Abstract
Here we outline how islet cells use autocrine and paracrine “circuits” of classical neurotransmitters and their corresponding receptors and transporters to communicate with vicinal β-cells to regulate glucose stimulated insulin secretion. Many of these same circuits operate in the CNS and can be visualized by molecular imaging. We discuss how these techniques might be applied to measuring the dynamics of β-cell function in real time.
Keywords: β-cells, imaging, GSIS, PET
Introduction
An important issue in the pathogenesis of diabetes, in all of its molecular incarnations, is the relative contributions of reductions of β-cell mass and impairments in function of individual islet cells. For example, while destruction of β-cells is the primary pathogenetic process in T1D, hypofunction of the remaining β-cells - due to the effects of ambient cytokines and metabolites – clearly contributes. In T2D, metabolic stress on β- cells is the major mechanism for relative hypoinsulinemia, but the mass of β-cells may ultimately decrease as a result of this process, exacerbating the defining systemic pathology [1]. These are not simply fine points. Ultimately, the management and prevention of the disease hinges on these distinctions. To make such distinctions, what is needed are methods that can quantify β-cell mass in vivo, while, ideally also characterizing functional competence of the β-cell.
Our group has taken advantage of the fact that many of the β-cell-restricted “neuroreceptors” and transporters are quantifiable by non-invasive PET imaging. The β-cell-restricted patterns of expression of these molecules allow us to make in vivo measurements of β-cell mass and function [2-4] within the pancreas. This overview is aimed at summarizing studies that have lead to a broader understanding of the possible roles of the “classical” neurotransmitters and receptors have in regulating GSIS and address how molecular imaging might be applied to capture information regarding BCM, function and dysfunction in vivo.
Monoamine Neurotransmitter Regulation of Insulin Secretion
We and others have proposed a novel autocrine “circuit” of regulation of glucose-stimulated insulin secretion (GSIS) [5,6], mediated by monoamine neurotransmitters stored within islet vesicles. This system is distinct from that mediated by intrinsic parasympathetic-sympathetic innervation of islets (summarized by Ahrén [7]) which plays a central role in islet hormone secretion and glucose homeostasis (reviewed in [8]). In man, these autocrine (or paracrine) circuits maybe particularly important because, relative to rodent islets, human islets appear more autonomous from CNS control, being sparsely innervated with few contacts to autonomic and cholinergic axons [9]. Moreover, in human islets, sympathetic axons are associated with the smooth muscle cells of blood vessels located around and deep within the islet rather than directly contacting β-cells. Effects on blood flow can, of course, have major impact on islet physiology [10].
The existence of an autocrine inhibitory circuit acting among the resident cells of islets is suggested by three lines of evidence: 1) islet cells contain vesicular stores of neurotransmitters (e.g. DA and 5-HT); 2) islet cells (i.e. β-cells) express receptors and transporters for neurotransmitters; 3) GSIS can be negatively regulated by DA and 5-HT and in vitro islets release specific monoamine neurotransmitters in proportion to ambient glucose concentrations [11-14]. Throughout the body, monoamine neurotransmitters including norepinephrine, dopamine, and serotonin are shuttled from the cytosol to secretory storage granules by vesicular monoamine transporters (VMATs) [15]. In the human pancreas, the expression of VMAT2 is largely confined to insulin-containing β-cell granules [16,17], and absent from islet cells staining for glucagon (α cells), somatostatin (δ cells); relatively minor VMAT2 expression occurs in PP cells and exocrine pancreas [17, 18]. Similarly, the pancreas also expresses dopamine type 2 receptors (D2R), and, like VMAT2, D2R expression within the pancreas is largely confined to β-cells [5]. On this basis, we propose a feed forward inhibitory circuit where DA, secreted along with insulin, down-regulates GSIS in islets via interaction with D2R (figure 1 and 2).
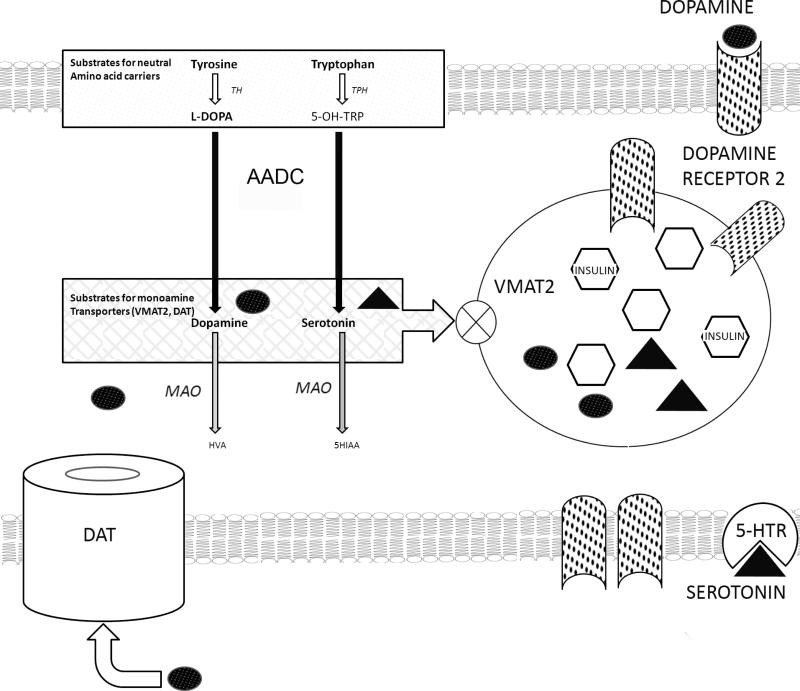
Figure 1.
Schematic of a putative dopamine mediated autocrine negative feedback circuit in β-cells. Import of aromatic amino acids (Tyrosine [Try] and Tryptophan [Trp]) by neutral amino acid carriers (LAT1) from extracellular space occurs, followed by transformation of Typ and Trp to L-DOPA and 5-hydroxy-tryptophan (5-OH-TRP) by the action of tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH). Both L-DOPA and 5-OH-TRP are transformed into dopamine (DA) and serotonin (5-HT) by the action of aromatic amino acid decarboxylase (AADC).Cytoplasmic DA or 5-HT are transported by VMAT2 into β-cell vesicles and later delivered to the intracellular space following glucose stimulated insulin secretion. Dopamine type 2 receptors are stored in vesicles, rendered inactive in the acid microenvironment. Extracellular dopamine (e.g. as elevated dopamine sulfate found in postprandial serum samples can shuttled inside the β-cell by the action of the dopamine transporter (DAT). DA and 5-HT not sequestered by the action of VMAT2 is exposed to the action of monoamine oxidase and destructively metabolized.
