Abstract
Background
Pulmonary vein isolation (PVI) for atrial fibrillation (AF) is associated with a transient increased risk of thromboembolic and hemorrhagic events. We hypothesized that dabigatran can be safely used as an alternative to continuous warfarin for the peri-procedural anticoagulation in PVI.
Methods and Results
999 consecutive patients undergoing PVI were included; 376 patients were on dabigatran (150 mg) and 623 were on warfarin with therapeutic INR. Dabigatran was held 1 to 2 doses prior to PVI and restarted at the conclusion of the procedure or as soon as patients were transferred to the nursing floor. Propensity score matching was applied to generate a cohort of 344 patients in each group with balanced baseline data. Total hemorrhagic and thromboembolic complications were similar in both groups, before (3.2% vs 3.9%; p = 0.59), and after (3.2% vs 4.1%; p = 0.53) matching. Major hemorrhage occurred in 1.1% vs 1.6% (p = 0.48) before, and 1.2% vs 1.5% (p = 0.74) after matching in the dabigatran vs warfarin group respectively. A single thromboembolic event occurred in each of the dabigatran and warfarin groups. Despite higher doses of intra-procedural heparin, the mean ACT was significantly lower in patients who held dabigatran for 1 or 2 doses than those on warfarin.
Conclusions
Our study found no evidence to suggest a higher risk of thromboembolic or hemorrhagic complications with use of dabigatran for peri-procedural anticoagulation in patients undergoing PVI compared to uninterrupted warfarin therapy.
Keywords: anticoagulants, fibrillation, ablation, catheter ablation, stroke
Introduction
AF ablation has evolved over the past decade providing patients with symptomatic AF an alternative to medical therapy.1,2 Thromboembolic and bleeding complications, however, represent rare but serious consequences.3 Sheaths and catheters in the left atrium (LA), atrial stunning, endothelial damage, and inflammation from ablation heightens the risk of thromboembolic complications during and early after ablation.4 Peri-procedural management of anticoagulation in patients undergoing PVI is critical to limit complications.
Warfarin has been the only effective oral anticoagulant available since 1950. Most centers prefer to discontinue warfarin prior to PVI and bridge anticoagulation before and after ablation.5,6 More recently several studies have shown that PVI can be safely performed in patients with a therapeutic INR.7–9 This strategy is gaining momentum and has been endorsed in the 2012 HRS/EHRA/ECAS expert consensus statement.10
Emergence of dabigatran as a safe and effective alternative to warfarin in patients with non-valvular AF,11 offers new challenges and possibilities for minimizing peri-operative thromboembolism and hemorrhage. The purpose of our study was to evaluate the use of dabigatran for peri-operative anticoagulation in patients undergoing AF ablation compared to uninterrupted warfarin therapy.
Methods
The study was a review of a prospectively collected registry of patients undergoing PVI between December 2010 and July 2012 at our center. All consecutive patients referred for PVI while on dabigatran etexilate (150 mg) were included and compared to consecutive patients undergoing PVI while on uninterrupted warfarin with a therapeutic INR during the same time period. The and hemorrhagic complications during the initial 30 days following ablation, and the intra-procedural heparin and ACT were compared between the two groups. The study was approved by the Cleveland Clinic Institutional Review Board and all patients gave written informed consent before ablation.
Major hemorrhage was defined as the occurrence of tamponade or hemopericardium that required intervention or caused symptoms, excessive bleeding (≥ 2 g/L decrease in hemoglobin or need for transfusion), hematoma requiring intervention or additional hospitalization, significant hemoptysis, hemothorax, or retroperitoneal bleeding. Minor hemorrhage was defined as the occurrence of a hematoma or any bleeding that did not require intervention or prolong hospitalization. Thromboembolic complications were defined as the occurrence of ischemic stroke, transient ischemic attack, peripheral embolic events, or deep venous thrombosis.
Ablation protocol
Our AF ablation approach was previously described in detail.12 In summary, two sheaths were placed in each of the femoral veins under ultrasound guidance. Intracardiac echocardiography (ICE) was used in all procedures to assist with trans-septal punctures, view catheters in the LA, and identify complications including pericardial effusion. Two catheters were advanced into the LA for mapping and ablation guided by ICE. All pulmonary veins were isolated using a 3.5 mm irrigated tip catheter using fluoroscopy and a 3D navigation system for guidance (CARTO, Biosense-Webster Inc. or Ensite NavXTM, St. Jude Medical Inc., MN, USA). Radiofrequency energy was limited to 35–40 W. Impedance and esophageal temperature were closely monitoring to avoid excessive heating and tissue injury. In patients with concomitant atrial flutter, activation mapping and entrainment were performed to locate and ablate the critical isthmus.
