Abstract
The mechanistic/mammalian target of rapamycin (mTOR) pathway plays a critical role in cellular metabolism, growth and proliferation, and has been evaluated as a target for therapy in various malignancies. The mTOR pathway is a major tumor-initiating pathway in hepatocellular carcinoma, with upregulation seen in up to 50% of (HCC) tumors. Metformin, which represses mTOR signaling by activating AMPK, has been shown to decrease liver carcinogenesis in population studies. mTOR inhibitors such as everolimus have been evaluated as adjunctive chemotherapy with some success, although efficacy has been limited by the lack of complete mTOR pathway inhibition. The active site mTOR inhibitors hold greater promise, given that they offer complete mTOR suppression. There is also evidence of mTOR pathway activation in cholangiocarcinoma, although its biological significance in initiating and promoting tumor progression remains ambiguous. This review article provides an overview of the complex biochemistry behind the mTOR pathway and its role in carcinogenesis, especially as it pertains to hepatic malignancies.
Keywords: mTOR pathway, hepatocellular carcinoma, cholangiocarcinoma, mTOR inhibitors
The mTOR pathway and its relevance to carcinogenesis
The liver is the principal organ regulating body metabolism, and the mTOR pathway is a key regulator of cellular metabolism. The mTOR pathway integrates upstream growth, metabolic and mitogenic signals that effect downstream activation of a broad array of intracellular processes. The mTOR pathway plays an important role in insulin resistance, type II diabetes, adipogenesis, angiogenesis, and tumor development1. In addition to potent antifungal and immunosuppressive properties, mTOR inhibitors strongly suppress cellular proliferation, making them attractive anti-cancer agents.
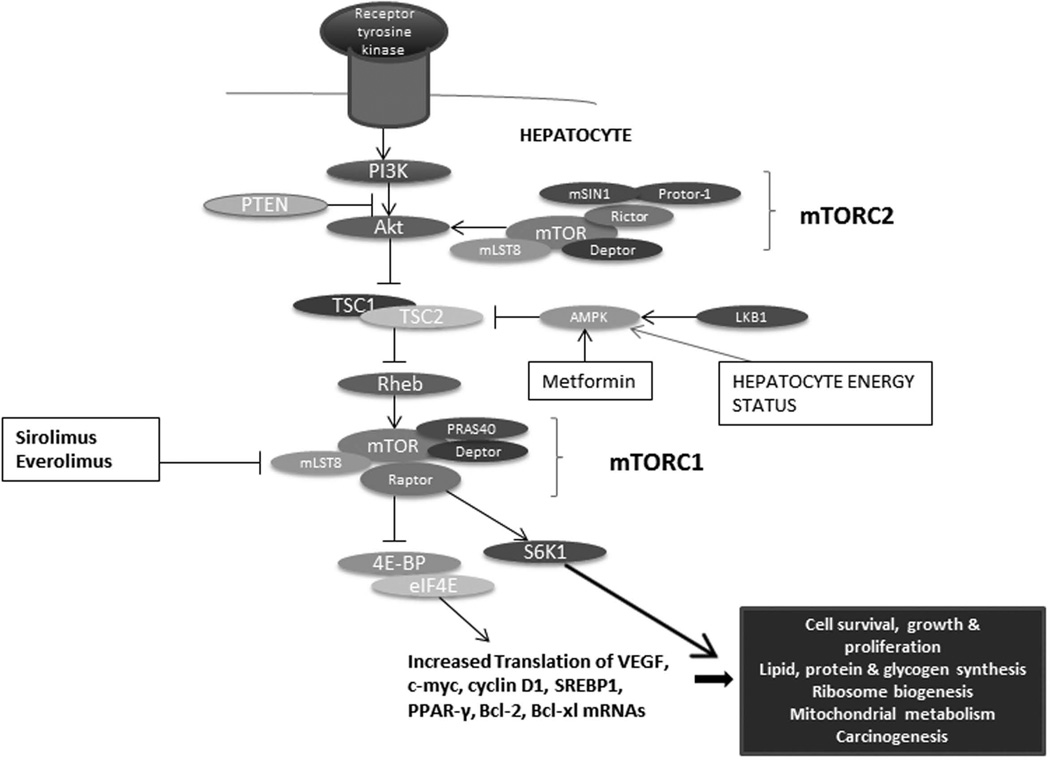
mTOR is a large 289 kDa protein that forms the nucleus of two functionally distinct multi-protein complexes, mTORC1 (mTOR complex 1) and mTORC2 (mTOR complex 2)2. References to mTOR in the literature predominantly pertain to the activity of mTORC1, which is sensitive to sirolimus (the official name for rapamycin). mTORC1 associates with at least four other proteins: mammalian lethal with SEC13 protein 8 (mLST8), regulatory-associated protein of mTOR (Raptor), proline-rich AKT substrate of 40 kDa (PRAS40), and DEP-domain-containing mTOR-interacting protein (Deptor). There is evidence to suggest that the latter two proteins inhibit mTORC1 complex activity. The best-studied and characterized substrates of mTORC1 are S6 kinase (S6K) and 4E-BPs (eukaryotic translation initiation factor-binding proteins 4E-BP1, 4E-BP2 and 4E-BP3), which control cell growth and proliferation (Figure 1).
