Abstract
Purpose
Our goal was to investigate utilization trends for advanced radiation therapy (RT) technologies, such as intensity-modulated radiation therapy (IMRT) and stereotactic radiosurgery (SRS), in the last year of life among patients diagnosed with metastatic cancer.
Methods
We used the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked databases to analyze claims data in the last 12 months of life for 64,525 patients diagnosed with metastatic breast, colorectal, lung, pancreas, and prostate cancers from 2000–2007. Logistic regression modeling was conducted to analyze potential demographic, health services, and treatment-related variables’ influences on receipt of advanced RT.
Results
Among the 19,161 (29.7%) patients who received radiation therapy, there was a significant decrease in the proportion of patients who received the simplest radiation technique (i.e., 2D-radiation therapy) (p<0.0001), and significant increases in the proportions of patients receiving more advanced radiation techniques (i.e., IMRT, and SRS; p<0.0001 for all curves); although the rates for use of IMRT and SRS in 2007 remained under 5%. On multivariate analyses, receipt of RT varied significantly by non-clinical characteristics such as race, marital status, neighborhood income, and SEER region. Patients who received hospice care in the last year of life were more likely to receive radiation therapy (OR=1.35, 95% CI: 1.30–1.40) but less likely to be treated with IMRT (OR=0.76, 95% CI: 0.62–0.92).
Conclusion
While the proportion of patients receiving RT in the last year of life for metastatic cancer did not change for most of the past decade, we observed significant trends toward more advanced radiation techniques.
INTRODUCTION
Radiation therapy (RT) can be administered via various technological approaches, some of which are very costly and untested in the setting of metastatic cancer as to whether they improve palliative or survival outcomes. Palliative RT regimens have historically been delivered using 2-dimensional planning (2D-RT) techniques which allow rapid initiation of treatment for symptom control for advanced cancer while also being less conformal, with reduced sparing of normal tissues. These 2D plans offer lower complexity in dose calculations, lower costs, and lower utilization of radiation oncology departmental resources than other RT techniques such as 3-dimensional RT (3D-RT), intensity-modulated radiation therapy (IMRT), and stereotactic radiosurgery (SRS).
Radiation therapy charges are among the fastest growing insurance claims in the past several years. 1 Emerging RT technologies delivered by evermore sophisticated and expensive treatment devices and planning systems have contributed to the increased costs of radiotherapy in recent years as the field of radiation oncology evolves to provide more conformal treatment capabilities. Investigators report rapid diffusion of use of IMRT for a variety of cancers. 2,3 Most of the indications for and studies of IMRT involve patients with localized, potentially curable cancer.3 However, survey data show that 57% of radiation oncologists have used IMRT for palliation.2 IMRT is more resource intensive to deliver and is thus more highly reimbursed compared to conventional RT.2–4 Thus, increasing IMRT utilization after metastatic (i.e., largely incurable) cancer diagnosis of common solid tumors may be contributing to the high cost of health care at the end of life, bearing particular relevance given that 25% of Medicare outlays are spent on the last year of life. 5
Stereotactic radiosurgery (SRS) is another advanced RT technique often used for treatment of metastatic cancer (e.g., brain metastases, spinal metastases, lung and liver metastases) and allows a highly conformal dose of radiotherapy to be delivered, usually via RT regimens that are shorter (i.e., fewer days) than conventional RT. To date, no population-based studies have been performed in the U.S. regarding the utilization of advanced RT techniques for advanced-stage cancer. In this study, our goal is to analyze trends in utilization of RT techniques for advanced cancer among Medicare beneficiaries. We will also explore variation in their use according to patient, tumor, socio-demographic or health services characteristics.
METHODS
Data source and cohort definition
We conducted this analysis using the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database, which links Medicare beneficiaries and their Medicare claims files with patients in the SEER tumor registries. The SEER program (a National Cancer Institute-supported database) includes tumor registries in 17 geographic areas. 6 The Medicare program provides payments for hospital, physician, and outpatient medical services for 97% of U.S. citizens who are ≥ 65 years of age. 7,8 The Patient Entitlement and Diagnosis Summary File (PEDSF) contains one record per person in the SEER database and provides information on basic socio-demographics and tumor characteristics; cancer patients in the PEDSF can be linked to the Medicare enrollment and claims files via encrypted person identifiers. We used all available Medicare claims files to obtain information on treatment utilization. All data were de-identified such that no protected health information can be linked to individual patients, and the University of Texas MD Anderson Cancer Center’s institutional review board exempted this study.
