Abstract
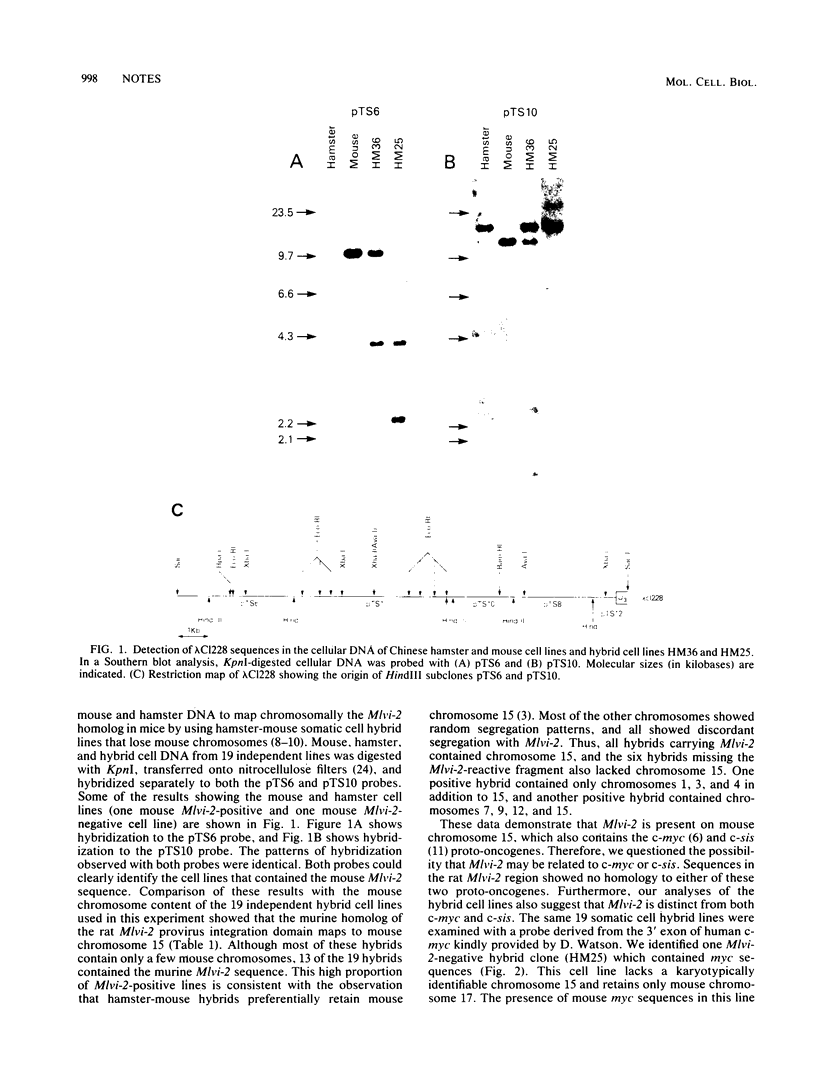
Two cellular DNA regions representing common domains for proviral DNA integration ( Mlvi -1 and Mlvi -2) have been identified in Moloney murine leukemia virus-induced rat thymic lymphomas. Cellular sequences which were free of repeated DNA derived from a clone that defines the Mlvi -2 integration domain (lambda Cl228 ) were found to be highly conserved in a variety of vertebrate species that we examined, including mice, hamsters, cats, and humans. In this study, we identified the chromosomal map location of the Mlvi -2 homologous sequences in mice by using hamster-mouse somatic cell hybrids. The results show that Mlvi -2 is present on mouse chromosome 15 but is unrelated to the c-myc and c-sis proto-oncogenes, which map on the same chromosome. Since aberrations on chromosome 15 have been observed reproducibly in mouse thymomas, our data suggest that Mlvi -2 may define a novel sequence involved in the induction or progression of murine thymic lymphomas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Corcoran L. M., Cory S. Cellular myc oncogene is altered by chromosome translocation to an immunoglobulin locus in murine plasmacytomas and is rearranged similarly in human Burkitt lymphomas. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1982–1986. doi: 10.1073/pnas.80.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dofuku R., Biedler J. L., Spengler B. A., Old L. J. Trisomy of chromosome 15 in spontaneous leukemia of AKR mice. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1515–1517. doi: 10.1073/pnas.72.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Lalley P. A., Moss W., Ivy J., Minna J. D. Gene mapping in Mus musculus by interspecific cell hybridization: assignment of the genes for tripeptidase-1 to chromosome 10, dipeptidase-2 to chromosome 18, acid phosphatase-1 to chromosome 12, and adenylate kinase-1 to chromosome 2. Cytogenet Cell Genet. 1977;19(2-3):57–84. doi: 10.1159/000130799. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983 Feb;32(2):311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic murine leukemia virus-inducing locus of BALB/c mouse to chromosome 5. Science. 1979 Apr 6;204(4388):69–71. doi: 10.1126/science.219475. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic virus-inducing locus Akv-2 of the AKR mouse. J Exp Med. 1980 Nov 1;152(5):1419–1423. doi: 10.1084/jem.152.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Sears J. F., Hoggan M. D. Genetic mapping of the mouse proto-oncogene c-sis to chromosome 15. Science. 1983 Aug 26;221(4613):867–869. doi: 10.1126/science.6308764. [DOI] [PubMed] [Google Scholar]
- Kozak C., Nichols E., Ruddle F. H. Gene linkage analysis in the mouse by somatic cell hybridization: assignment of adenine phosphoribosyltransferase to chromosome 8 and alpha-galactosidase to the X chromosome. Somatic Cell Genet. 1975 Oct;1(4):371–382. doi: 10.1007/BF01538668. [DOI] [PubMed] [Google Scholar]