Figure 1. The mTOR pathway in the liver.
The 4E-BPs are key proteins that regulate translation initiation through their binding to eIF4E (eukaryotic translation initiation factor-4E). When phosphorylated, 4E-BPs dissociate from eIF4E, which is then free to bind the 5' cap of mRNAs. eIF4E is critical to global protein synthesis, and is a crucial effector of translation of various malignancy-associated mRNAs. It promotes the production of pro-oncogenic proteins involved in cell cycle progression, proto-oncogenes (c-myc, VEGF), angiogenesis, cell survival, autocrine growth stimulation, communication with the extracellular environment and invasion3. The Ras pathway also contributes to activation of eIF4E, given that its signaling cascade culminates in stimulation of Mnk, a kinase that phosphorylates eIF4E3. The Ras and Akt signaling pathways, two principal effectors of carcinogenesis, thereby converge on eIF4E, which reflects its pivotal role in tumor development3. eIF4E is overexpressed in various malignancies, and is being studied as a prognostic marker and therapeutic target in cancer4. The mTORC2 complex is implicated in cellular functions such as protein synthesis, autophagy and metabolism, and actin cytoskeleton reorganization5. Much of the biology of the mTORC2 complex remains to be defined.
The mTOR pathway integrates signals from pro-oncogenic growth factors such as IGF-1 and VEGF as well as from various cytokines. Cellular energy levels, cellular stress, hypoxia, and DNA damage also modulate the mTOR pathway6. These upstream signals activate PI3 kinase, which in turn upregulates the protein kinase AKT through the second messenger phosphatidylinositol triphosphate (PIP3) and subsequently mTOR. In the context of low cellular energy levels, an increased AMP/ATP ratio activates AMPK, which thereby inhibits the mTOR pathway. PTEN is a tumor suppressor protein that also negatively regulates the mTOR pathway7 (Figure 1).
With respect to carcinogenesis at the cellular level, mTOR signaling promotes cell growth and proliferation by upregulating anabolic processes such as protein, glycogen, lipid and organelle synthesis and downregulating catabolic processes such as autophagy8. Insulin treatment triggers mTORC1 activation of sterol regulatory element binding protein 1 (SREBP1) and peroxisome proliferator-activated receptor- γ (PPARγ), key transcription factors in lipid and cholesterol biosynthesis9. Cancer cell proliferation is typically associated with de novo lipid synthesis: SREBP-1 induces lipid synthesis, needed for the membrane synthesis that enables cancer cell proliferation11. Sirolimus as an mTORC1 inhibitor decreases the amounts of many SREBP-1 target genes such as acetyl-CoA carboxylase and fatty acid synthase and downregulates PPARγ expression10. It has therefore been postulated that sirolimus may reduce carcinogenesis through inhibition of lipid and protein synthesis.
As tumors develop, they encounter significant stressors that impede their ability to grow. Genotoxic stressors in general induce DNA damage, which stimulates the mTORC1 inhibitor AMPK, thereby enabling apoptosis11. Although hypoxic conditions are the norm in rapidly growing cancers and hypoxia decreases mTOR signaling, cancer cells appear to circumvent the hypoxia-mediated mTOR limitation via preferential translation of Hypoxia Inducible Factor (HIF1α) and Vascular Endothelial Growth Factor A (VEGFA) by an mTOR-independent mechanism12. This confers hypoxia tolerance and restores control over protein synthesis and cell survival, particularly in more advanced tumors.
