shown), demonstrating induction of systemic memory CTL responses by vaginal immunization.
To examine CTL memory within the mucosal immune compartments of vaginally infected mice in vivo, we evaluated the rapid recall of memory T cells after reexposure to antigen. We therefore vaginally infected groups of naïve mice and those vaginally immunized with Flu/P18IIIB virus with a recombinant vaccinia virus expressing the HIV-1 IIIB envelope gene products (vPE16) (14) at 5 months postimmunization.
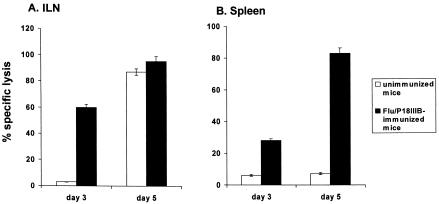
As shown in Fig. 3, the CTL response from ILN-derived cells of immunized mice at 3 days after vPE16 challenge resulted in significant lysis of 51Cr-labeled P815 cells pulsed with P18-I10 peptide, which was absent in the control groups of nonimmune mice. Immune and naive mice showed high CTL activity at 5 days after vaginal infection with vPE16 (Fig. 3A). The CTL response from in vitro-restimulated splenocyte effectors of immunized mice showed a time course typical of that of a secondary immune response. The detectable levels of CTL activity to P18-I10 peptide on day 3 increased to substantial levels on day 5 after challenge. By contrast, the primary response of splenocytes derived from the naive mice was not detectable even on day 5 after VPE16 infection (Fig. 3B). These results indicate that antigen-specific memory T cells were still present in mucosal as well as systemic lymphoid tissues at 5 months after Flu/P18IIIB virus immunization.
FIG. 3.
CTL memory upon vaginal infection with Flu/P18IIIB virus. Mice were vaginally infected with 3 × 105 PFU of Flu/P18IIIB virus and infected with 2 × 107 PFU of vaccinia vPE16 virus 5 months later. Naive (unimmunized) mice were also infected with vPE16 and served as a control representing the magnitude of a primary response. ILNs (A) and spleens (B) were removed at day 3 or 5 after vaginal vPE16 infection. The ILNs were cultured without antigen stimulation for 3 days before being tested in a CTL assay. Spleen cells were stimulated for 6 days in vitro with irradiated P18-I10 peptide-loaded stimulators before being examined for CTL activity. CTLs were assayed with P18-I10 peptide-pulsed P815 cells used as targets. Mean lysis values (± standard deviation) measured at an E:T ratio of 50:1 are shown.
Long-lived memory CTL activity in distant mucosa-associated lymph nodes.
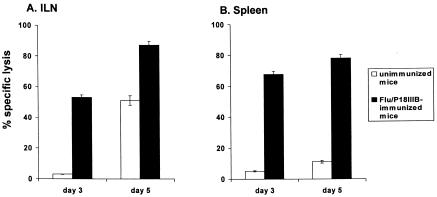
The mucosa of the genital tract is part of the common mucosal immune system; hence, intranasally administered antigens induce specific mucosal immune responses at local as well as distant sites, including genital tissues and draining lymph nodes (18, 34, 40). Therefore, we examined whether memory CTL activity, induced by intranasal immunization of anesthetized mice with 6 × 105 PFU of Flu/P18IIIB virus, is detectable as a rapid recall of effector CTLs in the ILN of mice infected vaginally with vPE16 4 months later. As described above, the CTL response of ILN-derived lymphocytes was determined for both naive and immunized groups of mice sacrificed on days 3 and 5 after vPE16 challenge. High levels of P18-I10-specific CTL activity were detectable in the ILN and spleen of immune mice as early as 3 days after vPE16 infection (Fig. 4). By contrast, naive mice showed specific CTL activity in the ILN only by day 5 (Fig. 4A) and in the spleen by day 7 (data not shown). These findings confirm the ability of influenza virus-based vaccines to prime the immune system for mucosal challenge, even from a distant site.
FIG. 4.