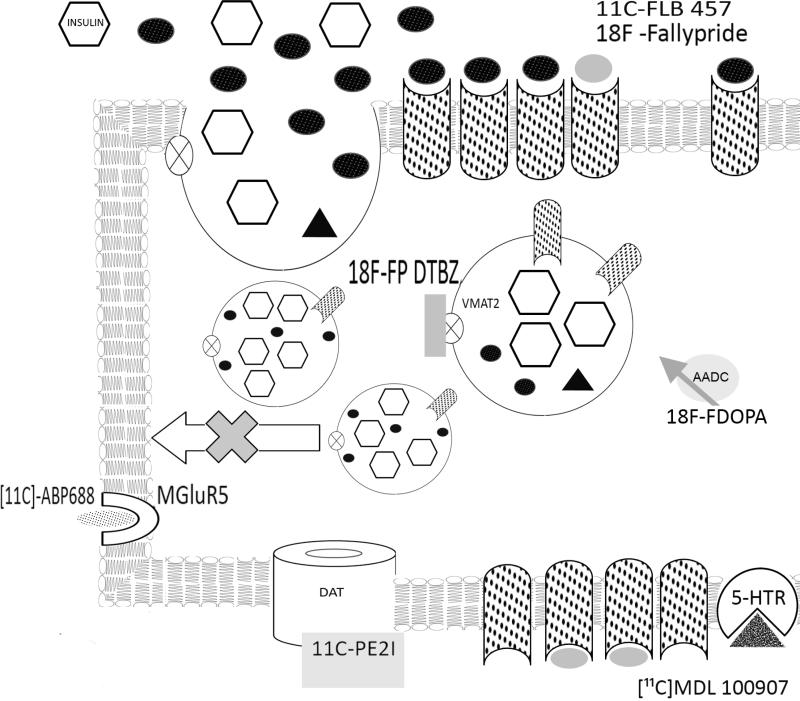
Figure 2.
Upon glucose stimulation, insulin (open hexagons), dopamine (black ovals) and dopamine type 2 receptors (stippled) are delivered to the extracellular space or plasma membrane. Dopamine diffuses to active cell surface D2R or D2R expressed on neighboring β-cell and binds to dopamine receptor (D2R) inhibiting further insulin secretion. The dopamine receptor type 2 PET ligands [11C]-FLB457 or 18F-Fallypride are shown in grey ovals. The VMAT2 antagonist dihydrotetrabenazine (DTBZ) prevents vesicular storage of dopamine by high affinity binding near the monoamine binding site. The VMAT2 PET ligand 18F-FP-DTBZ (grey rectangle) is shown bound to VMAT2 (open circle with X). The tyrosine hydroxylase substrate and PET ligand 18FDOPA is shown adjacent to its enzyme.PET ligands for the metabotropic glutamate receptor, 11C-APB 688 (stippled oval bound to open reversed C); dopamine transporter, 11C-PE2I; ( grey rectangle bound to open doughnut) and serotonin receptor 5HT2A, 11C MDL 100907( stippled triangle bound to open pie) are also shown.
Intra-islet communication via other classical neurotransmitters has also been documented. Autocrine and paracrine signaling via γ-amino butyric acid (GABA) and the GABA alpha type receptors (GABARs) has been studied in rodent, and recently in human islets[19]. GABA was found to be released by cultured islets in vitro, in a glucose-dependent manner. Of note was GABA-enhanced GSIS in human islets, seemingly distinct from GABA's role in the CNS as an “inhibitory neurotransmitter.” Lastly, GABAR expression was found predominantly on cells staining for somatostatin (δ-cells), with some staining on subpopulations of alpha and β-cells. A second paracrine circuit between α-cells and β-cells has been described by Caicedo et al [20]. As mentioned above, α-cells do not express VMAT2. Instead, α-cell vesicles express vesicular acetylcholine transporter (VAChT), the molecule responsible for the vesicular storage of the acetylcholine. Acetylcholine (ACh) released by α-cells primes the β-cell insulin secretion via muscarinic M3 receptors to respond optimally to subsequent increases in ambient glucose concentration [21].
Both dopamine and serotonin are stored in β-cell vesicles and regulate GSIS
An appreciation of the presence of monoamine neurotransmitters within islets and the role they play in regulation of glucose secretion began in the 1960s. With the development ofspecific and sensitive fluorescent methods of detecting catecholamines and other biogenic amines, Falk and Hellman in 1963 demonstrated the presence of a fluorescent signal with granular distribution in “alpha” cells and “beta” cells within the islets of Langerhans of pancreata obtained from rats, mice, cats, dogs, horse and ducks. Presaging research that would take place 45 years later, these investigators also showed that the vesicular signal was blocked by reserpine(a VMAT2inhibitor) [22]. Later these amines were shown to be 5-hydroxytryptamine (5-HT or serotonin) and dopamine (DA) [23,24]. Using EM and autoradiography, 5-HT was found to be located in β-cell secretory granules [25,26]. In subsequent studies in rodents and humans, both in vivo and using islets ex vivo [27-30], it was shown that both 5-HT,L-DOPA (the direct metabolic precursor to DA) as well as DA could inhibit GSIS.
Since then it has become clear that not only do β-cells contain monoamine neurotransmitters, but that most if not all of the enzymes needed for de novo synthesis and catabolism of monoamines, including: tyrosine hydroxylase (TH), the enzyme catalyzing the rate limiting synthetic step whereby tyrosine is converted to L-DOPA [31]; L-DOPA decarboxylase (a.k.a. Aromatic L-amino acid decarboxylase (AADC) [5] producing DA; the catabolic enzymes acting on DA, monoamine oxidase (MAO-B) [32,33], AADC, and catechol-O-methyl transferase (COMPT) [34]. Recently, the gene expression pattern needed for encoding all of the products necessary for synthesizing, packaging, and secreting serotonin, including both isoforms of the serotonin synthetic enzyme tryptophan hydroxylase, have been found in islets [35].
Role of β-cell expression of D2R and 5-HTR
While evidence that in vitro GSIS could be modulated by exogenous DA and 5-HTR was provided years ago, experimental proof that β-cells express the corresponding receptors for DA and 5-HTR is recent and incomplete. Both rat and human islets express mRNAs for the 5-HT type 2A/B receptor [36,37]; however demonstration of expression of 5-HTRs at the protein level is lacking or indirect [38].In contrast, the evidence for β-cell expression of dopamine type 2 receptors is solid. Rubi et al [5] have shown that rat islets express the dopamine type 1-3 and 5 receptor message, and that human islets express the dopamine type 2 receptor protein in a pattern that overlaps with insulin staining, consistent with an intravesicular location. Interestingly, in D2R global knockout mice, in vivo GSIS is impaired, suggesting that while dopamine clearly inhibits insulin secretion, some low level of D2R occupancy may be required for normal insulin secretion [39]. Consistent with this formulation, in vitro studies of GSIS show that micromolar concentrations of DA (10-6M) enhance insulin secretion while higher concentrations (10-5 to 10-3 M) inhibit secretion [30].
Role of monoamine transporters in GSIS
The key role of VMAT2 in transporting monoamines from the cytosol to the vesicular lumen for storage and the related medicinal chemistry of VMAT2 have been expertly reviewed elsewhere [40,41]. In the context of an autocrine circuit of regulation of GSIS, VMAT2 function constitutes a central element. In both rodent and human pancreata, VMAT2 is preferentially expressed by β-cells [6,16,17] relative to the exocrine pancreas. Tetrabenazine (TBZ) binds to VMAT2 with a Ki in the nanomolar range [42] and inhibits the transport of monoamines to vesicles from the cytosol [43]. In in vitro experiments with purified rodent islets exposed to20 mM glucose, concurrent treatment of cultured islets with TBZ significantly enhanced insulin secretion. Similar results were reported in vivo using a rat model with intraperitoneal glucose tolerance testing. In these experiments, both exogenous L-DOPA and DA partially reversed the effects of systemic TBZ [6]. Measurements of total tissue dopamine revealed that TBZ-treated pancreata and brains were dopamine depleted as predicted by a model where dopamine not sequestered into vesicles is catabolized by MAO [44]. These data fit well with the dopamine autocrine inhibitory circuit proposed in figures 1 and 2.