Upregulation of the mTOR pathway is seen in ~70% of all types of cancers13. The importance of the mTOR pathway in carcinogenesis is further underscored by the presence of mutations along the mTOR pathway in familial cancer syndromes. Examples of such syndromes include Cowden's syndrome (loss of PTEN), Peutz-Jegher's syndrome (loss of LKB1, an activator of AMPK which in turn inhibits the mTOR pathway), tuberous sclerosis and lymphangioleiomyomatosis (loss of TSC1 or TSC2)14.
The mTOR pathway in Hepatocellular carcinoma
Liver disease occurs as a result of complex insults, including viral hepatitis, alcohol and lipotoxicity. Liver cell death in these conditions occurs via apoptosis, necrosis or the two combined. Carcinogenesis is thought to occur as a result of mutations acquired in the context of rapid cell turnover triggered by these insults. Both processes, acquisition of genetic lesions and cell turnover, are required for development of liver cancer (Figure 2). mTOR as a survival pathway has been suggested to modulate apoptosis through eIF4E, by upregulating the translation of anti-apoptotic mRNAs, such as Bcl-2, Bcl-xL and Mcl-115. Inhibition of S6K1 the other branch downstream of mTOR unexpectedly prevented hepatocyte apoptosis, as demonstrated in an in vivo model of S6K1 knockout mice16. This is likely be due to loss of the negative feedback of S6K1 on Akt and hence the mTOR pathway17. Nonetheless, this paper further proved that the mTOR pathway is essential for hepatocyte cell survival. Mouse models of gene knockouts of components upstream of mTOR have demonstrated the importance of mTOR in liver regeneration after partial hepatectomy17.
Figure 2. mTOR pathway initiation and progression in HCC.
The mTOR pathway has been implicated in fibrogenesis and HCC initiation and progression in vitro and in vivo. There is also in vitro data on effective mTOR inhibition in these processes, as well as retrospective clinical data on metformin in preventing HCC initiation 40,56–63
Given its importance in both cell survival and proliferation, it is not surprising that mTOR appears to play a pivotal role in hepatic carcinogenesis. The mTOR pathway is aberrantly upregulated in up to 50% of HCC tumors, as determined by integrating data from direct sequencing, DNA copy number changes, mRNA levels, and immunohistochemistry in a large human HCC tissue sample cohort18. Increased mTOR signaling occurs downstream of receptor tyrosine kinase signaling cascades such as those initiated by Insulin Growth Factor (IGF) or Epidermal Growth Factor (EGF). The importance of mTOR in hepatocarcinogenesis has been further shown in a mouse model with a liver-specific knockout of Tsc1: the resulting chronic mTOR activation led to sporadic and sequential development of histological features associated with HCC (liver damage, inflammation, necrosis, and regeneration)19. PTEN, the tumor suppressor that inhibits the mTOR pathway, is inactivated in around half of HCC tumors20. A transgenic hepatocyte-specific PTEN-deficient mouse model exhibited histological features of non-alcoholic steatohepatitis (NASH) at 40 weeks, with adenomas developing in 60% and HCCs in 100% of the mice by 80 weeks of age20. An additional study has supported the concept of the mTOR pathway enabling the transition from NASH-related cirrhosis to HCC21. Aberrant lipogenesis was increasingly seen in a spectrum of human non-tumorous liver tissue to liver cancer, and was associated with mTOR pathway activation. Activation of the mTOR pathway may be the mechanism through which HCC develops on the basis of NASH without intervening cirrhosis22. This has been proposed based on the findings of animal and immunohistochemical studies, where activation of Akt and the mTOR pathway in turn triggered development of HCC in a NASH liver23. With respect to clinicopathologic parameters, AKT phosphorylation has been correlated with early HCC recurrence and poor prognosis23. All of the aforementioned in vivo and human histological studies strongly suggest the implication of the mTOR pathway in hepatocarcinogenesis. There has been growing interest in the use of mTOR inhibitors to treat HCC (Figure 3), especially as several mTOR inhibitors are in clinical use (Table 1) albeit not approved for HCC. It has been proposed that mTOR inhibitors would be effective in tumors where angiogenesis