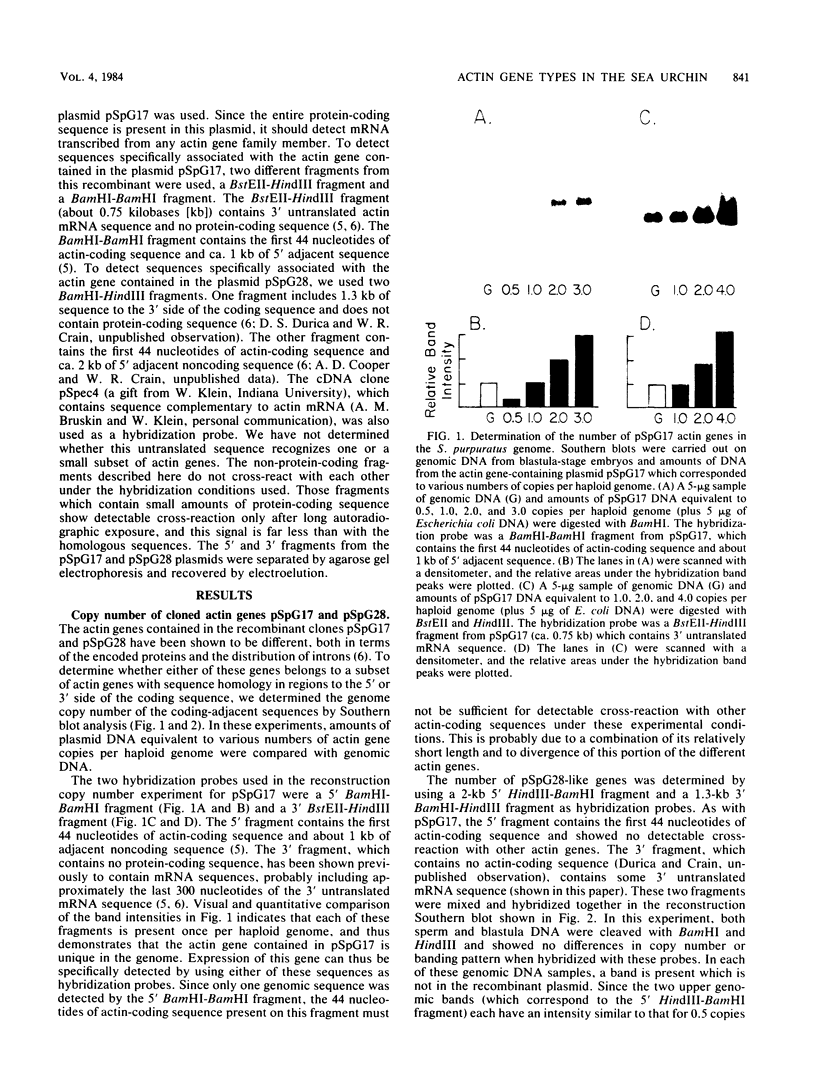
Abstract
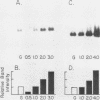
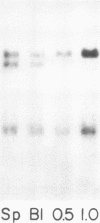
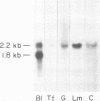
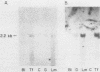
The expression of three different actin genes in the sea urchin, Strongylocentrotus purpuratus, was monitored in embryos and adult tissues by using untranslated mRNA sequences as specific hybridization probes. Three distinct patterns of expression were found: muscle specific, embryo specific, and constitutive (i.e., present in all tissues examined). The actin genes encoding the muscle-specific and constitutively expressed genes were each found to be present once in the haploid genome. The embryo-specific probe could derive from either a single gene or a small subset of actin genes. These data demonstrate that at least three members of the sea urchin actin gene family are expressed in distinct ways and thus are probably associated with different regulatory programs of gene expression necessary for development of this metazoan.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruskin A. M., Tyner A. L., Wells D. E., Showman R. M., Klein W. H. Accumulation in embryogenesis of five mRNAs enriched in the ectoderm of the sea urchin pluteus. Dev Biol. 1981 Oct 30;87(2):308–318. doi: 10.1016/0012-1606(81)90154-8. [DOI] [PubMed] [Google Scholar]
- Bushman F. D., Crain W. R., Jr Conserved pattern of embryonic actin gene expression in several sea urchins and a sand dollar. Dev Biol. 1983 Aug;98(2):429–436. doi: 10.1016/0012-1606(83)90372-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper A. D., Crain W. R., Jr Complete nucleotide sequence of a sea urchin actin gene. Nucleic Acids Res. 1982 Jul 10;10(13):4081–4092. doi: 10.1093/nar/10.13.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain W. R., Jr, Durica D. S., Van Doren K. Actin gene expression in developing sea urchin embryos. Mol Cell Biol. 1981 Aug;1(8):711–720. doi: 10.1128/mcb.1.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Durica D. S., Schloss J. A., Crain W. R., Jr Organization of actin gene sequences in the sea urchin: molecular cloning of an intron-containing DNA sequence coding for a cytoplasmic actin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5683–5687. doi: 10.1073/pnas.77.10.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Shott R. J., Rose S. J., 3rd, Thomas T. L., Britten R. J., Davidson E. H. Sea urchin actin gene subtypes. Gene number, linkage and evolution. J Mol Biol. 1984 Jan 15;172(2):149–176. doi: 10.1016/s0022-2836(84)80035-2. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Water R. D., Moore G. P., Kleinsmith L. J. Change in expression of the actin gene family during early sea urchin development. Dev Biol. 1981 Jul 30;85(2):505–508. doi: 10.1016/0012-1606(81)90280-3. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Caravatti M., Buckingham M. E. A fetal skeletal muscle actin mRNA in the mouse and its identity with cardiac actin mRNA. Cell. 1982 Aug;30(1):185–192. doi: 10.1016/0092-8674(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Sanchez F., Tobin S. L., Rdest U., Zulauf E., McCarthy B. J. Two Drosophila actin genes in detail. Gene structure, protein structure and transcription during development. J Mol Biol. 1983 Feb 5;163(4):533–551. doi: 10.1016/0022-2836(83)90111-0. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., McAllister L. B., Crain W. R., Jr, Durica D. S., Posakony J. W., Thomas T. L., Britten R. J., Davidson E. H. Organization and expression of multiple actin genes in the sea urchin. Mol Cell Biol. 1981 Jul;1(7):609–628. doi: 10.1128/mcb.1.7.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shott R. J., Lee J. J., Britten R. J., Davidson E. H. Differential expression of the actin gene family of Strongylocentrotus purpuratus. Dev Biol. 1984 Feb;101(2):295–306. doi: 10.1016/0012-1606(84)90143-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Horovitch S. J., Scott M. P., Rich A., Pardue M. L. Myogenesis in primary cell cultures from Drosophila melanogaster: protein synthesis and actin heterogeneity during development. Cell. 1978 Apr;13(4):589–598. doi: 10.1016/0092-8674(78)90210-6. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Rich A. Chick cytoplasmic actin and muscle actin have different structural genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2346–2350. doi: 10.1073/pnas.73.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Mammalian cytoplasmic actins are the products of at least two genes and differ in primary structure in at least 25 identified positions from skeletal muscle actins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1106–1110. doi: 10.1073/pnas.75.3.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]