The study cohort consisted of 64,525 patients, ≥ 65 years of age, who died from lung, breast, prostate, colorectal, and pancreas cancers between January 1, 2000 and December 31, 2007. These cancers were chosen because they accounted for the top five most common causes of cancer deaths and comprised almost 60% of cancer deaths in 2010. 9 Table 1 shows the algorithm of a series of inclusion and exclusion criteria we used to construct the study cohort. We identified the patients’ causes of death using the SEER cause of death recorded variable which is based on the International Classification of Diseases 9th and 10th revision (ICD-9 and ICD-10) codes: 153.XX, 154.0, 154.1, 154.8, C18–20 (colorectal); 174.XX, C50 (breast); 162.2–162.5, 162.8, 162.9, C34.0–C34.3, C34.8, C34.9 (lung); 157.XX, C25 (pancreas); and 185.XX, C61 (prostate).
Table 1.
Study cohort and exclusion criteria
| Step | Criteria | No. of remaining cases |
|---|---|---|
| 1 | Death from breast, colorectal, lung, pancreas or prostate cancer Between 2000–2007, with matched month/year of death in SEER and Medicare databases. | 355,283 |
| 2 | Exclude if reporting source for diagnosis was autopsy or death certificate | 355,205 |
| 3 | Pathologic confirmation of cancer | 346,823 |
| 4 | Age at death ≥ 65 | 317,917 |
| 5 | Diagnosis with distant metastatic disease at the time of cancer diagnosis and cancer cause of death matched the metastatic cancer diagnosis | 107,334 |
| 5 | Medicare coverage (Part A and B coverage) and without HMO Coverage in the 12 months prior to date of cancer diagnosis | 70,401 |
| 6 | Exclude if there are no claims data available the 12 months prior to date of diagnosis with metastatic cancer | 64,525 |
Dependent variables
We determined the proportion of patients who received external beam RT during last 12 months of life (or less if they survived less than 12 months after their diagnosis) from Medicare claims. Radiation therapy Current Procedural Terminology (CPT) codes 77400– 77416, 77418, G0174, 0197T, 77371–77373, 77435; G0173, G0251, G0338, G0339, G0340 from the Medicare claims files. We determined the type of RT technique (e.g. 2D-RT, 3D-RT, IMRT, or SRS) based upon the simulation and delivery claims present after diagnosis of metastatic cancer until death. A delivery code must have been present following a simulation code for a patient to be coded as having received RT. A patient was considered to have received 3D-RT if an RT delivery code and 3D simulation code were present. Patients were considered to have received IMRT if any codes were present for IMRT planning or delivery. Patients were considered to have received SRS if they had an SRS planning or delivery code present. Patients were considered to have received 2D RT if the RT delivery codes were present and 2D simulation codes and planning codes were present. All radiation therapy courses for each patient were counted as determined by the presence of both simulation and treatment codes; therefore patients could undergo more than one type of radiation therapy modality in their last year of life if they received more than one course of radiation therapy.
Independent variables
Independent variables in our analyses included year of death (2001–2007), age at death (categorical variable: 65–69, 70–74, 75–79, ≥80), gender, race/ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, and non-Hispanic Other races), cancer type, marital status, SEER geographic region, urban vs. rural residence, co-morbidity, neighborhood educational and income levels, and use of hospice care. We linked the SEER-Medicare database to the Area Resource File (ARF)10 via state and county codes provided in the databases to ascertain the number of radiation oncologists per 100,000 practicing within each patient’s health service area. Neighborhood education and income variables were measured at the census tract level (categorized in quartiles). Co-morbidity was constructed using Klabunde’s algorithm; this algorithm calculated a modified Charlson co-morbidity score 11 using inpatient and outpatient claims within a 12 month window prior to cancer diagnosis. 12–14 Whether the patient received hospice care was identified based on any hospice admission and/or service date in the Hospice claims file.
Analyses examining explanatory variables for the type of RT delivered were limited to the subset of patients who received RT. In addition to the independent variables listed above, we added a binary variable, type of RT facility because reimbursement for providers can be higher in freestanding facilities compared to those in hospital-associated centers, since providers in free-standing facilities potentially receive technical as well as professional fees for services provided. Using an algorithm developed by other investigators, 15 we considered that patients had their RT delivered at a hospital-associated facility if their claims for RT were only present in the OUTPT claims files. Those whose RT claims were present in the NCH file were considered to have had their treatments in a free-standing facility.
Statistical analyses
Statistical analyses were conducted using SAS Systems software for Windows (Version 9.3). The unadjusted association of each potential explanatory variable with the outcome of RT after metastatic cancer diagnosis was assessed using chi-square tests. We performed a Cochran-Armitage test for trend to assess any significant change in the proportion of patients receiving RT and also the various types of radiation therapy from 2001 to 2007. Logistic regression models were used to examine the independent association between each explanatory variable and the utilization of RT as well as the advanced RT technology use (e.g., IMRT or SRS). Multivariable models were constructed using a stepwise forward selection process with an entry criteria of (p <0.05). Final results are presented as odds ratios with 95% confidence intervals. All p values reported are two-sided.