Long-term memory in the genital organ-associated lymphoid tissues and spleens of mice intranasally infected with Flu/P18IIIB virus. Five mice intranasally infected with 3 × 105 PFU of Flu/P18IIIB virus were vaginally infected with 2 × 107 PFU of vPE16 4 months later. Naive (unimmunized) mice were also infected with vPE16 and served as a control representing the magnitude of a primary response. ILNs (A) and spleens (B) were removed at day 3 or 5 after vaginal challenge. Following 3 days of in vitro culture without antigen stimulation for ILN and 6 days of culture with irradiated P18-I10 peptide-loaded stimulators for spleen cells, the presence of CTL effectors was evaluated in a 51Cr release assay, with P18-I10 peptide-pulsed P815 cells used as targets. The results are plotted as the mean net percentage of specific lysis (± standard deviation) at an E:T ratio of 50:1.
Humoral response induced by vaginal influenza virus immunization.
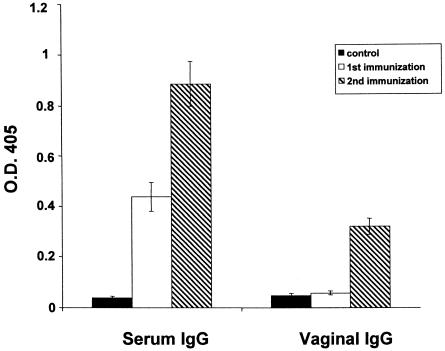
It was of interest to determine whether antibody responses were induced against the virus after vaginal infection. We therefore measured influenza virus-specific antibodies in serum samples and vaginal lavages by enzyme-linked immunosorbent assay (52). Immunoglobulin G (IgG) specific for influenza virus was detected in serum, but not in vaginal washes, at 2 weeks postinfection (Fig. 5), prompting us to vaginally infect mice twice (with a 2-week interval) and test for virus-specific antibodies 2 weeks after the second infection. The level of virus-specific IgG in serum was higher in mice receiving the virus twice than in those receiving it once, and vaginal IgG was also detected in the former group. Virus-specific IgA antibodies in vaginal washes were detected at lower levels than IgG and not in all mice tested (data not shown).
FIG. 5.
Mucosal and systemic anti-influenza virus IgG antibodies after vaginal infection with A/WSN/33 virus. The IgG response in samples from mice (n = 4) was measured 2 weeks after the first immunization and 2 weeks after the second immunization. Values for vaginal washings and sera were obtained with use of 4- and 50-fold-diluted samples, respectively. At these dilutions, the absorbance values for control samples were <0.03. Control refers to uninfected animals.
Here we demonstrate that upon vaginal infection, influenza virus can induce long-term cellular immune responses in mice, at both mucosal and systemic sites, against its own as well as foreign epitopes. The vaginal inoculation of mice with influenza virus also induced humoral responses in sera and vaginal secretions.
In mammals, most influenza A viruses produce acute infections that usually remain confined to epithelia lining the respiratory tract. In this study, we found that progesterone-treated BALB/c mice support vaginal replication of the influenza viruses tested. The effect of the estrous cycle on the progesterone-induced susceptibility to vaginal infection by HSV-2 in adult BALB/c mice has been previously reported and has been suggested to be related to the increased permeability of the epithelial layer or to other factors, such as possible changes in virus receptors on the epithelial cells (42, 43). Studies are in progress to characterize the sites of virus replication and the extent of inflammation (by immunohistochemistry) in the female genital organs of mice after influenza virus infection.
Much effort has been exerted to induce strong HIV-specific mucosal immunity. HIV-specific segretory IgA antibody with neutralizing activity in secretions on the mucosal surface is thought to be important for preventing HIV infection (9, 13). In addition, an ideal vaccine capable of preventing transmission or dissemination of sexually acquired pathogens may rely on the induction of antigen-specific CTLs in genital and rectal tissues and draining lymph nodes (3, 5, 29, 30). ILNs function as an inductive site from which T and B cells home preferentially to the vaginal, cervical, and rectal mucosa (36). Thus, localization of sensitized T and B cells in these lymph nodes might be essential to preventing HIV transmission by virus-infected Langherans cells, dendritic cells, or macrophages from the mucosal tissues to the lymph nodes. In this context, rectal and vaginal immunization of mice with peptides, plasmid DNA, or viral vectors has been shown to induce detectable CTLs in ILNs, in some cases in association with reduced replication in the ovaries of vaccinia virus expressing the HIV-1 gene (2, 50). For the most part, this CTL response was enhanced by the coadministration of cytokines or cytokine-expressing plasmids, suggesting that appropriate costimulators play an essential role in inducing an appropriate immune response (4, 24). Our studies suggest an alternative approach: the use of influenza virus for vaginal immunization. Influenza A viruses are strong inducers of cellular immune responses. In particular, the capacity of influenza viruses to infect dendritic cells and to express viral genes at high levels allows the antigen-presenting cells to initiate an appropriate response that may have practical implications for local vaginal immunization strategies (6, 12).