is an important feature of the pathogenesis24. For example, sirolimus and temsirolimus have been shown to inhibit angiogenesis, correlating with decreased VEGF production and HIF-1 activity in cancer cells24. Sirolimus has potent antiproliferative activity against HCC cell lines in vitrohowever in vivo evidence is currently lacking25. The growth of hepatomas induced by DNA damage is curtailed by treatment with everolimus, apparently due to its effect on cell cycle related proteins26. A recently completed Phase I/II study of everolimus in advanced HCC patients, most of whom had received prior systemic chemotherapy, confirmed its safety at 10 mg/day27. Although there was no placebo arm for comparison and the study was not continued to phase II, some encouraging antitumor effects were seen with a progression-free survival of 28.6% at 24 weeks. A Phase I trial attempting to combine the gold standard of sorafenib (a Ras/Raf kinase/VEGF inhibitor) with the mTOR inhibitor temsirolimus in patients with advanced HCC was closed by the data safety committee due to excess toxicity, particularly dose-limiting thrombocytopenia28. This adverse event was likely due to use of the maximal dose of temsirolimus without an attempt to dose the drug by determining serum levels28. The efficacy of mTOR inhibition has been limited, most likely because the inhibitors studied bind to mTORC1 solely. This allows for an escape mechanism through mTORC2, with maintained cancer cell survival. This prompted the development of active site mTOR inhibitors, which exert dual inhibition of mTORC1 and mTORC2 with striking anti-proliferative effects. A multinational phase I/II trial of the active-site mTOR inhibitor AZD8055 in Asian patients with advanced HCC and mild to moderate hepatic impairment has just been completed, and results are pending (NC00999882). Other active site mTOR inhibitors including PI-103 and PKI-587 have been tested in combination with sorafenib on in vitro and in vivo mouse models of HCC with success29. A recent novel concept is that the ratio of eIF4E to 4E-BP in cancer cells affects response to active site mTOR inhibitors, with a higher ratio resulting in greater resistance to these agents30. The dual PI3K/mTOR inhibitors, which theoretically could provide more potent suppression of the mTOR pathway through additional inhibition of PI3K upstream of mTOR, are also being investigated in HCC. NVP-BEZ235 is such a dual inhibitor: it inhibits PI3K at its ATP-binding domain in addition to preventing the catalytic activity of mTORC1 and mTORC2 complexes. This compound was shown to significantly affected HCC cell proliferation in vitro and diminished tumor volume in a dose-dependent manner in vivo31. This dual PI3K/mTOR inhibitor has also been shown to act synergistically with the catalytic active site inhibitor everolimus in suppressing HCC cell proliferation, with this combination being much more potent than either agent alone32. This was attributed to the suppression of phosphorylation of Akt and 4E-BP by both mTORC1 and mTORC2. This potent suppression is in contradistinction to the weaker effect of sirolimus, where inhibition of the mTORC1/S6K1 negative feedback loop activates the PI3K/Akt/mTOR pathway to a certain extent33.
Figure 3. Effects of mTOR inhibition on liver physiology and metabolism.
This figure illustrates the different targets of mTOR, and how mTOR inhibitors affect the various metabolic and physiologic processes in the liver, leading to decreased cell survival and autophagy, decreased protein and lipid synthesis, and decreased ribosomal biogenesis.
Table 1.
mTOR inhibitors clinically studied in malignancies
| mTOR inhibitor | Mechanism of action | FDA-approved indications in clinical practice (www.fda.gov) |
|---|---|---|
| Sirolimus | Allosteric mTOR inhibitor |
|
| Everolimus | Allosteric mTOR inhibitor |
|
| Temsirolimus | Allosteric mTOR inhibitor |
|
A limitation of mTOR inhibitors in clinical trials has been their significant side effect profile (Figure 4). mTOR inhibitors are associated with certain side effects common with other immunosuppressants (mucositis, rash, anemia, thrombocytopenia). Other unique side effects (hyperlipidemia, hyperglycemia and hypophosphatemia) occur as a result of the metabolic impact of inhibiting the mTOR pathway34.
Figure 4. Side effects of mTOR inhibitors.