RESULTS
Receipt of RT in the last year of life after diagnosis of metastatic cancer
The median survival time of patients in this cohort was 123 days (95% CI: 121–125 days). Of the 64,525 patients, 55,602 (86.2%) died within a year of their diagnosis, and 19,161 (29.7%) received RT after their metastatic cancer diagnosis. Characteristics of the cohort and univariate analyses of the associations of these characteristics are shown in Table 2. The overall trend in any RT use remained stable from 2000 to 2007 (Figure 1). Multivariate analysis (Table 3) revealed that the likelihood of receiving RT was greater for the following: younger patients; non-Hispanic white, Hispanic, and non-Hispanic other patients compared to non-Hispanic black patients; married patients; patients living in higher income neighborhoods; patients with low co-morbidities; and patients living in the Southern SEER region. Lung and prostate cancer patients were more likely to receive radiotherapy than breast cancer patients, whereas patients with colorectal and pancreatic cancer were less likely to receive RT. Patients who elected the Medicare hospice benefit were also more likely to have received RT.
Table 2.
Associations between receipt of radiation therapy in the last year of life after metastatic cancer diagnosis and socio-demographic, disease, and health services characteristics
| Characteristic | total no. | n (%) treated with RT | p value |
|---|---|---|---|
| Entire cohort | 64,525 | 19,161 (29.7) | |
| Year of death | |||
| 2000 | 5564 | 1551 (27.9) | 0.11 |
| 2001 | 7448 | 2277 (30.6) | |
| 2002 | 8186 | 2378 (29.1) | |
| 2003 | 8534 | 2487 (29.1) | |
| 2004 | 8621 | 2675 (31.0) | |
| 2005 | 8798 | 2626 (29.9) | |
| 2006 | 8827 | 2612 (29.6) | |
| 2007 | 8547 | 2555 (29.9) | |
| Age, years | |||
| 65–69 | 11,563 | 4395 (38.0) | <0.0001 |
| 70–74 | 15,830 | 5453 (34.5) | |
| 75–79 | 16,786 | 4984 (29.7) | |
| ≥80 | 20,346 | 4329 (21.3) | |
| Gender | |||
| Male | 33,554 | 10,514 (31.3) | <0.0001 |
| Female | 30,971 | 8647 (27.9) | |
| Race/Ethnicity | |||
| Non-Hispanic White | 53,497 | 16,230 (30.3) | <0.0001 |
| Non-Hispanic Black | 5412 | 1373 (25.4) | |
| Hispanic | 2791 | 770 (27.6) | |
| Other | 2825 | 788 (27.9) | |
| Marital status | |||
| Married | 33,444 | 10,952 (32.8) | <0.0001 |
| Unmarried | 10,275 | 2801 (27.3) | |
| Unknown | 20,806 | 5408 (26.0) | |
| Cancer type | |||
| Breast | 3018 | 803 (26.6) | <0.0001 |
| Colorectal | 9977 | 787 (7.9) | |
| Lung | 38,842 | 15,898 (40.9) | |
| Pancreas | 9564 | 639 (6.7) | |
| Prostate | 3124 | 1034 (33.1) | |
| Comorbidity index | |||
| 0 | 32,066 | 10,280 (26.6) | <0.0001 |
| 1 | 18,545 | 5506 (29.7) | |
| ≥2 | 13,914 | 3375 (24.3) | |
| SEER region | |||
| West/Hawaii | 26,092 | 7551 (28.9) | <0.0001 |
| Northeast | 15,003 | 4410 (29.4) | |
| Midwest | 11,321 | 3299 (29.1) | |
| South | 12,109 | 3901 (32.2) | |
| Urban vs Rural residence | |||
| Urban | 63,283 | 18,794 (29.7) | 0.9237 |
| Rural | 1241 | 367 (29.6) | |
| Median income in census tract | |||
| Lowest quartile | 15,234 | 4336 (28.5) | <0.0001 |
| Second quartile | 15,203 | 4573 (30.1) | |
| Third quartile | 15,240 | 4589 (30.1) | |
| Highest quartile | 15,230 | 4651 (30.5) | |
| Unknown | 3618 | 1012(30.0) | |
| Education level of census tract--% in the neighborhood w < 12 yrs of education | |||
| 1st quartile (highest level) | 15,444 | 4601 (29.8) | 0.0016 |
| 2nd quartile | 15,415 | 4727 (30.7) | |
| 3rd quartile | 15,444 | 4528 (29.3) | |
| 4th quartile | 15,441 | 4443 (28.8) | |
| Unknown | 1913 | 868(31.2) | |
| Radiation Oncologists per 100,000 | |||
| 0 | 23,744 | 7203 (30.3) | 0.0138 |
| 1–3 | 8228 | 2427 (29.5) | |
| 4–24 | 16,320 | 4851 (29.7) | |
| 25–147 | 16,233 | 4680 (28.8) | |
| Hospice | |||
| No | 25,388 | 6964 (27.4) | <0.0001 |
| Yes | 39,147 | 12,207 (31.2) | |
RT-radiation therapy
Figure 1.