In the CNS, DA released at the synapse is recycled into vesicles by the action of the dopamine transporter (DAT a.k.a. SLC6A3) [45]. There are few published data supporting the preferential expression of dopamine transporters (DAT) in β-cells or islets (relative to the pancreas as a whole)beyond those found in the public gene expression data bases [46]. These data are, however, consistent with the preferential expression of DAT by islets relative to the total pancreas. Preliminary experiments with rodent islets suggest that DAT inhibitors have effects on GSIS similar to dopamine depleting agents (e.g. augment insulin secretion in response to glucose) (Simpson et al, personal communication). In DAT knockout mice there is a generalized depletion of dopamine levels in the CNS [47]. If inhibition of DAT in β-cells functions analogously to DAT knock out in the CNS and results in a depletion of dopamine levels in the islets, then enhanced insulin secretion is predicted by our model.
Role of neutral amino acid carriers
Given the importance of DA and 5-HT in regulating GSIS, and the observation that β-cells express the biosynthetic machinery to synthesize DA and 5-HT from metabolic precursors, it seems reasonable to posit that mechanisms related to import of tryptophan and tyrosine into β-cells could play an important role in the physiology of GSIS. The large neutral amino acid transporter (LAT1) (a.k.a. CD98) is a heterodimeric glycoprotein formed from SLC3A2 and SLC7A5 subunits that preferentially transports branched-chain and aromatic (i.e. tryptophan, tyrosine) amino acids [48]. In gene expression profiling using rat islets cultured in either high or low glucose, SLC3A2 is down regulated in response to high glucose [49], as might be predicted by the model given in figures 1 and 2. The collection of more information regarding β-cell expression and regulation of these transporters seems warranted.
Lastly, it is interesting to note that genes participating in pathways regulating the biosynthesis, catabolism and response to DA and 5-HT have been implicated in diabetes- and/or obesity-related phenotypes by genetic association studies [50].
Molecular Imaging of β-Cell Restricted Targets To Capture Dynamics of GSIS
Imaging β-cell mass via VMAT2
Because for VMAT2: 1) transcription appears to be stable in vitro under a variety conditions including a range of glucose and lipid concentrations (e.g. up to 20 mM glucose and 0.5 mM palmitic acid) ; 2) protein expression parallels insulin staining in pancreata of T2D and T1D patients [17]; and 3) density can be quantified by PET imaging [51], we proposed the use ofVMAT2 as a biomarker of insulin producing β-cells mass (BCM) in the study of the pathogenesis and management of type 1 and type 2 diabetes. PET imaging of BCM via VMAT2 has been recently reviewed [3]. Recent data by two groups suggest that VMAT2 quantitation in the pancreas by PET (with 18F-FP-DTBZ) can readily distinguish between the BCM phenotypes found in healthy controls and individuals with long standing T1D [52] (Freeby et al, submitted).
The pet ligand currently in use to test the feasibility of measuring human BCM (18F-FP-(+)-DTBZ) binds to VMAT2 with high affinity [53]. Past clinical studies of PET imaging of BCM, however, used a different tracer ([11C]-(+)-DTBZ) with lower affinity for the VMAT2 transporter, a consequence of which would be less precise BCM measurements [2]. Following the development of 18F-FP-(+)-DTBZ, two papers questioned VMAT2 measurements as a stable dopamine (DA) neuron biomarker [54, 55]. One study demonstrated that in DOPA-responsive dystonia, a human neurological disorder resulting from the inability to synthesize DA, there was an apparent 20% increase in ([11C]-(+)DTBZ binding in pathologically relevant regions (e.g. striatum) of the CNS. Both investigative teams concluded that the increased [11C]-(+)-DTBZ binding reflected the greater accessibility of the radioligand to VMAT2 binding sites due to lack of competition from endogenous DA. Together these papers suggested that [11C]-(+)-DTBZ binding to VMAT2 might be sensitive to changes in vesicular DA storage levels. In instances of dopamine depletion (i.e. chronic methamphetamine users and DOPA responsive dystonia), these reports found increased [[11C]-(+)-DTBZ binding (about 20%), but not increased VMAT2 density. As a follow up to these studies, we performed VMAT2 imaging in the rat brain with [11C]-(+)-DTBZ. Prior to imaging, rodents were treated with alpha-methyl-p-tyrosine (AMPT, a tyrosine hydroxylase inhibitor) at doses that significantly (-75%) depleted brain tissue dopamine levels. Imaging revealed increased striatal binding of the [11C]-(+)-DTBZ VMAT2 tracer [56] in the AMPT-treated cohort relative to untreated controls. This reciprocal effect of ambient dopamine on [11C]-(+)-DTBZ as a quantitative VMAT2 tracer may have partially confounded our previous PET measurements of BCM based on DTBZ binding to VMAT2 [2]. Differences in pancreatic dopamine content of the clinical cohorts studied could have influenced apparent BCM. Accordingly, we next examined the relationship between dopamine depletion and pancreatic VMAT2 - 18F-FP-(+)-DTBZ binding in vivo. First we documented the in vivo effects of alpha methyl p- tyrosine (AMPT) administration in Lewis Rats. Adult rats were administered either a saline control injection or AMPT for several days and the tissues were analyzed for DA content. As expected, administration of AMPT reduced brain and pancreas DA content to 10-20% of the controls. To determine whether tissue DA content influenced the binding of 18F-FP-(+)-DTBZ to VMAT2, we applied a serial pancreatic imaging strategy. Rodents were imaged at baseline with 18F-FP-(+)-DTBZ and then again following the end of the course of AMPT. We found no statistically significant differences in the mean % change distribution volume ratio (DVR) in the dopamine depletion study (Table 1), suggesting that the dopamine status in the pancreas does not affect 18F-FP-(+)-DTBZ tracer binding to VMAT2. In parallel studies no effects of dopamine depletion on tracer binding were seen in the CNS as well. A power analysis of the sample sizes used in this study indicated that there was at least an eighty percent chance to detect differences of 20% at 95% confidence interval. These results suggest that 18F-FP-(+)-DTBZ binding to VMAT2 (Kd = 0.5 nM) are not as sensitive to local dopamine levels as was observed for [11C]-(+)-DTBZ binding to VMAT2 (Kd = 1.7 nM) in the CNS because of the higher affinity of the former tracer for its target.
Table 1.