Peri-procedural and Intra-procedural anticoagulation
Early in our experience, patients were instructed to hold 1 or 2 doses of dabigatran before ablation according to the preference of the electrophysiologist performing the procedure. More recently most patients are instructed to hold only one dose on the morning of the procedure. Patients on warfarin were instructed to continue taking the therapeutic dose. No heparin was administered to any patient in either group prior to ablation. Transesophageal Echocardiography (TEE) was performed immediately prior to PVI in patients presenting in AF with compliance issues on dabigatran, or sub-therapeutic INR on warfarin within 4 weeks of the procedure. For PVI, an initial unfractionated heparin (UFH) bolus (80–150 units/kg) was administered before transseptal puncture. During the procedure, UFH was continuously given to all patients via intravenous infusion. ACT was monitored every 10–30 minutes (Hemochron Jr. Signature + Micro coagulation System, ITC Medical, Edison, NJ, USA). Additional heparin boluses were given and the infusion rate was adjusted to target an ACT of 350–450 seconds. After ablation, catheters were withdrawn, and heparin was stopped and partially reversed with protamine before sheaths were pulled. PVI was performed under conscious sedation with fentanyl and Midazolam in the majority of patients. This allowed for safe administration of dabigatran (150 mg) and aspirin (325 mg) in the EP lab at the conclusion of the procedure. Patients who underwent PVI under general anesthesia were extubated in the EP lab, transferred to the post-anesthesia care unit, and received dabigatran as soon as they were transferred to the nursing floor. Patients on warfarin received their evening dose following PVI to ensure continuous therapy.
Peri-procedural monitoring and Post procedural follow-up
Patients were monitored for thromboembolic and hemorrhagic complications throughout the procedure, overnight, and prior to discharge the following day, using frequent symptom, neurologic, vascular access site, heart and peripheral pulsation evaluations. Transthoracic echocardiography (TTE) and ultrasound were performed as needed. Patients on dabigatran were discharged on 150 mg twice daily. Patients on warfarin had INR checks the day of the procedure and were followed by their local doctors and Coumadin clinics to maintain therapeutic INR. Follow-up weekly telephone calls were conducted in the first three months post-discharge by dedicated AF-electrophysiology registered nurses to assess progress of recovery and symptoms. In addition, all patients were instructed to call our center for AF if any symptoms developed and to send weekly trans-telephonic electrocardiogram transmissions for the first 3 months after ablation. Patients with suspected complications were asked to seek the nearest emergency department or their local physician. Documentation from these visits were obtained and added to our records. All patients had scheduled follow-up appointments with their electrophysiologist three months following PVI or earlier if symptoms arise to evaluate success and exclude complications.
Statistical Analysis
Demographic and baseline characteristics were summarized, categorical variables were compared using χ2 tests, and continuous variables were compared using analysis of variance if normally distributed and Kruskal Wallis test if not normally distributed.
Because of significant differences in some baseline characteristics between the dabigatran and warfarin groups, propensity score matching was applied. By constructing a logistic regression model in which the dabigatran vs. warfarin treatment was regressed on baseline characteristics related to dabigatran treatment and/or outcome of PVI. The estimated propensity score was obtained as the predicted probability of exposure of each patient to dabigatran. Matching was based on the logit of propensity score, using calipers of width 0.2 of the standard deviation of the logit of the propensity score.13 To assess bias reduction achieved by propensity matching, the absolute standardized differences of the 11 covariates included in propensity score calculation were compared before and after matching, with a value < 10% indicating between-group balance (Supplemental Figure 1).14 The matched baseline data is estimated by paired t-test or Wilcoxon singed rank test for normally or non-normally distributed continuous variables, and McNemar test or the Stuart-Maxwell statistics for binary or polytomous categorical variables.13 Complications after matching were compared between the dabigatran and warfarin groups using the McNemar test and a special method for estimating relative risks (RR) and their 95% confidence intervals (CI),15 to account for the dependent nature of the matched pairs.
To evaluate intra-procedural ACT values and heparin requirements, a greedy 5→1 digit matching of patients on warfarin to those who held dabigatran for 1 dose and those who held it for 2 doses without replacement was respectively conducted on a population of patients with complete ACT values up to 165 minutes, based on logistic regression models for deriving propensity scores in which dabigatran 1 dose or 2 doses was regressed on age and AF duration years that were related to dabigatran and ACT measures. A cohort of triple matched samples (warfarin, dabigatran 1 dose and 2 doses) was subsequently formed by merging these matched samples using the common warfarin patients. A random coefficient mixed model repeated measures analysis for ACT against measurement time across the treatment groups was performed in this matched cohort, and least-squares means (mean ± SE) were calculated. Furthermore, a growth curve model was built showing regression lines of the treatment groups. A time-to-event analysis was performed to examine time to first ACT > 350 seconds in a cohort of triple matched samples from the 3 treatment groups constructed on the same greedy 5→1 digit matching method with main effects in the propensity score model being age, males, AF duration, and hypertension that were related to dabigatran and time to first ACT > 350 seconds, and merged on common warfarin patients. Cumulative Kaplan-Meier estimates were plotted and compared.