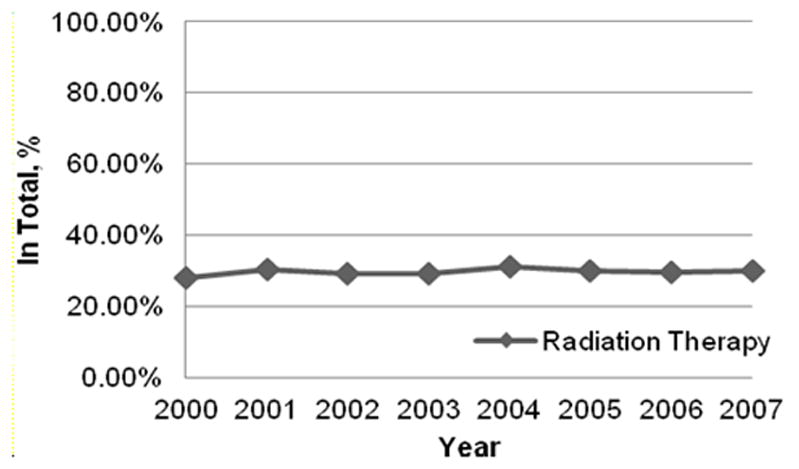
Percent of patients receiving any RT in the last year of life after cancer diagnosis from 2000–2007. (Cochrane Armitage test of trend, p=0.11)
TABLE 3.
Multivariate analysis of predictors of receipt of RT in the last 12 months of life after metastatic cancer diagnosis for the entire cohort
| Variable | Adjusted OR | 95% CI | p value |
|---|---|---|---|
| Year of death | |||
| 2000 | 1.00 | ||
| 2001 | 1.10 | 1.02–1.20 | 0.02 |
| 2002 | 1.03 | 0.94–1.11 | 0.49 |
| 2003 | 1.03 | 0.95–1.12 | 0.48 |
| 2004 | 1.08 | 1.00–1.17 | 0.07 |
| 2005 | 0.98 | 0.91–1.06 | 0.66 |
| 2006 | 1.00 | 0.92–1.09 | 0.96 |
| 2007 | 1.01 | 0.93–1.09 | 0.86 |
| Age, years | |||
| 65–69 | 1.00 | ||
| 70–74 | 0.88 | 0.82–0.91 | <0.0001 |
| 75–79 | 0.70 | 0.66–0.74 | <0.0001 |
| 80+ | 0.49 | 0.47–0.52 | <0.0001 |
| Race/Ethnicity | |||
| Non-Hispanic White | 1.00 | ||
| Non-Hispanic Black | 0.88 | 0.82–0.95 | 0.0005 |
| Hispanic | 1.01 | 0.92–1.11 | 0.81 |
| Other | 1.00 | 0.91–1.10 | 0.99 |
| Marital status | |||
| Married | 1.00 | ||
| Unmarried | 0.73 | 0.69–0.77 | <0.0001 |
| Unknown | 0.81 | 0.78–0.84 | <0.0001 |
| Cancer type | |||
| Breast | 1.00 | ||
| Colorectal | 0.22 | 0.19–0.24 | <0.0001 |
| Lung | 1.74 | 1.60–1.89 | <0.0001 |
| Pancreas | 0.17 | 0.15–0.19 | <0.0001 |
| Prostate | 1.36 | 1.22–1.53 | <0.0001 |
| Comorbidity score | |||
| 0 | 1.00 | ||
| 1 | 0.84 | 0.80–0.87 | <0.0001 |
| 2+ | 0.63 | 0.60–0.66 | <0.0001 |
| SEER region | |||
| Midwest | 1.00 | ||
| Northeast | 1.07 | 1.00–1.15 | 0.05 |
| South | 1.14 | 1.07–1.22 | <0.0001 |
| West/Hawaii | 1.02 | 0.96–1.08 | 0.58 |
| Median income in census tract | |||
| Lowest quartile | 1.00 | ||
| Second quartile | 1.07 | 1.01–1.11 | 0.02 |
| Third quartile | 1.09 | 1.02–1.13 | 0.01 |
| Highest quartile | 1.14 | 1.06–1.19 | <0.0001 |
| Unknown | 1.06 | 0.97–1.16 | 0.21 |
| Radiation Oncologists per 100,000 | |||
| 0 | 1.00 | ||
| 1–3 | 0.96 | 0.90–1.02 | 0.15 |
| 4–24 | 1.07 | 1.01–1.13 | 0.03 |
| 25–147 | 1.06 | 1.00–1.12 | 0.05 |
| Hospice | |||
| No | 1.00 | ||
| Yes | 1.35 | 1.30–1.40 | <0.0001 |
Gender, educational level and urban versus rural residence did not reach significance in the adjusted model and thus results for these variables are not shown here.