The concept of the common mucosal immune system implies that an immune response may occur in distal mucosal sites in addition to the site of vaccine administration (26, 28, 35, 51, 53). The induction of specific humoral immune responses in the genital tract of intranasally immunized mice is well documented (7, 34). Muster et al. (40) showed that replication of a chimeric influenza virus carrying the neutralizing gp41 epitope ELDKWA in the upper respiratory tract was sufficient to induce a systemic antibody response as well as a local immune response in the genital tract.
The induction and maintenance of antiviral long-term CTL memory responses in mucosa-associated lymphoid organs depend on mucosal immunization (18-20, 27). By examining rapid recall responses after reexposure to antigen in vivo, Gallichan and Rosenthal (18) demonstrated that intranasal immunization of mice with an adenoviral vector expressing the gB (AdgB8) antigen of HSV-2 resulted in long-term CTL memory not only in the local mucosal tissues of the respiratory tract but also in the mucosal tissues of the genital tract. Long-term memory CTL responses to foreign epitopes have been previously reported upon intranasal immunization with recombinant influenza viruses (11, 48). Ferko et al. (17) showed that a recombinant influenza virus containing an insertion of the 137 C-terminal amino acids of the HIV-1 Nef protein into the NS1 reading frame was able to induce a CD8+-T-cell response in mouse spleen and lymph nodes draining the urogenital tract. Excellent priming induced in the distal genitorectal draining lymph nodes by recombinant influenza virus has also been recently reported (21). Thus, the influenza virus system is a robust model for induction of a CTL response to protective CTL epitopes. Studies are in progress to determine the effect of the mucosal immunity induced by recombinant influenza virus given vaginally on long-term protection from challenge with pathogens.
Recent efforts in AIDS vaccine research focus on stimulation of strong cellular immune responses, with prime-boost regimens relying on viral vectors for antigen delivery. There will likely be no optimal type of mucosal vaccine; rather, the immunization protocol may specify combinations of different vaccine vectors, which could present the same or related antigens differently to the immune system or to alternative sites, to induce optimal immunity against the pathogen of interest (1, 16, 23, 25, 44, 54). Thus, mucosal vaccination represents one of several methods that may prove useful in establishing effective immunization protocols. The positive effect of recombinant influenza and vaccinia viruses on the immune response against a foreign epitope has already been described (21, 22, 32, 38, 41). However, our success in inducing a specific immune response via the vaginal route of immunization with a recombinant influenza virus provides a basis for further research on combination vaccines for sexually transmitted diseases. Direct application of an antigen to the target mucosa may be the most efficient way to induce a local protective immune response. The administration of large doses of recombinant virus by this route is likely to be of practical interest because of the magnitude of systemic and local immune responses that may block, or at least limit, the entry of sexually transmitted pathogens.
Acknowledgments
We thank Patrizia Di Zeo for assistance in the animal experiments and John Gilbert for scientific editing.
This work was supported in part by grants from the Istituto Superiore di Sanità, the Italian Ministry of Health, the Italian Project on AIDS, Consiglio Nazionale delle Ricerche, by National Institute of Allergy and Infectious Diseases Public Health Service research grants, by CREST (Japan Science and Technology Corporation), by Grants-in-Aid by the Ministry of Education, Culture, Sports, Science and Technology, and by the Ministry of Health, Labor and Welfare, Japan.