Some variables that are related to the repeated measures or time-to-event were compared pair-wisely among the treatment groups using the Bonferroni adjustment, in the respective matched cohorts. All statistical analyses were performed using the SAS software (version 9.2, SAS Institute, Cary, NC). A 2-sided probability value of 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 999 patients undergoing PVI at our institution between December 2010 and July 2012 were included in the study, of which 376 patients were on dabigatran and 623 patients were on uninterrupted warfarin with a therapeutic INR. Dabigatran was started 62 days [median; IQR (34, 120)] before PVI, and was held for one dose in 203 patients and for 2 doses in 173 patients prior to PVI. Baseline patient characteristics are summarized in Table 1. Propensity score logit matching identified 344 dabigatran (92%) and the same number of warfarin patients who were comparable with respect to age, gender, body mass index, common comorbidities, CHADS II score, prevalence of persistent AF, and aspirin intake. INR was not included in matching because it is inherently higher in the warfarin group.
Table 1.
Baseline characteristics* of the study population before and after propensity matching
| Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|
| Variable | Dabigatran (n = 376) | Warfarin (n = 623) | P | Dabigatran (n = 344) | Warfarin (n = 344) | P |
| Age (yrs) | 58.6 ± 11.0 | 62.7 ± 9.6 | < 0.001 | 60.0 ± 10.0 | 60.1 ± 10.4 | 0.82 |
| Male gender | 282 (75.0) | 457 (73.4) | 0.57 | 256 (74.4) | 252 (73.3) | 0.72 |
| BMI (kg/m2) | 30.3 ± 5.3 | 30.6 ± 6.2 | 0.95 | 30.5 ± 5.4 | 30.5 ± 6.4 | 0.85 |
| AF duration (yrs) | 3.0 (1.0, 6.5) | 4.5 (2.0, 8.5) | < 0.001 | 3.0 (1.0, 7.0) | 3.0 (1.5, 6.0) | 0.59 |
| Persistent AF | 161 (42.8) | 279 (44.9) | 0.53 | 155 (45.1) | 136 (39.7) | 0.16 |
| CAD | 45 (12.0) | 130 (20.9) | < 0.001 | 44 (12.8) | 48 (14.0) | 0.61 |
| Diabetes mellitus | 40 (10.6) | 92 (14.8) | 0.06 | 39 (11.3) | 33 (9.6) | 0.44 |
| Hypertension | 183 (48.7) | 370 (59.4) | 0.001 | 176 (51.2) | 169 (49.1) | 0.55 |
| CHF | 32 (8.5) | 88 (14.1) | 0.01 | 32 (9.3) | 34(9.9) | 0.79 |
| Prior stroke/TIA | 24 (6.4) | 52 (8.3) | 0.26 | 23 (6.7) | 25 (7.3) | 0.75 |
| CHADS2 score | < 0.001 | 0.92 | ||||
| – 0 | 160 (42.6) | 181(29.1) | 135 (39.2) | 137 (39.8) | ||
| – 1 | 139 (37.0) | 259 (41.6) | 134 (39.0) | 137 (39.8) | ||
| – ≥ 2 | 77 (20.5) | 183(29.4) | 75(21.8) | 70 (20.3) | ||
| SR at time of PVI | 228 (60.6) | 334 (53.6) | 0.03 | 201 (58.4) | 204 (59.3) | 0.81 |
| Creatinine (mg/dL) | 0.9 (0.8,1.1) | 0.9 (0.8, 1.1) | 0.29 | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.1) | 0.67 |
| INR | 1.1(1.0,1.2) | 2.4(2.1, 2.8) | < 0.001 | 1.1(1.0, 1.2) | 2.4(2.1,2.8) | < 0.001 |
| Aspirin usage | 125 (33.2) | 246 (39.5) | 0.048 | 120 (34.9) | 114 (33.1) | 0.63 |
| LV EF (%) | 55.0 (54.0, 60.0) | 55.0 (50.0, 60.0) | 0.06 | 55.0 (52.5, 60.0) | 55.0 (52.0, 60.0) | 0.34 |
| LAVI (ml/m2) | 32.2 (28.3, 41.0) | 34.4 (27.5, 42.0) | 0.42 | 32.7 (28.4, 42.1) | 33.6 (26.0, 41.4) | 0.74 |
| TEE | 44 (11.9) | 136 (25.4) | < 0.001 | 40 (11.9) | 70 (23.4) | < 0.001 |
BMI indicates body mass index; CAD, coronary artery disease; CHF, congestive heart failure; SR, sinus rhythm; INR, international normalization ration; LVEF, left ventricular ejection fraction; and LAVI, left atrium volume index, TEE; transesophageal echocardiography.
Categorical variables are expressed as frequency (percentage), continuous variables are expressed as mean ± SD if normally distributed or as median (IQR) if not normally distributed.