Receipt of advanced RT techniques
Among the 19,161 patients who received some form of RT in this cohort, we were unable to determine the type of RT delivered (due to absence of simulation or planning codes) in 443 patients. Among the remaining 18,718 who were analyzed to ascertain trends in utilization of types of RT, there was significant (p < 0.0001) increase in the proportion of patients receiving IMRT from 0% in 2000 to 4.93% in 2007. Similarly, the proportion of patients who received SRS after their diagnosis of metastatic cancer increased from 0.13% in 2000 to 3.72% in 2007 (p < 0.0001). Figure 2 illustrates the trends in proportions of patients treated with IMRT and SRS from 2000 to 2007. Figure 3 shows that there was a decrease in the use of 2D-RT (p < 0.0001) and an increase in the use of 3D-RT from 2000 to 2007 (p < 0.0001).
Figure 2.
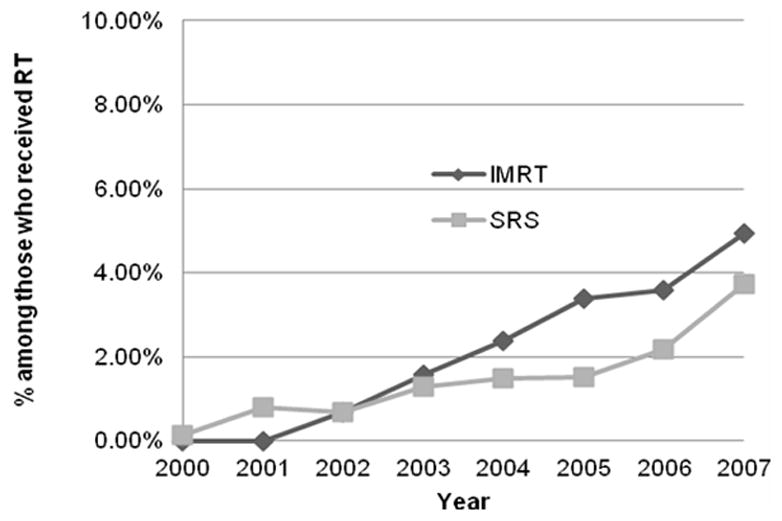
Percent of patients receiving RT who were treated with SRS or IMRT in the last 12 months of life after a metastatic cancer diagnosis. (Cochrane Armitage tests of trend, p<0.0001 for both curves).
Figure 3.
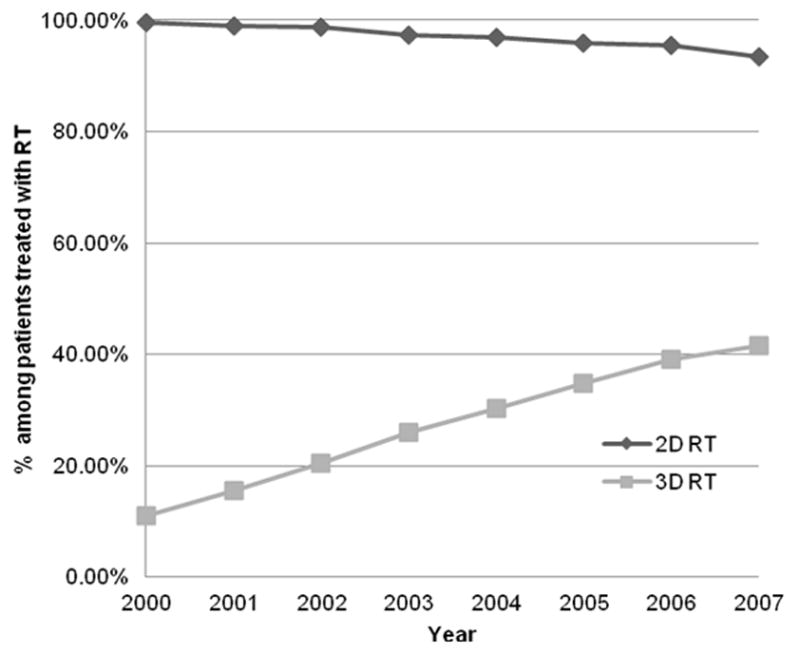
Percent of patients receiving RT who were treated with 2-dimensional or 3-dimensional RT in the last 12 months of life after a metastatic cancer diagnosis. (Cochrane Armitage tests of trend, p<0.0001 for both curves). The receipt of 2D-RT and 3D-RT are not mutually exclusive.