Average Pancreatic VMAT2 Density as DVR measured with 18F-FP-DTBZ
| Rodent ID | Baseline | AMPT | % Change in DVR | |
| 14F | 2.00 | 1.92 | 4.00 | |
| 15E | 1.97 | 2.11 | -7.11 | |
| 39C | 1.75 | 1.78 | -1.71 | |
| 3A8 | 2.10 | 2.50 | -19.05 | |
| 330 | 1.50 | 1.27 | 15.33 | |
| Mean % Change DVR | -1.71 | |||
| Variability | 12.77 | |||
If one reconsiders the imaging properties of [11C]-(+)-DTBZ, in terms of its sensitivity to the local concentration of monoamines, the possibility of imaging the dynamics of GSIS (e.g. changing concentrations of monoamines within the islet) becomes apparent. While this area is as yet unexplored in terms of measuring β-cell function, precedents in molecular imaging of the CNS support its feasibility. If β-cell VMAT2 expression is truly as invariant as evidence suggests, then the BCM measures provided by PET scans with 18F-FP-(+)-DTBZ might serve as a convenient denominator of combined measures of both functional (e.g. using [11C]-(+)-DTBZ as a displaceable tracer) and anatomical BCM (i.e. using the non-displaceable tracer 18F-FP-(+)-DTBZ). In the field of CNS imaging, the majority of functional studies have centered on dopaminergic neurons and the measurement of changes in occupancy of the dopamine receptor.
Imaging D2R occupancy in islets as a measure of β-cell function
Dopaminergic neurotransmission has a critical role in movement coordination, and degeneration of the nigrostriatal dopamine system causes Parkinson's disease. Dopamine secretion and changes in D2R receptor occupancy in the CNS have been associated with a reward system that may impact drug abuse and food intake [57]. Imaging of neurotransmission and changes in D2R occupancy using PET and single photon emission computed tomography (SPECT) has been reviewed [58] and is an accepted technique in the study of CNS disorders such as schizophrenia. For example, healthy control subjects were imaged at baseline with the D2R SPECT tracer [123I] IBZM, after the first imaging session, but while the subject was still in the camera, dextroamphetamine sulfate, a compound know to increase synaptic dopamine concentration, was administered and the subject reimaged. A third scan was performed after a three day course of oral AMPT. The model that was applied to the analysis of the data predicts that challenges (e.g. GSIS) that increased local (i.e. synaptic or inter β-cell) DA concentration will increase occupancy of D2 receptors, reducing availability of D2 receptors for binding of the radiotracer. Conversely, conditions that decrease DA synaptic concentration (e.g. treatment with tetrabenzine) will reduce D2 receptor occupancy by DA and increase D2 receptor availability for radiotracer binding [58]. Comparison of the apparent binding density of the tracer revealed that relative to baseline, amphetamine decreased in the amount of tracer bound, while dopamine depletion by AMPT was associated with an increase in the amount of bound tracer [59].
Within the human pancreas, D2R expression is restricted to β-cells and is at least 50 fold higher in islets relative to the surrounding exocrine tissue (Maffei, personal communication). .As posited in our model of the dopaminergic regulation of insulin homeostasis, GSIS may be accompanied by changes in numbers of functional D2R as well as by changes in D2R occupancy by dopamine. The feasibility of imaging of dopamine receptors as a biomarker of β-cell mass has already been demonstrated in rodents by Garcia et al [60], but our interest in this imaging approach is to capture in real time possible changes in pancreatic D2R occupancy that may accompany GSIS. If feasible, such measurements would yield information regarding β-cell function relevant to a phenotype distinct from that measured by current metabolic assessment measures such as mixed meal or oral glucose tolerance testing.
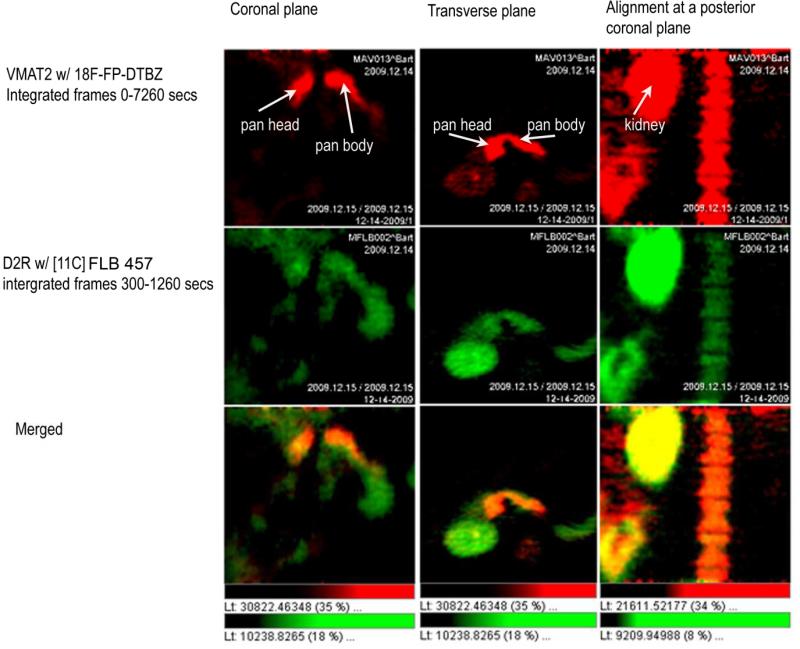
We selected a high affinity D2R tracer, [11C] isoremoxipride (a.k.a.[11C] FLB 457) (Kd=0.02 nM),whose binding to D2Rhas been reported to be less sensitive to local DA concentration compared to other D2R tracers such as [11C] raclopride (Kd=1.3 nM)[61,62]. We also included in the study the VMAT2 tracer 18F-FP-(+)-DTBZ as a control probe known to be selective for pancreatic β-cells. The quantitation of D2R in the pancreas was assessed using the following strategy: we dynamically imaged the abdomen of male baboons for 60 minutes. From reconstructed images of the PET data, we placed regions of interest on the pancreas and other abdominal organs to generate time-activity curves (TACs) from which tracer kinetics could be studied. From the TACs, using a reference region method, we estimated the tracer target density using the distribution volume ratio (DVR) as the outcome measure. Using this approach we performed serial imaging experiments and quantitiated the apparent D2R density in the pancreas region of interest. Reconstructed PET images from scans performed with D2R tracer ( i.e. [11C] FLB 457) and VMAT2 tracer (18F-FP-(+)-DTBZ) showed exact overlap of their respective pancreatic signals (figure 3) suggesting that that D2R and VMAT2 coexist within the pancreas consistent with immunohistochemistry of β-cells in pancreatic sections usingD2R- and VMAT2-specific antibodies. Serial scans of the same baboon performed with D2R tracer alone, and then following prior administration of a D2R blocker (i.e. the D2R antagonist haloperidol), demonstrated that a significant portion (>50%) of the pancreas PET signal was specific (figure 4)
Figure 3.
Papio Ursinus was imaged twice in one session for a study comparing the region of interest defined by a VMAT2 probe, 18F-FP-(+)-DTBZ, and a D2R probe [11C] FLB 457.The [11C] study was performed first and activity from the probe allowed to decay for six half lives (11C T1/2 = 21 minutes) before injection of the secondVMAT2 probe, 18F-FP-(+)-DTBZ. Following reconstruction of the images the pancreatic region of interest was defined using the VMAT2 probe. The overlap of the D2R and VMAT2 signals was then examined (bottom row-Merged). All studies were approved by the Columbia Institutional Animal Care and Use Committees.