REFERENCES
- 1.Barnett, S. W., J. M. Klinger, B. Doe, C. M. Walker, L. Hansen, A. M. Duliege, and F. M. Sinangil. 1998. Prime-boost immunization strategies against HIV. AIDS Res. Hum. Retrovir. 14(Suppl. 3):299-309. [PubMed] [Google Scholar]
- 2.Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95:1709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belyakov, I. M., L. S. Wyatt, J. D. Ahlers, P. Earl, C. D. Pendleton, B. L. Kelsall, W. Strober, B. Moss, and J. A. Berzofsky. 1998. Induction of mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J. Virol. 72:8264-8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., J. D. Ahlers, J. D. Clements, W. Strober, and J. A. Berzofsky. 2000. Interplay of cytokines and adjuvants in the regulation of mucosal and systemic HIV-specific CTL. J. Immunol. 165:6454-6462. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj, N., A. Bender, N. Gonzales, L. K. Bui, M. C. Garrett, and R. M. Steinman. 1994. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J. Clin. Investig. 94:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bienestock, J., A. D. Defus, M. McDermott, S. Mirski, K. Rosenthal, and A. Tagliabue. 1992. The mucosal immunologic network: compartmentalization of lymphocytes, natural killer cells and mast cells. Ann. N. Y. Acad. Sci. 409:164-170. [DOI] [PubMed] [Google Scholar]
- 8.Bodmer, H. C., R. M. Pemberton, J. B. Rothbard, and B. A. Askonas. 1988. Enhanced recognition of a modified peptide antigen by cytotoxic T cells specific for influenza nucleoprotein. Cell 52:253-258. [DOI] [PubMed] [Google Scholar]
- 9.Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277-287. [DOI] [PubMed] [Google Scholar]
- 10.Castrucci, M. R., P. Bilsel, and Y. Kawaoka. 1992. Attenuation of influenza A virus by insertion of a foreign epitope into the neuraminidase. J. Virol. 66:4647-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castrucci, M. R., S. Hou, P. C. Doherty, and Y. Kawaoka. 1994. Protection against lethal lymphocytic choriomeningitis virus (LCMV) infection by immunization of mice with an influenza virus containing an LCMV epitope recognized by cytotoxic T lymphocytes. J. Virol. 68:3486-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devito, C., K. Broliden, R. Kaul, L. Svensson, K. Johansen, P. Kiama, J. Kimani, L. Lopalco, S. Piconi, J. J. Bwayo, F. Plummer, M. Clerici, and J. Hinkula. 2000. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 165:5170-5176. [DOI] [PubMed] [Google Scholar]
- 14.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enami, M., W. Luytjes, M. Krystal, and P. Palese. 1990. Introduction of site-specific mutations into the genome of influenza virus. Proc. Natl. Acad. Sci. USA 87:3802-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eo, S. K., M. Gierynska, A. A. Kamar, and B. T. Rouse. 2001. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J. Immunol. 166:5473-5479. [DOI] [PubMed] [Google Scholar]
- 17.Ferko, B., J. Stasakova, S. Sereinig, J. Romanova, D. Katinger, B. Niebler, H. Katinger, and A. Egorov. 2001. Hyperattenuated recombinant influenza A virus nonstructural-protein-encoding vectors induce human immunodeficiency virus type 1 Nef-specific systemic and mucosal immune responses in mice. J. Virol. 75:8899-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallichan, W. S., and K. L. Rosenthal. 1996. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 184:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallichan, W. S., and K. L. Rosenthal. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177:1155-1161. [DOI] [PubMed] [Google Scholar]
- 20.Gallichan, W. S., R. N. Woolstenencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 21.Gherardi, M. M., J. L. Najera, E. Perez-Jimenez, S. Guerra, A. Garcia-Sastre, and M. Esteban. 2003. Prime-boost immunization schedules based on influenza virus and vaccinia virus vectors potentiate cellular immune responses against human immunodeficiency virus Env protein systemically and in the genitorectal draining lymph nodes. J. Virol. 77:7048-7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalo, R. M., D. Rodriguez, A. Garcia-Sastre, J. R. Rodriguez, P. Palese, and M. Esteban. 1999. Enhanced CD8+ T cell response to HIV-1 Env by combined immunization with influenza and vaccinia virus recombinants. Vaccine 17:887-892. [DOI] [PubMed] [Google Scholar]