There were 428 patients (2.3% of those who received radiotherapy) treated with IMRT after their metastatic cancer diagnosis. In an adjusted analysis of receipt of IMRT among patients treated with RT (Table 4), pancreatic cancer patients were more likely to receive IMRT than those with other cancer types. Non-clinical factors associated with increased likelihood of receiving IMRT included non-white race, married status, being in the lowest neighborhood income quartile, receiving radiotherapy at a freestanding radiation oncology facility, and residing in the Southern SEER region. Patients living in areas where density of radiation oncologists was the highest were more likely to be treated with IMRT. Patients who received hospice care were significantly less likely to be treated with IMRT than patients not electing hospice care during their last year of life.
TABLE 4.
Multivariate analysis of predictors of receipt of IMRT in the last 12 months of life after metastatic cancer diagnosis among those who received RT
| Variable | Adjusted OR | 95% CI | p value |
|---|---|---|---|
| Year of death | |||
| 2002 | 1.00 | ||
| 2003 | 1.60 | 0.95–2.70 | 0.08 |
| 2004 | 2.49 | 1.53–4.03 | 0.0002 |
| 2005 | 3.76 | 2.36–5.99 | <0.0001 |
| 2006 | 4.18 | 2.63–6.64 | <0.0001 |
| 2007 | 5.85 | 3.72–9.20 | <0.0001 |
| Race/Ethnicity | |||
| Non-Hispanic White | 1.00 | ||
| Non-Hispanic Black | 1.41 | 1.01–1.97 | 0.05 |
| Hispanic | 1.67 | 1.12–2.50 | 0.01 |
| Other | 1.99 | 1.36–2.92 | 0.0004 |
| Marital status | |||
| Married | 1.00 | ||
| Unmarried | 0.95 | 0.73–1.25 | 0.72 |
| Unknown | 0.67 | 0.53–0.86 | 0.0018 |
| Cancer type | |||
| Breast | 1.00 | ||
| Colorectal | 1.37 | 0.72–2.65 | 0.34 |
| Lung | 0.92 | 0.56–1.52 | 0.74 |
| Pancreas | 4.89 | 2.76–8.68 | <0.0001 |
| Prostate | 0.87 | 0.45–1.70 | 0.70 |
| SEER region | |||
| Midwest | 1.00 | ||
| Northeast | 1.50 | 0.99–2.27 | 0.05 |
| South | 2.15 | 1.45–3.19 | 0.0001 |
| West/Hawaii | 1.41 | 1.01–1.97 | 0.05 |
| Median income in census tract | |||
| Lowest quartile | 1.00 | ||
| Second quartile | 0.76 | 0.58–1.00 | 0.05 |
| Third quartile | 0.51 | 0.38–0.70 | <0.0001 |
| Highest quartile | 0.64 | 0.47–0.86 | 0.0030 |
| Unknown | 0.87 | 0.54–1.40 | 0.56 |
| Radiation Oncologists per 100,000 | |||
| 0 | 1.00 | ||
| 1–3 | 0.82 | 0.57–1.17 | 0.28 |
| 4–24 | 1.08 | 0.76–1.52 | 0.68 |
| 25–147 | 2.11 | 1.54–2.89 | <0.0001 |
| Hospice | |||
| No | 1.00 | ||
| Yes | 0.76 | 0.62–0.92 | 0.01 |
| Type of RT facility | |||
| Free-standing | 1.00 | ||
| Hospital-based | 0.50 | 0.41–0.61 | <0.0001 |
Age, gender, co-morbidities, educational level and urban versus rural residence did not reach significance in the adjusted model and thus results for these variables are not shown here.
Of those treated with RT after metastatic cancer diagnosis, 300 patients received SRS (1.6%). In the adjusted model, factors predictive for higher likelihood of receipt of SRS after metastatic cancer diagnosis (Table 5) included: advancing calendar year, treatment in a hospital-based facility, and Midwest or West/Hawaii SEER region.
TABLE 5.
Multivariate analysis of predictors of receipt of SRS in the last 12 months of life after metastatic cancer diagnosis among those who received RT
| Variable | Adjusted OR | 95% CI | p value |
|---|---|---|---|
| Year of death | |||
| 2000 | 1.00 | ||
| 2001 | 6.43 | 1.49–27.74 | 0.01 |
| 2002 | 5.56 | 1.28–24.22 | 0.02 |
| 2003 | 10.60 | 2.54–44.29 | <0.0001 |
| 2004 | 12.23 | 2.95–50.67 | <0.0001 |
| 2005 | 12.65 | 3.05–52.40 | <0.0001 |
| 2006 | 18.11 | 4.42–74.30 | <0.0001 |
| 2007 | 31.39 | 7.73–127.54 | <0.0001 |
| SEER region | |||
| Midwest | 1.00 | ||
| Northeast | 0.63 | 0.44–0.90 | 0.01 |
| South | 0.50 | 0.33–0.76 | <0.0001 |
| West/Hawaii | 1.01 | 0.75–1.38 | 0. 94 |
| Type of RT facility | |||
| Free-standing | 1.00 | ||
| Hospital-based | 3.01 | 2.16–4.21 | <0.0001 |
Age, gender, race/ethnicity, urban vs. rural residence, marital status, type of cancer causing death, co-morbidities, income level, educational level, radiation oncologist density in health service area, and hospice care did not reach significance in the adjusted model and thus results for these variables are not shown here.