Complications
Total hemorrhagic and thromboembolic complications were similar in the dabigatran and warfarin groups before [3.2% vs 3.9%; p = 0.59; RR (95% CI) 0.828 (0.420, 1.636)] and after matching [3.2% vs 4.1%; p = 0.53; RR (95% CI) 0.786 (0.368, 1.676)] (Table 2). Thromboembolic events occurred in one patient in each of the warfarin and dabigatran groups. In the warfarin group, a 71-year-old male with a CHADS II score of 2 (hypertension and heart failure) developed right upper extremity weakness and expressive aphasia one hour following PVI secondary to a small left middle cerebral artery thromboembolic event. TEE was performed prior to PVI for subtherapeutic INR in the preceding week, revealing spontaneous echo contrast (SEC) but no LA thrombi. He was managed conservatively and no TPA was given due to therapeutic INR on the day of the procedure. The right upper extremity weakness completely resolved within few hours and minimal neurological deficits were noted on the 3 months follow up visit. In the dabigatran group, one patient who held 1 dose before ablation reportedly complained of pleuritic chest pain and was diagnosed with pulmonary embolism at an outside hospital within 2 weeks of discharge. Investigations that supported this diagnosis were not available for our review. He was managed by switching to warfarin in addition to aspirin.
Table 2.
Complications before and after propensity matching
| Before propensity score matching | After propensity score matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (999) | Dabigatran (n = 376) | Warfarin (n = 623) | Relative Risk (95% Confidence Interval) | P | Total (688) | Dabigatran (n = 344) | Warfarin (n = 344) | Relative Risk (95% Confidence Interval) | P | |
| Total Hemorrhagic/thromboembolic events, n (%) | 36 (3.6%) | 12 (3.2%) | 24 (3.9%) | 0.828 (0.420, 1.636) | 0.59 | 25 (3.6%) | 11 (3.2%) | 14 (4.1%) | 0.786 (0.368, 1.676) | 0.53 |
| Major hemorrhagic events, n (%) | 14 (1.4%) | 4 (1.1%) | 10 (1.6%) | 0.663 (0.209, 2.099) | 0.48 | 9 (1.3%) | 4 (1.2%) | 5 (1.5%) | 0.800 (0.215, 2.981) | 0.74 |
| Minor hemorrhagic events, n (%) | 20 (2.0%) | 7 (1.9%) | 13 (2.1%) | 0.892(0.359,2.215) | 0.81 | 15 (2.2%) | 6(1.7%) | 9 (2.6%) | 0.667 (0.237, 1.873) | 0.44 |
| Acute tamponade, n (%) | 10 (1.0%) | 3 (0.8%) | 7 (1.1%) | 0.710 (0.185, 2.730) | 0.75 | 6 (0.9%) | 3 (0.9%) | 3 (0.9%) | 1.000 (0.202, 4.955) | 1.00 |
| Small pericardial effusion, n (%) | 3 (0.3%) | 1 (0.3%) | 2 (0.3%) | 0.828 (0.075, 9.107) | 1.00 | 2 (0.3%) | 1 (0.3%) | 1 (0.3%) | 1.000 (0.063, 15.982) | 1.00 |
| Total groin hematoma, n (%) | 15 (1.5%) | 5 (1.3%) | 10 (1.6%) | 0.828 (0.285, 2.405) | 0.73 | 11 (1.6%) | 4 (1.2%) | 7 (2.0%) | 0.571 (0.167, 1.951) | 0.37 |
| Gastrointestinal bleeding, n (%) | 3 (0.3%) | 1 (0.3%) | 2 (0.3%) | 0.828 (0.075, 9.107) | 1.00 | 3 (0.4%) | 1 (0.3%) | 2 (0.6%) | 0.500 (0.045, 5.515) | 0.56 |
Major hemorrhagic complications were similar between the dabigatran and warfarin groups before [1.1% vs 1.6%; p = 0.48; RR (95% CI) 0.663 (0.209, 2.099)] and after propensity matching [1.2% vs 1.5%; p = 0.74; RR (95% CI) 0.800 (0.215, 2.981)]. A 40-year-old male patient in the dabigatran group with CHADS II score of 2 (hypertension and diabetes) developed a small hemorrhagic stroke that presented 48 hours after PVI with severe headache, dizziness, and ear ringing without any focal deficit. Symptoms resolved by the time he was evaluated in the emergency department. Brain CT showed a small right hemispheric hemorrhagic stoke. Brain MRI showed a cavernous malformation associated with a congenital venous anomaly. Dabigatran was discontinued and the patient was started on warfarin one week later after a repeat brain CT showed no evidence of further bleeding.