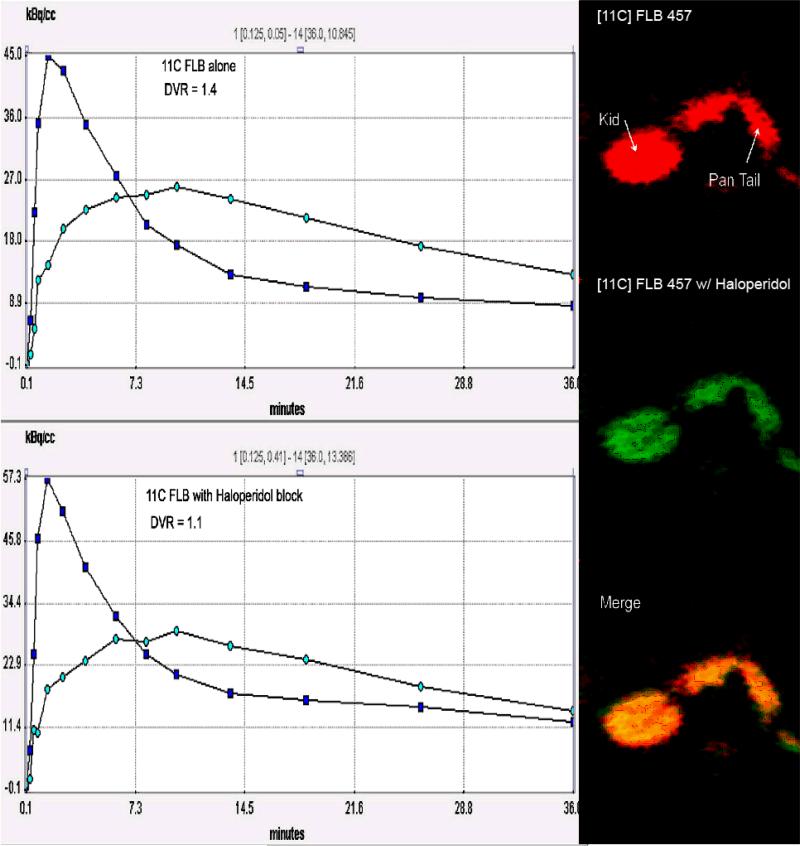
Figure 4.
[11C] FLB 457 study in one baboon at the level of the pancreas at baseline and following D2R blockade with haloperidol. Images are summed axial frames from 6 to 30 min post-injection of a bolus of [11C] FLB 457. Data are not normalized to injected activity. Time activity curves (blue squares–ROI on kidney, light blue dots – ROI on pancreas) were used to calculate a distribution volume ratio (DVR) using a reference region method. The dose of haloperidol (a D2R antagonist) administered (1 mg/kg) was expected to displace at least 30% of the specifically bound PET ligand. A kidney reference region was used to calculate the DVR. Haloperidol administration caused the apparent DVR to drop to 1.1 from 1.4.
The next step in this series of experiments will be to image subjects serially before and after an intravenous glucose tolerance test (IVGTT). We hypothesize that the PET signal will change in the pancreas in a manner analogous to that observed in the brain following amphetamine challenge. For example, in the resting pancreas, PET scans with D2R tracers may reveal time-activity curves very similar to that illustrated in figure 3 (left top panel).Once β-cells are stimulated in vivo by IVGTT and reimaged, a muted time-activity curve similar to that illustrated in figure 3 (left bottom panel) may be produced. It is possible that the IVGTT will need to be performed during the imaging session; in this case, a change in slope of the time activity curve might be expected during stimulation. The magnitude of the changes in the time-activity curves are expected to reflect a unique facet of β-cell function (i.e. changes in D2R receptor occupancy) related to, but distinct from, metabolic measures based on insulin production.
Imaging monoamine precursor metabolism with 18F-DOPA
Since insulin resistance and hyperinsulinemia generally precede the onset of obesity-induced T2D, molecular imaging of these processes could hypothetically be used in diagnosis and the evaluation of existing and novel therapeutic interventions. Congenital hyperinsulinism of infancy (CHI) manifests as persistent hyperinsulinism and severe hypoglycemia (reviewed in [63]) and represent an example where molecular imaging has been successfully applied as a diagnostic measure. The metabolic changes that accompany CHI have been studied in vivo [64] and in vitro [65] and it is believed that the insulin hypersecretory phenotype is accompanied by hyperactivity of L-DOPA metabolism. As might be expected on the basis of clinical findings, in vitro, CHI β-cells secrete insulin tonically. In vitro, CHI β-cells release about 5x more insulin under basal glucose stimulation and under longer culture periods release 10-40 % more insulin than control tissue [65]. Severe CHI is frequently treated by near total pancreatectomy which often results in later development of diabetes [66]. CHI is most often associated with recessive, loss of function mutations in ABCC8 and KCNJ11.ABCC8 and KCNJ11 encode two subunits of the pancreatic ATP-sensitive potassium (KATP) channel [67] . There are two histopathological types of CHI, categorized as diffuse and focal. Diffuse CHI arises when mutations are present in both alleles of the KATP channel genes, resulting in insulin over-secretion from all β-cells in the pancreas. In focal CHI, there is specific loss of the maternal 11p15 region accompanied bya paternally inherited mutation of the KCNJ11 gene. The lost region of maternal 11p15 carries silenced genes regulating cell proliferation. The resultant paternal disomy at 11p15 and loss of maternal silenced genes leads to a growth advantage for insulin overproducing β-cells resulting in a focal lesion in the pancreas [68]. The critical difference is that focal CHI can often be treated by selective resection, rather than near total pancreatectomy.
PET scans with 18F-DOPA are very useful in distinguishing focal from diffuse CHI [69,70]. The principle of the 18F-DOPA PET scan is based on β-cell uptake of L-dihydroxyphenylalanine (L-DOPA) via the L system transporter previously mentioned for later conversion to DA.
A major question that needs to be addressed is whether 18F-DOPA metabolism is sufficiently specific to visualize normal but hyperfunctioning β-cells. The entire normal pancreas shows uptake of 18F-DOPA as demonstrated by the fact that the normal pancreas has a standardized uptake value (SUV) of about 4 [71]. SUV is a relative measure of tracer concentration in the region of interest (ROI) where the ROI uptake is compared to what the tracer concentration (activity/cc) would be had the total tracer dose been dispersed in a volume of water equivalent to the subject's weight. In focal CHI disease, the ratio of lesion SUV to normal tissue SUV is 1.5 [72]. Both observations suggest that β-cell use of monoamines can be followed using 18F-DOPA imaging. Another concern is that while β-cells express AADC, the enzyme responsible for metabolizing L-DOPA to DA, adjacent alpha cells and exocrine tissue also express AADC [73]. Possibly a “pulse-chase” paradigm (e.g. administer 18F-DOPA, then follow with L-DOPA) could be applied to selectively label β-cell accumulation of 18F-DA. If feasible, changes in β-cell vesicular 18F-DA contents could then be visualized during in vivo GSIS.
Other neurofunctional β-cell targets
Figure 2 shows three additional cell surface molecules that may provide means to image β-cell mass and/or function. However, the β-cell specificity and abundance of expression of these targets are not documented.