- 23.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. Robust recall and long-term memory T-cell responses induced by prime-boost regimens with heterologous live viral vectors expressing human immunodeficiency virus type 1 Gag and Env proteins. J. Virol. 76:7506-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamajima, K., Y. Hoshino, K. Q. Xin, F. Hayashi, K. Tadokoro, and K. Okuda. 2002. Systemic and mucosal immune responses in mice after rectal and vaginal immunization with HIV-DNA vaccine. Clin. Immunol. 102:12-18. [DOI] [PubMed] [Google Scholar]
- 25.Heeney, J. L., G. Koopman, B. Rosenwirth, W. Bogers, J. Van Dijk, I. Nieuwenhuis, H. Niphuis, P. ten Haaft, T. Hanke, G. Rhodes, P. Berglund, A. Burny, F. Bex, G. Sutter, and P. Liljestrom. 2000. A vaccine strategy utilizing a combination of three different chimeric vectors which share specific vaccine antigens. J. Med. Primatol. 29:268-273. [DOI] [PubMed] [Google Scholar]
- 26.Kiyono, H., C. J. Miller, Y. Lu, T. Lehner, M. Cranage, Y. T. Huang, S. Kawabata, M. Marthas, B. Roberts, J. G. Nedrud, M. E. Lamm, L. Bergmeier, R. Brooks, L. Tao, and J. R. McGhee. 1995. The common mucosal immune system for the reproductive tract: basic principles applied toward an AIDS vaccine. Adv. Drug Deliv. Rev. 18:23-51. [Google Scholar]
- 27.Klavinskis, L. S., C. Barnfield, L. Gao, and S. Parker. 1999. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J. Immunol. 162:254-262. [PubMed] [Google Scholar]
- 28.Kozlowski, P. A., S. Cu-Uvin, M. R. Neutra, and T. P. Flanigan. 1997. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions. Infect. Immun. 65:1387-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehner, T., Y. Wang, M. Cranage, L. A. Bergmeier, E. Mitchell, L. Tao, G. Hall, M. Dennis, N. Cook, R. Brookes, L. Klavinskis, I. Jones, C. Doyle, and R. Wards. 1996. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat. Med. 2:767-775. [DOI] [PubMed] [Google Scholar]
- 30.Lehner, T., L. Bergmeier, Y. Wang, L. Tao, and E. Mitchell. 1999. A rational basis for mucosal vaccination against HIV infection. Immunol. Rev. 170:183-196. [DOI] [PubMed] [Google Scholar]
- 31.Li, S., V. Polonis, H. Isobe, H. Zaghouani, R. Guinea, T. Moran, C. Bona, and P. Palese. 1993. Chimeric influenza virus induces neutralizing antibodies and cytotoxic T cells against human immunodeficiency virus type 1. J. Virol. 67:6659-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, R. S. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA 90:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marx, P. A., A. I. Spira, A. Gettie, P. J. Dailey, R. S. Veazey, A. A. Lackner, C. J. Mahoney, C. J. Miller, L. E. Claypool, D. D. Ho, and N. J. Alexander. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084-1089. [DOI] [PubMed] [Google Scholar]
- 34.McDermott, M. R., and J. Bienenstock. 1979. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory and genital tissues. J. Immunol. 122:1892-1898. [PubMed] [Google Scholar]
- 35.Menge, A. C., S. M. Michalek, M. W. Russell, and J. Mestecky. 1993. Immune response of the female rat genital tract after oral and local immunization with keyhole limpet hemocyanin conjugated to the cholera toxin B subunit. Infect. Immun. 61:2162-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell, E. A., L. A. Bergmeier, C. Doyle, R. Brooks, L. A. Hussain, Wang, Y., and T. Lehner. 1998. Homing of mononuclear cells from iliac lymph nodes to the genital and rectal mucosa in non-human primates. Eur. J. Immunol. 28:3066-3074. [DOI] [PubMed] [Google Scholar]
- 37.Murali-Krishna, K., J. D. Altman, M. Suresh, D. Sourdive, A. Zajac, J. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 38.Murata, K., A. Garcia-Sastre, M. Tsuji, M. Rodrigues, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, P. Palese, M. Esteban, and F. Zavala. 1996. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia virus. Cell. Immunol. 173:96-107. [DOI] [PubMed] [Google Scholar]