DISCUSSION
The percentage of patients treated with radiotherapy in their last year of life after diagnosis with metastatic cancer remained stable from 2000 to 2007. However, there was a shift in the types of RT delivered away from the simplest, least costly technique (e.g., 2D-RT) to more advanced and expensive technologies (e.g., 3D-RT, IMRT, SRS). The increased use of 3D-RT was based on improved imaging capabilities (e.g., computed tomography) for planning which aid in visualization of tumors and surrounding anatomy, but beam placement processes and dosing regimens remain similar to 2D-RT. While 3D-RT is an intermediately more complex and resource intensive approach than 2D-RT, we will focus in this discussion on the more advanced and newer techniques of IMRT and SRS. The overall rates of advanced radiation technology use were relatively low; however, the steepness of the slopes of their rise may have significant future cost implications for radiation oncology in advanced cancer care. Our findings might partially explain a recent observation that radiation oncology costs are outpacing their expected contribution to Medicare expenditures. 16
Whether IMRT might improve outcomes for patients with metastatic cancer has not been proven or even explicitly evaluated in comparison to conventional techniques. Dosing regimens for IMRT are similar to those for conventional treatment (e.g., days to weeks of daily treatment), and the advantage of IMRT arises in higher conformality of the area targeted for full radiotherapy dose. The studies on IMRT have been performed for non-metastatic presentations of various cancers, i.e., those for whom treatment intent was curative, and have shown reduced rates of late toxicity (i.e., those occurring months to years after treatment) such as improved salivary function after treatment for head and neck cancer patients or decreased late rectal and genitourinary toxicities for treatment of prostate cancer.3 Moreover, a report from the American College of Radiology Appropriateness Criteria Expert Panel consistently listed IMRT as among the least appropriate treatment options for palliation of bone metastases for various clinical scenarios.17 However, some patients with oligometastatic breast or prostate cancer may have prolonged expected survival, and thus benefit from the reduction of late toxicity through IMRT.
Stereotactic radiosurgery was, in large part, developed specifically for treatment of metastases. 18–21 Therefore, its rapid diffusion into radiation oncology practice for patients with metastatic cancer is not surprising. In contrast to IMRT, SRS techniques are often delivered in fewer treatment days than conventional radiotherapy techniques, and therefore offer a more convenient treatment course for eligible patients than multiple weeks of daily radiotherapy. An example would be a single day course of SRS for oligometastases in the brain rather than a 10-day regimen of whole brain RT; and indeed, there are comparative studies emerging evaluating the role of SRS versus conventional radiotherapy or surgical approaches for patients with brain metastases, with some evidence that neurological function may be improved with SRS in some patients. 22–24 With respect to SRS to other anatomic sites, such as liver or lung oligometastases, delivery of comparable high dose radiation with conventional radiation techniques (i.e., 2D-RT or 3D-RT) is not feasible. Thus, there are no valid comparative effectiveness studies for some indications for SRS, which essentially opened up new roles for RT in the management of metastatic cancer. However, SRS may offer a less morbid alternative to thoracic surgery for patients with lung lesions.21
Characteristics predictive of receipt of RT reflected clinically logical patterns, with younger and lung cancer patients being the most likely to receive radiation therapy. Our finding that pancreatic cancer patients were more likely to receive IMRT may reflect anatomical challenges of treating pancreatic tumors that are situated among multiple critical normal structures with relatively low radiation dose tolerances. Our findings that receipt of RT varied by non-clinical factors, such as race or income, are consistent with findings by others revealing disparate distribution of cancer treatments among socio-economic and racial subgroups. 25,26
Certain health services characteristics were significantly predictive of receipt of RT or an advanced radiotherapy technique. Patients who elected hospice care were more likely to receive RT; however, they were less likely to receive IMRT. Patients treated at a freestanding facility were more likely to receive IMRT; a finding consistent with other investigators who found adoption of breast IMRT to be more likely in freestanding radiation oncology centers.15 Conversely, patients treated at hospital-associated facility were three times more likely than those treated at a freestanding center to be treated with SRS. This may reflect that fact treatment machines that deliver conventional RT can also deliver IMRT, whereas stereotactic units often involve special equipment or imaging capabilities whose cost might be better absorbed by a hospital system than by freestanding radiation oncology practices. Geographic variation in receipt of RT, as was observed in our study, has been documented by other investigators. 25 Finally, patients living in areas of higher density of radiation oncologists were more likely to receive RT and more likely to undergo IMRT, possibly reflecting better access to care or competitive market influences for higher technology availability that might attract patients which could have a downstream effect of greater diffusion into practice.