Brice et al [74] recently demonstrated that in vitro insulin secretion could be modulated by agonists of the mGluR5 receptor as well as by agonists to mGluR2/3 and mGluR4/6-8 families. At present, PET ligand radiochemists are working to develop novel ligands with which to image receptors of the glutamate and γ-aminobutric acid (GABA) system, but few ligands are available. We selected the [3H]-APB 688, specific for the MGluR5 receptor [75], to explore the feasibility of imaging human β-cell mass or function. Using human pancreas membranes and a radioreceptor assay, we found that the Bmax (molar amount of ligand bound per mass of protein) was too low to support feasibility of PET imaging of this target. There are several lines of evidence that other receptors in the GABA system are abundant targets on β-cells and may be worthy of a second look once the appropriate tracer is found.
Two additional putative targets for imaging β-cell dynamics during GSIS are shown in figures 1 and 2: the dopamine “reuptake” transporter (DAT) and the serotonin receptor (5-HTR2A/B).Again evidence for the expression of DAT is based on in vitro GSIS experiments using purified islets in combination with specific inhibitors of DAT that show enhanced insulin secretion when DAT is inhibited. Most of the evidence for β-cell or islet expression of 5-HTRs is based in vitro GSIS experiments using agonists and antagonists of serotonin receptors or gene profiling experiments of islets and β-cells [49]. If imaging of serotonin receptors in islet β- cells is practical, it is possible that measurements of serotonin receptor occupancy in the pancreas will provide further insight into the real-time function of β-cells. PET ligands for these receptors have already been developed for quantitation of targets in the CNS (see examples in figure 2) and await testing of applicability in β-cell imaging. The critical questions regarding feasibility of these approaches will be: 1) what is the specificity of expression on β-cells relative to exocrine tissue; 2) what is their abundance of expression; and 3) what is the magnitude of non-specific binding of the tracer relative to the specific and saturable tracer binding in the pancreas taken as a whole.
Summary
As emphasized in the introduction, the issue of the relative contributions of impairments of BCM and function to the pathogenesis, natural history and responses to interventions of the various clinical forms of diabetes remains unresolved. But answers to these questions are important to guiding strategies for prophylaxis and prevention. For example, ongoing debates regarding the mechanism(s) by which bariatric/ ”metabolic” surgery meliorates T2D might be enlightened by the ability to monitor in vivo the functional and structural consequences to the β-cell of such interventions [76,77]. While the absorbed radiation doses associated with these PET studies are not trivial (3-30x absorbed radiation of typical chest x ray), two or three of these studies could be performed per year without exceeding federal exposure guidelines for research volunteers and patients.
It is interesting to note that the brain is only a minor source of peripheral DA.A major source of peripheral DA is the gastrointestinal tract [78]. This fact, coupled with the finding that there are large post prandial elevations in serum DA in man [79], suggests that DA in its role of regulating β-cell insulin secretion represents an “anti incretin” hypothesized to account, in part, for the beneficial effects of bariatric surgery on T2D [80]. Here, surgical procedures that possibly lower circulating and pancreatic DA levels (e.g. sleeve gastrectomy with duodenal switch) might be predicted to enhance GSIS. Such changes in pancreatic DA levels following bariatric surgery, as well as changes in anatomical β-cell mass, could be monitored both prior to and after bariatric surgery using the imaging techniques outlined above. Monitoring these novel phenotypes might provide insights to the mechanisms at work in metabolic surgery for reversal of T2D.
Acknowledgements
This work was supported by the PHS, NIH, NIDDK (5 R01 DK063567; P30 DK63608) and the Helmsley Charitable Trust (09PG-T1D020) (http://helmsleytrust.org). Some of the research performed here was performed with the support of Integrated Islet Distribution Program (IIDP) and the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International (JDRF) and or the NIDDK. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at www.jdrfnpod.org/our-partners.php.
Footnotes
6. Conflict of Interest Statement
P. E. H. is an inventor on patent application #20100204258 and # 20090202428 and has received a research award from AVID Radiopharmaceuticals, Inc. R. L. L. has nothing to declare.
References
- 1.Ashcroft FM, Rorsman P. Diabetes mellitus and the beta cell: The last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goland R, Freeby M, Parsey R, et al. 11C-dihydrotetrabenazine pet of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J Nucl Med. 2009;50:382–389. doi: 10.2967/jnumed.108.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichise M, Harris PE. Imaging of beta-cell mass and function. J Nucl Med. 2010;51:1001–1004. doi: 10.2967/jnumed.109.068999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souza F, Freeby M, Hultman K, et al. Current progress in non-invasive imaging of beta cell mass of the endocrine pancreas. Curr Med Chem. 2006;13:2761–2773. doi: 10.2174/092986706778521940. [DOI] [PubMed] [Google Scholar]
- 5.Rubi B, Ljubicic S, Pournourmohammadi S, et al. Dopamine d2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280:36824–36832. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- 6.Raffo A, Hancock K, Polito T, et al. Role of vesicular monoamine transporter type 2 in rodent insulin secretion and glucose metabolism revealed by its specific antagonist tetrabenazine. J Endocrinol. 2008;198:41–49. doi: 10.1677/JOE-07-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 8.Henquin JC. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in beta-cells. Diabetes Res Clin Pract. 2011;93(Suppl 1):S27–S31. doi: 10.1016/S0168-8227(11)70010-9. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyman LR, Ford E, Powers AC, Piston DW. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. Am J Physiol Endocrinol Metab. 2010;298:E807–E814. doi: 10.1152/ajpendo.00715.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith PA, Proks P, Ashcroft FM. Quantal analysis of 5-hydroxytryptamine release from mouse pancreatic beta-cells. J Physiol. 1999;521:651–64. doi: 10.1111/j.1469-7793.1999.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aspinwall CA, Huang L, Lakey JR, Kennedy RT. Comparison of amperometric methods for detection of exocytosis from single pancreatic beta-cells of different species. Anal Chem. 1999;71:5551–5556. doi: 10.1021/ac990817e. [DOI] [PubMed] [Google Scholar]
- 13.Zawalich WS, Tesz GJ, Zawalich KC. Effects of prior 5-hydroxytryptamine exposure on rat islet insulin secretory and phospholipase C responses. Endocrine. 2004;23:11–16. doi: 10.1385/ENDO:23:1:11. [DOI] [PubMed] [Google Scholar]