Tamponade occurred in 3 patients on dabigatran and 7 on warfarin before [0.8% vs 1.1%; p = 0.75; RR (95% CI) 0.710 (0.185, 2.730)] and in 3 patients on dabigatran and 3 on warfarin after propensity matching [0.9% vs 0.9%; p = 1.00; RR 1.000 (0.202, 4.955)]. In the dabigatran group, tamponade was recognized during mapping of the LA in one patient and after PVI completion in 2 patients. One patient held dabigatran for 1 dose and two patients held it for 2 doses before ablation. In all patients on dabigatran, tamponade was managed by reversing heparin with protamine and performing percutaneous pericardiocentesis in the EP lab. No re-accumulation was detected on follow up TTEs, and no surgery or dialysis were required in any patient. In the warfarin group, 7 patients developed tamponade; 3 detected during LA mapping or ablation and 4 following PVI completion. Six patients were managed with heparin reversal and pericardiocentesis. One patient developed tamponade several minutes following a steam pop that occurred during ablation along the LA roof. He remained hemodynamically unstable despite heparin reversal and pericardiocentesis. Surgical exploration showed an intact LA roof and a right ventricular laceration at the site of the pericardial sheath. He stabilized following repair of the laceration and no re-accumulation was detected on follow up TTE.
Hemoptysis occurred in 2 patients on warfarin following PVI. One patient had major bleeding that required blood and fresh frozen plasma transfusion and intubation. Chest CT and bronchoscopy showed no evidence of bronchial injury and bleeding resolved spontaneously without further interventions. The second patient had minor hemoptysis that resolved overnight without any interventions. One patient on warfarin developed epistaxis one month after PVI that required an ED and cauterization.
Incidence of groin hematoma was similar between the dabigatran and warfarin groups before [1.3% vs 1.6%; p = 0.73; RR (95% CI) 0.828 (0.285, 2.405)] and after matching [1.2% vs 2.0%; p = 0.37; RR (95% CI) 0.571 (0.167, 1.951)]. Only 2 patients on warfarin required thrombin injections for pseudoaneurysm. No interventions were required in any of the patients on dabigatran.
One patient on dabigatran developed bleeding secondary to hemorrhoids 5 days after PVI and was managed by holding 2 doses of dabigatran and a banding procedure. Two patients on warfarin developed limited lower gastrointestinal bleeding that did not require transfusion or interruption of the oral anticoagulation.
Intra-procedural activated clotting time and heparin requirements
Intra-procedural ACT and heparin requirements were evaluated in a population of 184 patients (70 held 1 dose of dabigatran, 63 held 2 doses of dabigatran, and 51 on warfarin). 5→1 digit propensity matching identified a cohort of 42 patients with balanced baseline characteristics in each of the 3 groups. A mixed model repeated measures analysis that incorporated linear random coefficients and quadratic fixed time effects (Figure 1) revealed that ACT was significantly lower across time in patients who held dabigatran for 2 doses prior to the PVI procedure (least-squares mean ± SE: 336.05 ± 4.76) than those who held it for only one dose (351.68 ± 5.61; p = 0.037), and those on warfarin therapy (391.68 ± 7.34; p < 0.001).
Figure 1.
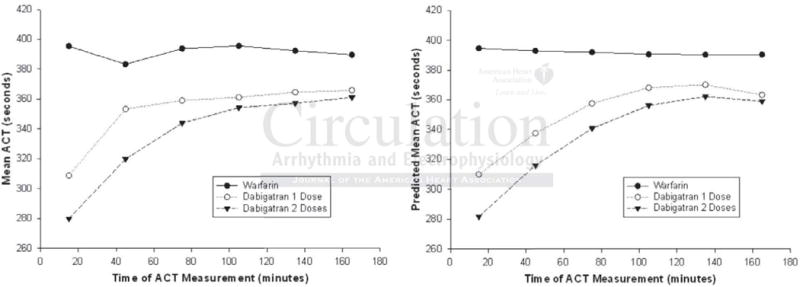
Mean intraprocedural ACT measurements throughout the PVI procedure. (Left) Mean ACT measurements across the time categorized by the 3 treatment groups. (Right) Growth curves generated from the random coefficient mixed model repeated measures analysis showing predicted mean ACT measurements across the time stratified by the 3 treatment groups.
The heparin dose throughout ablation was significantly higher in patients who held dabigatran for 1 or 2 doses (mean ± SD 225.2 ± 64.37 U/kg vs 239.0 ± 64.99 U/kg) than those on warfarin (164.9 ± 36.06 U/kg; p < 0.001). The mean heparin dose required to achieve target ACT (> 350 seconds) was significantly higher in patients who held dabigatran for 1 or 2 doses versus those on warfarin (153.3 ± 42.74 U/kg and 175.1 ± 57.65 U/kg, vs 103.4 ± 23.57 U/kg respectively; p < 0.001). All these multiple comparisons remained significant after the Bonferroni adjustment.