Our study has limitations inherent to retrospective analyses with large registry and claims data. Specifically, there are no data regarding performance status, patient and physician preferences, nor whether treatment intent was radical or palliative in nature. Therefore it is beyond the scope of these data to ascertain the appropriateness of therapies. However, our goal in this paper was not to comment upon the appropriateness of radiotherapy services rendered but rather only to investigate the changing trends in radiation oncology practice for patients with metastatic cancer. Also, while the SEER registries cover approximately one-quarter of the US population, investigators have recently pointed out that the geographic distribution of these databases coverage may not offer a nationally representative picture of practice patterns.27
In conclusion, our study showed significant shifting trends in the technological approaches to radiation oncology care for patients diagnosed with metastatic cancer who received radiotherapy in their last twelve months of life. Our findings may partially explain the observed increasing costs associated with radiation oncology care over the past decade. More research is needed into the role of emerging radiation technologies in advanced cancer care with respect to improvements in patient outcomes, physician incentives for advanced technology use, and cost implications for radiation oncology care.
Acknowledgments
We acknowledge that the study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the SEER Program registries in the creation of the SEER-Medicare database. This manuscript as been approved by IMS as compliant with the database user agreement.
FUNDING SOURCE:
This study was supported in part by a grant from the National Institutes of Health/National Cancer Insitute (1R21CA164449-01A1 to B.A.G.)
Footnotes
There are no conflicts of interest and no external funding sources.
References
- 1.Carreyrou J, Tamman M. A Device to Kill Cancer, Lift Revenue. Wall Street Journal. 2010 http://online.wsj.com/article/SB10001424052748703904804575631222900534954.html.
- 2.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S., 2004. Cancer. 2005;104(6):1296–303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 3.Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, De Neve W. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol. 2008;9(4):367–75. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 4.Sher DJ. Cost-effectiveness studies in radiation therapy. Expert review of pharmacoeconomics & outcomes research. 10(5):567–82. doi: 10.1586/erp.10.51. [DOI] [PubMed] [Google Scholar]
- 5.Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45(2):565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anonymous. National Cancer Institute. [Last accessed, May 26, 2009];Surveillance Epidemiology and End Results. http://seer.cancer.gov/index.html. Available at: http://seer.cancer.gov/index.html.
- 7.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31(8):732–48. [PubMed] [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. Area Resource File (ARF) US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Professions; Rockville, MD: 2009–2010. www.arf.hrsa.gov. [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 13.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006;44(10):921–8. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–90. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Smith BD, Pan IW, Shih YC, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. Journal of the National Cancer Institute. 103(10):798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 16.Alhassani A, Chandra A, Chernew ME. The sources of the SGR “hole”. N Engl J Med. 2012;366(4):289–291. doi: 10.1056/NEJMp1113059. [DOI] [PubMed] [Google Scholar]
- 17.Janjan N, Lutz ST, Bedwinek JM, et al. Therapeutic guidelines for the treatment of bone metastasis: a report from the American College of Radiology Appropriateness Criteria Expert Panel on Radiation Oncology. Journal of palliative medicine. 2009;12(5):417–26. doi: 10.1089/jpm.2009.9633. [DOI] [PubMed] [Google Scholar]
- 18.Park HS, Chiang VL, Knisely JP, Raldow AC, Yu JB. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: an update. Expert Rev Anticancer Ther. 2011;11(11):1731–1738. doi: 10.1586/era.11.165. [DOI] [PubMed] [Google Scholar]
- 19.Chawla S, Schell MC, Milano MT. Stereotactic Body Radiation for the Spine: A Review. Am J Clin Oncol. 2011 doi: 10.1097/COC.0b013e31822dfd71. [DOI] [PubMed] [Google Scholar]
- 20.Høyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82(3):1047–1057. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Dahele M, Senan S. The role of stereotactic ablative radiotherapy for early-stage and oligometastatic non-small cell lung cancer: evidence for changing paradigms. Cancer Res Treat. 2011;43(2):75–82. doi: 10.4143/crt.2011.43.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 24.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 25.Tuttle TM, Jarosek S, Habermann EB, Yee D, Yuan J, Virnig BA. Omission of radiation therapy after breast-conserving surgery in the United States: A population-based analysis of clinicopathologic factors. [Accessed January 26, 2012];Cancer. 2011 doi: 10.1002/cncr.26505. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21952948. [DOI] [PubMed]
- 26.Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non-small cell lung cancer among black and white Medicare beneficiaries, 2000–2003. J Natl Med Assoc. 2011;103(8):711–718. doi: 10.1016/s0027-9684(15)30410-7. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson M, Earle CC, Newhouse JP. Geographic variation in physicians’ responses to a reimbursement change. N Engl J Med. 2011;365(22):2049–2052. doi: 10.1056/NEJMp1110117. [DOI] [PMC free article] [PubMed] [Google Scholar]