- 14.Rosario LM, Barbosa RM, Antunes CM, et al. Regulation by glucose of oscillatory electrical activity and 5-HT/insulin release from single mouse pancreatic islets in absence of functional K(ATP) channels. Endocr J. 2008;55:639–650. doi: 10.1507/endocrj.k07e-131. [DOI] [PubMed] [Google Scholar]
- 15.Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anlauf M, Eissele R, Schafer MK, et al. Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. J Histochem Cytochem. 2003;51:1027–1040. doi: 10.1177/002215540305100806. [DOI] [PubMed] [Google Scholar]
- 17.Saisho Y, Harris PE, Butler AE, et al. Relationship between pancreatic vesicular monoamine transporter 2 (VMAT2) and insulin expression in human pancreas. J Mol Histol. 2008;39:543–551. doi: 10.1007/s10735-008-9195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mezey E, Eisenhofer G, Harta G, et al. A novel nonneuronal catecholaminergic system: Exocrine pancreas synthesizes and releases dopamine. Proc Natl Acad Sci U S A. 1996;93:10377–10382. doi: 10.1073/pnas.93.19.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun M, Ramracheya R, Bengtsson M, et al. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes. 2010;59:1694–1701. doi: 10.2337/db09-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Diaz R, Dando R, Jacques-Silva MC, et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med. 2011;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz de Azua I, Gautam D, Guettier JM, Wess J. Novel insights into the function of beta-cell m3 muscarinic acetylcholine receptors: Therapeutic implications. Trends Endocrinol Metab. 2011;22:74–80. doi: 10.1016/j.tem.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falck B, Hellman B. A fluorescent reaction for monoamines in the insulin producing cells of the guinea-pig. Acta Endocrinol. 1964;45:133–138. doi: 10.1530/acta.0.0450133. [DOI] [PubMed] [Google Scholar]
- 23.Cegrell L, Falck B, Rosengren AM. Dopa-decarboxylase and monoamine oxidase activities in a transplantable islet cell tumor of the golden hamster. Experientia. 1969;25:969–970. doi: 10.1007/BF01898094. [DOI] [PubMed] [Google Scholar]
- 24.Cegrell L. Monoamine-containing cells in the fetal and newborn guinea-pig pancreas. Life Sci. 1967;6:1647–1652. doi: 10.1016/0024-3205(67)90175-0. [DOI] [PubMed] [Google Scholar]
- 25.Ekholm R, Ericson LE, Lundquist I. Monoamines in the pancreatic islets of the mouse. Subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia. 1971;7:339–348. doi: 10.1007/BF01219468. [DOI] [PubMed] [Google Scholar]
- 26.Jaim-Etcheverry G, Zieher LM. Electron microscopic cytochemistry of 5-hydroxytryptamine (5-HT) in the beta cells of guinea pig endocrine pancreas. Endocrinology. 1968;83:917–923. doi: 10.1210/endo-83-5-917. [DOI] [PubMed] [Google Scholar]
- 27.Ericson LE, Hakanson R, Lundquist I. Accumulation of dopamine in mouse pancreatic b-cells following injection of l-dopa. Localization to secretory granules and inhibition of insulin secretion. Diabetologia. 1977;13:117–124. doi: 10.1007/BF00745138. [DOI] [PubMed] [Google Scholar]
- 28.Lernmark A. The significance of 5-hydroxytryptamine for insulin secretion in the mouse. Horm Metab Res. 1971;3:305–309. doi: 10.1055/s-0028-1094131. [DOI] [PubMed] [Google Scholar]
- 29.Coulie B, Tack J, Bouillon R, Peeters T, Janssens J. 5-hydroxytryptamine-1 receptor activation inhibits endocrine pancreatic secretion in humans. Am J Physiol. 1998;274:E317–E20. doi: 10.1152/ajpendo.1998.274.2.E317. [DOI] [PubMed] [Google Scholar]
- 30.Shankar E, Santhosh KT, Paulose CS. Dopaminergic regulation of glucose-induced insulin secretion through dopamine d2 receptors in the pancreatic islets in vitro. IUBMB Life. 2006;58:157–163. doi: 10.1080/15216540600687993. [DOI] [PubMed] [Google Scholar]
- 31.Iturriza FC, Thibault J. Immunohistochemical investigation of tyrosine-hydroxylase in the islets of langerhans of adult mice, rats and guinea pigs. Neuroendocrinology. 1993;57:476–480. doi: 10.1159/000126394. [DOI] [PubMed] [Google Scholar]
- 32.Feldman JM, White-Owen C, Klatt C. Golden hamster pancreatic islets: A tissue rich in monoamine oxidase. Endocrinology. 1980;107:1504–1511. doi: 10.1210/endo-107-5-1504. [DOI] [PubMed] [Google Scholar]
- 33.Ito K, Hirose H, Maruyama H, et al. Neurotransmitters partially restore glucose sensitivity of insulin and glucagon secretion from perfused streptozotocin-induced diabetic rat pancreas. Diabetologia. 1995;38:1276–1284. doi: 10.1007/BF00401759. [DOI] [PubMed] [Google Scholar]
- 34.Karhunen T, Tilgmann C, Ulmanen I, Julkunen I, Panula P. Distribution of catechol-o-methyltransferase enzyme in rat tissues. J Histochem Cytochem. 1994;42:1079–1090. doi: 10.1177/42.8.8027527. [DOI] [PubMed] [Google Scholar]
- 35.Ohta Y, Kosaka Y, Kishimoto N, et al. Convergence of the insulin and serotonin programs in the pancreatic beta-cell. Diabetes. 2011;60:3208–3216. doi: 10.2337/db10-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacDonald MJ. Glucose-stimulated expressed sequence tags from rat pancreatic islets. Mol Cell Endocrinol. 1996;123:199–204. doi: 10.1016/s0303-7207(96)03918-4. [DOI] [PubMed] [Google Scholar]
- 37.Maffei A, Liu Z, Witkowski P, et al. Identification of tissue-restricted transcripts in human islets. Endocrinology. 2004;145:4513–4521. doi: 10.1210/en.2004-0691. [DOI] [PubMed] [Google Scholar]
- 38.Papadopoulos KP, Colovai AI, Maffei A, et al. Tissue-specific self-peptides bound by major histocompatibility complex class i molecules of a human pancreatic beta-cell line. Diabetes. 1996;45:1761–1765. doi: 10.2337/diab.45.12.1761. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Tornadu I, Ornstein AM, Chamson-Reig A, et al. Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology. 2010;151:1441–1450. doi: 10.1210/en.2009-0996. [DOI] [PubMed] [Google Scholar]
- 40.Eiden LE, Weihe E. VMAT2: A dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann N Y Acad Sci. 2011;1216:86–98. doi: 10.1111/j.1749-6632.2010.05906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wimalasena K. Vesicular monoamine transporters: Structure-function, pharmacology, and medicinal chemistry. Med Res Rev. 2011;31:483–519. doi: 10.1002/med.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kilbourn M, Lee L, Vander Borght T, Jewett D, Frey K. Binding of alpha-dihydrotetrabenazine to the vesicular monoamine transporter is stereospecific. Eur J Pharmacol. 1995;278:249–252. doi: 10.1016/0014-2999(95)00162-e. [DOI] [PubMed] [Google Scholar]
- 43.Howell M, Shirvan A, Stern-Bach Y, et al. Cloning and functional expression of a tetrabenazine sensitive vesicular monoamine transporter from bovine chromaffin granules. FEBS Lett. 1994;338:16–22. doi: 10.1016/0014-5793(94)80108-8. [DOI] [PubMed] [Google Scholar]
- 44.Fuenmayor LD, Vogt M. Production of catalepsy and depletion of brain monoamines by a butyrophenone derivative. Br J Pharmacol. 1979;67:115–22. [PMC free article] [PubMed] [Google Scholar]