- MOLONEY J. B. Properties of a leukemia virus. Natl Cancer Inst Monogr. 1960 Sep;4:7–37. [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Noori-Daloii M. R., Swift R. A., Kung H. J., Crittenden L. B., Witter R. L. Specific integration of REV proviruses in avian bursal lymphomas. Nature. 1981 Dec 10;294(5841):574–576. doi: 10.1038/294574a0. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Blais B. M., Tsichlis P. N., Coffin J. M. At least two regions of the viral genome determine the oncogenic potential of avian leukosis viruses. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1225–1229. doi: 10.1073/pnas.79.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Pearson M. N., DeSimone D. W., Tsichlis P. N., Coffin J. M. Subgroup-E avian-leukosis-virus-associated disease in chickens. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1133–1141. doi: 10.1101/sqb.1980.044.01.122. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Human oncogene locations and chromosome aberrations. Nature. 1983 Jan 27;301(5898):290–291. doi: 10.1038/301290a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spira J., Babonits M., Wiener F., Ohno S., Wirschubski Z., Haran-Ghera N., Klein G. Nonrandom chromosomal changes in thy-1-positive and thy-1-negative lymphomas induced by 7,12-dimethylbenzanthracene in SJL mice. Cancer Res. 1980 Jul;40(7):2609–2616. [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombinants between endogenous and exogenous avian tumor viruses: role of the C region and other portions of the genome in the control of replication and transformation. J Virol. 1980 Jan;33(1):238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Role of the C region in relative growth rates of endogenous and exogenous avian oncoviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1123–1132. doi: 10.1101/sqb.1980.044.01.121. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Donehower L., Hager G., Zeller N., Malavarca R., Astrin S., Skalka A. M. Sequence comparison in the crossover region of an oncogenic avian retrovirus recombinant and its nononcogenic parent: genetic regions that control growth rate and oncogenic potential. Mol Cell Biol. 1982 Nov;2(11):1331–1338. doi: 10.1128/mcb.2.11.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Hu L. F. A common region for proviral DNA integration in MoMuLV-induced rat thymic lymphomas. 1983 Mar 31-Apr 6Nature. 302(5907):445–449. doi: 10.1038/302445a0. [DOI] [PubMed] [Google Scholar]
- Wiener F., Ohno S., Spira J., Haran-Ghera N., Klein G. Cytogenetic mapping of the trisomic segment of chromosome 15 in murine T-cell leukaemia. Nature. 1978 Oct 19;275(5681):658–660. doi: 10.1038/275658a0. [DOI] [PubMed] [Google Scholar]