Time to first ACT > 350 seconds was analyzed in 261 patients (91 in warfarin, 102 in dabigatran 1 dose, 68 in dabigatran 2 doses). 5→1 digit matching was used to identify a cohort of 52 patients with balanced baseline characteristics in each of the 3 groups. Kaplan-Meier curves (Figure 2) and Z statistics13 demonstrated that time to first ACT > 350 seconds was significantly longer in patients who held two doses of dabigatran prior to PVI [median (IQR): 50 min (35, 72.5)] than those who held one dose [20 (15, 40); Z = 2.94, p-value = 0.003] and those who were on warfarin [20 (15, 30); Z = 4.71, p < 0.001]
Figure 2.
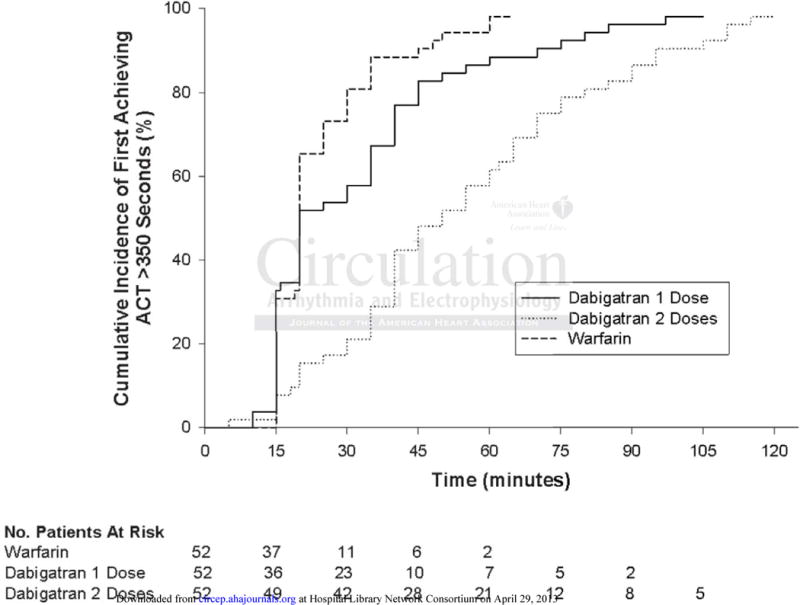
Kaplan-Meier estimates of the rates of first achieving ACT > 350 seconds following initial heparin bolus dose, stratified by the 3 treatment groups.
Discussion
In this study, there was no evidence of increased risk of thromboembolic or hemorrhagic complications with use of dabigatran for peri-procedural anticoagulation in patients undergoing AF ablation. However, compared to patients who underwent AF ablation on uninterrupted warfarin therapy, patients who were on dabigatran had higher intra-procedural heparin requirements, lower mean ACT, and more prolonged time to reach the target ACT.
Peri-procedural Safety of Dabigatran
In our study, dabigatran was held for only 1–2 dose before ablation and restarted immediately after sheaths were pulled or once patients were on the floor. Major hemorrhagic and thromboembolic complications were rare. In the dabigatran group, a single hemorrhagic cerebrovascular event occurred in a young patient with cerebral cavernous malformation. In contrast, a single thromboembolic stroke occurred in a patient on warfarin with long-standing persistent AF and a very large atrium with SEC on TEE as described in the results section.
Tamponade occurred in three patients in the dabigatran group, and although the medication was held briefly prior to PVI bleeding was self-limited with no need for surgical intervention or dialysis. Despite the similarity of the peri-procedural anticoagulation strategies; dabigatran held one dose prior to ablation and restarted 3 hours afterwards, Lakkireddy et al16 recently reported a higher risk of bleeding with use of dabigatran in a multicenter study involving 145 patient undergoing PVI compared with a similar number of patients on uninterrupted warfarin. In their study, the incidence of hemorrhagic and thromboembolic complications was generally high in both the dabigatran (16%) and the uninterrupted warfarin (6%) groups. Tamponade occurred in 9 patients (6%) on dabigatran and one patient on warfarin (1%). Procedural techniques were operator dependent and high radiofrequency energy outputs (up to 45 W) were used. It is unclear if complications occurred predominantly in one or more of the 8 involved centers. In addition, a higher percentage of patients on dabigatran were older than 75 years of age compared to our patient population (7% vs 4.5%). Interestingly, they reported an alarming 31% complication rate in this age group, which is consistent with recent published data suggesting increased risk of major bleeding in patients ≥ 75 years old with use of dabigatran 150 mg.17 Thromboembolic complications occurred in 2% of patients on dabigatran and none in the warfarin group. Target ACT was lower (300–400 seconds) and all patients were empirically given a bolus of 10,000 U of heparin prior to the trans-septal puncture. Intra-procedural ACT levels and heparin requirements were not reported.
Early in our study, significantly low intra-procedural ACT was noted in patients on dabigatran and subsequently more aggressive anticoagulation with higher initial and more frequent boluses of heparin were administered to avoid this situation as we gained experience with managing the heparin requirements in 376 consecutive ablation procedures on dabigatran. This may not have been possible in a multicenter study, in which 145 cases were distributed over 8 centers and performed simultaneously, and may have contributed to the higher complication rates in patients on dabigatran.