- 45.Kristensen AS, Andersen J, Jorgensen TN, et al. Slc6 neurotransmitter transporters: Structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 46.Barrett T, Troup DB, Wilhite SE, et al. NCBI GEO: Archive for functional genomics data sets--10 years on. Nucleic Acids Res. 2011;39:D1005–D1010. doi: 10.1093/nar/gkq1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 48.Verrey F. System l: Heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–533. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- 49.Bensellam M, Van Lommel L, Overbergh L, Schuit FC, Jonas JC. Cluster analysis of rat pancreatic islet gene mrna levels after culture in low-, intermediate- and high-glucose concentrations. Diabetologia. 2009;52:463–476. doi: 10.1007/s00125-008-1245-z. [DOI] [PubMed] [Google Scholar]
- 50.Becker KG, Barnes KC, Bright TJ, Wang SA. The genetic association database. Nat Genet. 2004;36:431–432. doi: 10.1038/ng0504-431. [DOI] [PubMed] [Google Scholar]
- 51.Frey KA, Koeppe RA, Kilbourn MR. Imaging the vesicular monoamine transporter. Adv Neurol. 2001;86:237–247. [PubMed] [Google Scholar]
- 52.Normandin M, Petersen KF, Ding YS, et al. In vivo imaging of endogenous pancreatic beta cell mass in healthy and type 1 diabetic subjects using [18F]fp-(+)-dtbz and pet. J Nucl Med. 2012;53:908–916. doi: 10.2967/jnumed.111.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goswami R, Ponde DE, Kung MP, et al. Fluoroalkyl derivatives of dihydrotetrabenazine as positron emission tomography imaging agents targeting vesicular monoamine transporters. Nucl Med Biol. 2006;33:685–694. doi: 10.1016/j.nucmedbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Boileau I, Rusjan P, Houle S, et al. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: Is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28:9850–9856. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De La Fuente-Fernandez R, Furtado S, Guttman M, et al. VMAT2 binding is elevated in dopa-responsive dystonia: Visualizing empty vesicles by pet. Synapse. 2003;49:20–28. doi: 10.1002/syn.10199. [DOI] [PubMed] [Google Scholar]
- 56.Kilbourn MR, Butch ER, Desmond T, et al. In vivo [11C]dihydrotetrabenazine binding in rat striatum: Sensitivity to dopamine concentrations. Nucl Med Biol. 2010;37:3–8. doi: 10.1016/j.nucmedbio.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal d2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry. 2009;65:1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Garcia A, Mirbolooki MR, Constantinescu C, et al. 18F-fallypride pet of pancreatic islets: In vitro and in vivo rodent studies. J Nucl Med. 2011;52:1125–1132. doi: 10.2967/jnumed.111.088583. [DOI] [PubMed] [Google Scholar]
- 61.Narendran R, Frankle WG, Mason NS, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: A comparative evaluation of the high affinity dopamine d2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63:447–461. doi: 10.1002/syn.20628. [DOI] [PubMed] [Google Scholar]
- 62.Aalto S, Hirvonen J, Kaasinen V, et al. The effects of d-amphetamine on extrastriatal dopamine d2/d3 receptors: A randomized, double-blind, placebo-controlled pet study with [11C]FLB 457 in healthy subjects. Eur J Nucl Med Mol Imaging. 2009;36:475–483. doi: 10.1007/s00259-008-0969-9. [DOI] [PubMed] [Google Scholar]
- 63.Stoy J, Steiner DF, Park SY, et al. Clinical and molecular genetics of neonatal diabetes due to mutations in the insulin gene. Rev Endocr Metab Disord. 2010;11:205–215. doi: 10.1007/s11154-010-9151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sempoux C, Guiot Y, Lefevre A, et al. Neonatal hyperinsulinemic hypoglycemia: Heterogeneity of the syndrome and keys for differential diagnosis. J Clin Endocrinol Metab. 1998;83:1455–1461. doi: 10.1210/jcem.83.5.4768. [DOI] [PubMed] [Google Scholar]
- 65.Henquin JC, Nenquin M, Sempoux C, et al. In vitro insulin secretion by pancreatic tissue from infants with diazoxide-resistant congenital hyperinsulinism deviates from model predictions. J Clin Invest. 2011;121:3932–3942. doi: 10.1172/JCI58400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cherian MP, Abduljabbar MA. Persistent hyperinsulinemic hypoglycemia of infancy (PHHI): Long-term outcome following 95% pancreatectomy. J Pediatr Endocrinol Metab. 2005;18:1441–1448. doi: 10.1515/jpem.2005.18.12.1441. [DOI] [PubMed] [Google Scholar]
- 67.Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cdna encoding a novel atp-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- 68.Verkarre V, Fournet JC, de Lonlay P, et al. Paternal mutation of the sulfonylurea receptor (sur1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest. 1998;102:1286–1291. doi: 10.1172/JCI4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribeiro MJ, De Lonlay P, Delzescaux T, et al. Characterization of hyperinsulinism in infancy assessed with pet and 18f-fluoro-l-dopa. J Nucl Med. 2005;46:560–6. [PubMed] [Google Scholar]
- 70.Hardy OT, Hernandez-Pampaloni M, Saffer JR, et al. Diagnosis and localization of focal congenital hyperinsulinism by 18f-fluorodopa pet scan. J Pediatr. 2007;150:140–145. doi: 10.1016/j.jpeds.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 71.Chondrogiannis S, Grassetto G, Marzola MC, et al. 18F-dopa pet/ct biodistribution consideration in 107 consecutive patients with neuroendocrine tumours. Nucl Med Commun. 2012;33:179–184. doi: 10.1097/MNM.0b013e32834e0974. [DOI] [PubMed] [Google Scholar]
- 72.Kauhanen S, Seppanen M, Minn H, Nuutila P. Clinical pet imaging of insulinoma and beta-cell hyperplasia. Curr Pharm Des. 2010;16:1550–1560. doi: 10.2174/138161210791164090. [DOI] [PubMed] [Google Scholar]
- 73.de Lonlay P, Simon-Carre A, Ribeiro MJ, et al. Congenital hyperinsulinism: Pancreatic [18F]fluoro-l-dihydroxyphenylalanine (DOPA) positron emission tomography and immunohistochemistry study of dopa decarboxylase and insulin secretion. J Clin Endocrinol Metab. 2006;91:933–940. doi: 10.1210/jc.2005-1713. [DOI] [PubMed] [Google Scholar]
- 74.Brice NL, Varadi A, Ashcroft SJ, Molnar E. Metabotropic glutamate and GABA(b) receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia. 2002;45:242–252. doi: 10.1007/s00125-001-0750-0. [DOI] [PubMed] [Google Scholar]
- 75.Burger C, Deschwanden A, Ametamey S, et al. Evaluation of a bolus/infusion protocol for [11C]-ABP688, a PET tracer for MGluR5. Nucl Med Biol. 2010;37:845–851. doi: 10.1016/j.nucmedbio.2010.04.107. [DOI] [PubMed] [Google Scholar]
- 76.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 78.Eisenhofer G, Aneman A, Friberg P, et al. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82:3864–3871. doi: 10.1210/jcem.82.11.4339. [DOI] [PubMed] [Google Scholar]
- 79.Goldstein DS, Swoboda KJ, Miles JM, et al. Sources and physiological significance of plasma dopamine sulfate. J Clin Endocrinol Metab. 1999;84:2523–2531. doi: 10.1210/jcem.84.7.5864. [DOI] [PubMed] [Google Scholar]
- 80.Rubino F, R'Bibo S L, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: The role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6:102–109. doi: 10.1038/nrendo.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]