- 39.Murphy, B. R., and M. L. Clements. 1989. The systemic and mucosal immune response of humans to influenza A virus. Curr. Top. Microbiol. Immunol. 146:107-116. [DOI] [PubMed] [Google Scholar]
- 40.Muster, T., B. Ferko, A. Klima, M. Purtscher, A. Trkola, P. Schulz, A. Grassauer, O. G. Engelhardt, A. Garcia-Sastre, P. Palese, and H. Katinger. 1995. Mucosal model of immunization against human immunodeficiency virus type 1 with a chimeric influenza virus. J. Virol. 69:6678-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakaya, Y., H. Zheng, and A. Garcia-Sastre. 2003. Enhanced cellular immune responses to SIV Gag by immunization with influenza and vaccinia virus recombinants. Vaccine 21:2097-2106. [DOI] [PubMed] [Google Scholar]
- 42.Parr, E. L., J. J. Bozzola, and M. B. Parr. 1998. Immunity to vaginal infection by herpes simplex virus type 2 in adult mice: characterization of the immunoglobulins in vaginal mucus. J. Reprod. Immunol. 38:15-30. [DOI] [PubMed] [Google Scholar]
- 43.Parr, M. B., L. Kepple, M. R. McDermott, M. D. Drew, J. J. Bozzola, and E. L. Parr. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. 70:369-379. [PubMed] [Google Scholar]
- 44.Rayevskaya, M. V., and F. R. Frankel. 2001. Systemic immunity and mucosal immunity are induced against human immunodeficiency virus Gag protein in mice by a new hyperattenuated strain of Listeria monocytogenes. J. Virol. 75:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirai, M., C. D. Pendleton, and J. A. Berzofsky. 1992. Broad recognition of cytotoxic T cell epitopes from the HIV-1 envelope protein with multiple class I histocompatibility molecules. J. Immunol. 148:1657-1667. [PubMed] [Google Scholar]
- 46.Staats, H. F., R. J. Jackson, M. Marinaro, I. Takahashi, H. Kiyono, and J. R. McGhee. 1994. Mucosal immunity to infection with implications for vaccine development. Curr. Opin. Immunol. 6:572-583. [DOI] [PubMed] [Google Scholar]
- 47.Staczek, J., H. E. Gilleland, Jr., L. B. Gilleland, N. Harty, A. Garcia-Sastre, O. G. Engelhardt, and P. Palese. 1998. A chimeric influenza virus expressing an epitope of outer membrane protein F of Pseudomonas aeruginosa affords protection against challenge with P. aeruginosa in a murine model of chronic pulmonary infection. Infect. Immun. 66:3990-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Topham, D. J., M. R. Castrucci, F. S. Wingo, G. T. Belz, and P. C. Doherty. 2001. The role of antigen in the localization of naive, acutely activated, and memory CD8+ T cells to the lung during influenza pneumonia. J. Immunol. 167:6983-6990. [DOI] [PubMed] [Google Scholar]
- 49.Tuffrey, M., and D. Taylor-Robinson. 1981. Progesterone as a key factor in the development of a mouse model for genital tract infection with Chlamydia trachomatis. FEMS Microbiol. Lett. 12:111-115. [Google Scholar]
- 50.Vajdy, M., J. Gardner, J. Neidleman, L. Cuadra, C. Greer, S. Perri, D. O'Hagan, and J. M. Polo. 2001. Human immunodeficiency virus type 1 Gag-specific vaginal immunity and protection after local immunizations with Sindbis virus-based replicon particles. J. Infect. Dis. 184:1613-1616. [DOI] [PubMed] [Google Scholar]
- 51.Wassen, L., K. Schon, J. Holmgren, M. Jertborn, and N. Lycke. 1996. Local intravaginal vaccination of the female genital tract. Scand. J. Immunol. 44:408-414. [DOI] [PubMed] [Google Scholar]
- 52.Webster, R. G., L. E. Brown, and D. C. Jackson. 1983. Change in antigenicity of the hemagglutinin molecule of H3 influenza virus at acidic pH. Virology 126:587-599. [DOI] [PubMed] [Google Scholar]
- 53.Wu, H. Y., S. Abdu, D. Stinson, and M. W. Russell. 2000. Generation of female genital tract antibody responses by local and central (common) mucosal immunization. Infect. Immun. 68:5539-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zdenek, H., W. P. Tsai, A. Thornton, J. Nacsa, L. Giuliani, E. Tryniszewska, M. Poudyal, D. Venzon, X. Wang, J. Altman, D. I. Watkins, W. Lu, A. von Gegerfelt, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2001. Potentiation of simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J. Immunol. 167:7180-7191. [DOI] [PubMed] [Google Scholar]