Perioperative anticoagulation strategies
The optimal perioperative anticoagulation strategy in patients undergoing AF ablation remains unclear. Historically, interruption of oral anticoagulation, insufficient intra-procedural anticoagulation, and use of non-irrigated catheters were associated with up to 5% risk of thromboembolic complications.18 More recently, a world-wide survey of AF ablation reported an incidence of 0.94%, 1.31% and 1.47% of stroke/TIA, tamponade, and pseudoaneurysm or arterio-venous fistulae, respectively.3 Interruption of warfarin and bridging with full dose enoxaparin is associated with a higher bleeding risk,6 while bridging with half dose enoxaparin is inconvenient and expensive. PVI can be safely performed on uninterrupted warfarin with therapeutic INR throughout the peri-procedural period with less stroke and major hemorrhagic complications.7–9 This strategy is currently gaining wider acceptance and has been endorsed in the 2012 HRS/EHRA/ECAS expert consensus statement.10
Dabigatran’s efficacy and rapid onset and offset of action make it an ideal candidate for peri-procedural anticoagulation in PVI. Anticoagulant effects of dabigatran parallel its plasma concentrations. Onset of action is within 1 hour of oral administration, peak is within 2–3 hours, and terminal half-life is 12–17 hours. To minimize time spent with sub-therapeutic anticoagulation, in our study dabigatran was held for 1–2 doses prior to PVI and restarted immediately afterwards. This strategy was associated with no cerebral thromboembolic complications and a similar incidence of major hemorrhagic events compared to warfarin. It is important to note that it is not the anticoagulant that causes spontaneous bleeding but rather this is an inherent risk of the procedure. The concern is management of bleeding once it occurs especially in the dabigatran group given the absence of a reversal agent. However, in the three patients in our study and the nine patients in Lakkireddy’s study who did develop tamponade, bleeding was self-limited requiring no surgical intervention or hemodialysis for elimination of dabigatran.
Intra-procedural heparin requirements and ACT measurements
Despite the minimal interruption of dabigatran for only 1–2 doses, intra-procedural heparin requirements to achieve the target ACT of 350–450 seconds were significantly higher compared to those on continuous warfarin group. Possible explanations of these findings include; interaction between dabigatran and heparin, inaccuracy of the ACT test with dabigatran use, or diminution of anticoagulation effects of dabigatran after holding 1–2 doses compared to continuous warfarin.
The fact that patients who held 1 dose of dabigatran before PVI had relatively less heparin requirements, earlier and more consistent achievement of target ACT, and higher mean ACT values compared to those who held dabigatran for 2 doses suggests that rapid elimination of dabigatran is the most likely explanation for the higher heparin requirements. If interactions between dabigatran and heparin or the ACT test were responsible, we would have expected higher heparin requirements and lower ACT in patients who held dabigatran for 1 dose compared to 2 doses as higher levels of the drug remain in the circulation leading to more interaction.
Our results suggest that heparin requirements are indirectly proportionate to the intensity of therapeutic oral anticoagulation at the time of PVI, with lower anticoagulation intensity after holding 1–2 doses of dabigatran compared to uninterrupted warfarin. Interestingly, intra-procedural heparin requirements in patients who held dabigatran for 1–2 doses were comparable to historical heparin needs reported in patients who underwent PVI with a subtherapeutic or normal INR after interruption of warfarin. Our current recommendation is to hold dabigatran for only one dose prior to PVI as this strategy was associated with more consistent achievement of target ACT without significant increase in hemorrhagic complications.
Limitations
This was not a randomized trial. A much larger randomized study would be required to detect any differences if present in thromboembolic and hemorrhagic events between patients on dabigatran and those on continuous warfarin given the low incidence of complications with either strategy. The majority of our patients were younger than 75 years old with normal renal functions. Further studies are needed to assess safety of dabigatran for peri-procedural anticoagulation in other patient populations. Unlike warfarin, it is currently impossible to confirm patient’s compliance to dabigatran with a lab test. TEE was performed in patients who may have missed doses in the days to weeks prior to PVI. Nevertheless in this study of our current practice, that included all patients on dabigatran, the results are encouraging and were not associated with higher complication rates.
Conclusion
There was no evidence of increased thromboembolic or hemorrhagic complications with use of dabigatran for peri-procedural anticoagulation in patients undergoing AF ablation compared to uninterrupted warfarin therapy. Proper procedural techniques and vigilant monitoring of intra-procedural ACT are needed with use of dabigatran to avoid the inherent procedural risks. Larger studies are needed to confirm these findings and assess safety in sub-populations including patients older than 75 and those with renal impairment.
Supplementary Material
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Haissaguerre M, Jais P, Shah DC, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng JT, Roudaut R, Clementy J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary vein foci. Circulation. 2000;101:1409–1417. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 2.Wazni OM, Marrouche NF, Martin DO, Verma A, Bharqava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J, Gutleben K, Pisano E, Potenza D, Fanelli R, Raviele A, Themistoclakis S, Rossillo A, Bonso A, Natale A. Radiofrequency ablation vs. antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 3.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 4.Dorbala S, Cohen AJ, Hutchinson LA, Menchavez-Tan E, Steinberg JS. Does radiofrequency ablation induce a prethrombotic state? Analysis of coagulation system activation and comparison to electrophysiology study. J Cardiovasc Electrophysiol. 1998;9:1152–1160. doi: 10.1111/j.1540-8167.1998.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 5.Ren JF, Marchlinski FE, Callans DJ, Gerstenfeld EP, Dixit S, Lin D, Nayak HM, Hsia HH. Increased intensity of anticoagulation may reduce risk of thrombus during atrial fibrillation ablation procedures in patients with spontaneous echo contrast. J Cardiovasc Electrophysiol. 2005;16:474–477. doi: 10.1046/j.1540-8167.2005.40465.x. [DOI] [PubMed] [Google Scholar]
- 6.Oral H, Chugh A, Ozaydin M, Good E, Fortino J, Sankaran S, Reich S, Igic P, Elmouchi D, Tschopp D, Wimmer A, Dey S, Crawford T, Pelosi F, Jr, Jongnarangsin K, Bogun F, Morady F. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation for atrial fibrillation. Circulation. 2006;114:759–765. doi: 10.1161/CIRCULATIONAHA.106.641225. [DOI] [PubMed] [Google Scholar]
- 7.Wazni OM, Beheiry S, Fahmy T, Barrett C, Hao S, Patel D, Di Biase L, Martin DO, Kanj M, Arruda M, Cummings J, Schweikert R, Saliba W, Natale A. Atrial fibrillation ablation in patients with therapeutic international normalized ratio comparison of strategies of anticoagulation management in the peri-procedural period. Circulation. 2007;116:2531–2534. doi: 10.1161/CIRCULATIONAHA.107.727784. [DOI] [PubMed] [Google Scholar]
- 8.Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Horton R, Gallinghouse GJ, Lakkireddy D, Verma A, Khaykin Y, Hongo R, Hao S, Beheiry S, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Santangeli P, Wang P, Al-Ahmad A, Patel D, Themistoclakis S, Bonso A, Rossillo A, Corrado A, Raviele A, Cummings JE, Schweikert RA, Lewis WR, Natale A. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation. The impact of periprocedural therapeutic international normalized ratio. Circulation. 2010;121:2550–2556. doi: 10.1161/CIRCULATIONAHA.109.921320. [DOI] [PubMed] [Google Scholar]
- 9.Kwak JJ, Pak HN, Jang JK, Kim SK, Park JH, Choi JI, Hwang C, Kim YH. Safety and convenience of continuous warfarin strategy during the periprocedural period in patients who underwent catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:620–625. doi: 10.1111/j.1540-8167.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 10.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen S, Crijns HJG, Damiano RJ, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design. Heart Rhythm. 2012;9:632–696. [Google Scholar]
- 11.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. RE-LY Steering Committee and Investigators. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 12.Kanj M, Wazni O, Natale A. How to do circular mapping catheter-guided pulmonary vein antrum isolation: the Cleveland Clinic approach. Heart Rhythm. 2006;3:866–869. doi: 10.1016/j.hrthm.2006.02.1033. [DOI] [PubMed] [Google Scholar]
- 13.Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: A systematic review and suggestions for improvement. J Thorac Cardiovasc Surg. 2007;134:1128–1135. doi: 10.1016/j.jtcvs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 15.Agresti A, Min Y. Effects and non-effects of paired identical observations in comparing proportions with binary matched-pairs data. Stat Med. 2004;23:65–75. doi: 10.1002/sim.1589. [DOI] [PubMed] [Google Scholar]
- 16.Lakkireddy D, Reddy YM, Di Biase L, Vanga SR, Santangeli P, Swarup V, Pimentel R, Mansour M, D’Avila A, Sanchez JE, Burkhardt JD, Chalhoub F, Mohanty P, Coffey J, Shaik N, Monir G, Reddy VY, Ruskin J, Natale A. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2012;59:1168–74. doi: 10.1016/j.jacc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, Diener HC, Franzosi MG, Huber K, Reilly P, Varrone J, Yusuf S. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the Randomized Evaluation of Long-Term Anticoagulant Therapy (RE-LY) Trial. Circulation. 2011;123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 18.Kok LC, Mangrum JM, Haines DE, Mounsey JP. Cerebrovascular complication associated with pulmonary vein ablation. J Cardiovasc Electrophysiol. 2002;13:764–767. doi: 10.1046/j.1540-8167.2002.00764.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