mTOR inhibitors have also been used as immunosuppression after liver transplantation for HCC to prevent recurrence35. The theory behind using an mTOR inhibitor is its additional role as adjuvant chemotherapy, whereby circulating HCC cells that have engrafted into the newly transplanted liver or extrahepatic sites could be quenched. However, the evidence supporting mTOR inhibitors in this context remains quite limited, given that there is a lack of randomized double-blinded trials of sirolimus as immunosuppression following liver transplant for HCC. There are likely two reasons for this: firstly, the recurrence rate of HCC after liver transplant is low enough that it would be difficult to demonstrate a significant difference in recurrence rates within a randomized trial; secondly, sirolimus is associated with significant intolerable side effects for patients, which has historically led to significant study dropout. None of the retrospective studies thus far have managed to look at immunosuppression involving only mTOR inhibitors, because of side effects. Nonetheless, a meta-analysis of 5 retrospective studies, wherein sirolimus-based immunosuppression was used following liver transplantation for HCC, revealed that this preventive strategy resulted in decreased post-transplant cancer recurrence (OR = 0.42, 95% CI = 0.21-0.83) as compared to sirolimus-free regimens36. Additionally, patient survival was significantly improved at 1 [OR = 4.53, 95% CI = 2.31-8.89], 3 (OR = 1.97, 95% CI = 1.29-3.00), and 5 years (OR = 2.47, 95% CI = 1.72-3.55) among liver transplant recipients on sirolimus. There is currently an ongoing prospective randomized, open-labeled, trial entitled the SiLVER study (NCT00355862) comparing sirolimus-containing versus mTOR-inhibitor-free immunosuppression in patients undergoing liver transplantation for HCC. This study commenced in 2008, with a 3-year enrolment period and a subsequent 5-year follow-up period, and will hopefully provide a more conclusive answer as to whether sirolimus offers decreased risk of HCC recurrence and improved survival. This trial includes patients whose explants are found to have tumors beyond Milan criteria. In the context of diagnosed HCC recurrence after transplantation, a retrospective uncontrolled study of 31 patients where immunosuppression was switched to an mTOR inhibitor and sorafenib was added subsequent to diagnosis of recurrent HCC suggested that this combination may be effective37. There was an overall response rate to this combination therapy of 3.8% according to the Response Evaluation Criteria in Solid Tumors, with stabilization of the disease in 50% of patients.
A promising adjunct therapy that affects the mTOR pathway is metformin, a biguanide medication commonly prescribed to diabetic patients. It is known to inhibit the mTOR pathway by two mechanisms: 1) through inhibition of mitochondrial oxidative phosphorylation which activates AMPK thereby resulting in mTOR pathway inhibition and 2) through decreased serum glycemia, which inhibits IGF-R thereby preventing downstream mTOR pathway activation in insulin-responsive cancers38. Retrospective studies have suggested that metformin prevents development of HCC among patients with diabetes39 and those with chronic liver disease40. Metformin has been found to inhibit DEN-induced hepatocarcinogenesis in vivo by affecting lipogenesis41, and to induce apoptosis of HCC cells in vitro although the underlying mechanism is unclear42,43.
Targeting microRNAs that regulate mTOR gene expression is another novel approach being developed for cancer therapy. miR-99a/100, which targets the 3’UTR of mTOR in a post-transcriptional manner, has been shown to induce apoptosis in renal cell carcinoma44. miR-221 acts as a proto-oncogene in HCC by targeting a protein that modulates the mTOR pathway45. A recent study of the miRNA transcriptome in cancer cells subjected to long-term treatment with sirolimus demonstrated that resistance to sirolimus was mediated by upregulation of pro-oncogenic miRNAs and downregulation of tumor suppressor miRNAs46. This phenomenon was further confirmed when sensitivity to sirolimus was re-established with the introduction of inhibitors of these miRNAs.
The mTOR pathway in Cholangiocarcinoma
Cholangiocarcinoma is a rare type of primary liver cancer arising within the biliary tree. In the Western world, it arises most commonly in the context of primary sclerosing cholangitis. Cholangiocarcinoma tumors are heterogeneous in terms of their disease behavior and progression, and are categorized based on their anatomic location: intrahepatic, perihilar and extrahepatic, with perihilar tumors comprising 50% of cholangiocarcinomas. The approach to management currently varies based on anatomic location.
The evidence implicating the mTOR pathway in the development of cholangiocarcinoma is quite limited. A study of comparative genomic hybridization of DNA extracted from 32 cholangiocarcinoma tumors identified copy number gains in various genes along the mTOR pathway47. There were frequently gains in genes including mTOR, VEGFR, PDGF, and EGFR, the latter three of which are receptors that activate the mTOR pathway. An in vivo mouse model with liver-specific targeted disruption of PTEN and SMAD4 was shown to induce development of hyperplastic foci within the bile ducts exclusively48. Cholangiocarcinoma tumors progressed with stepwise histopathological changes, with increased levels of AKT and mTOR detected. Immunohistochemical evaluation of 101 intrahepatic cholangiocarcinomas revealed that overexpression of phospho-mTOR was significantly associated with well- to moderately-differentiated tumors, lack of metastases, and better survival49. In contrast, a tissue microarray study of 221 extrahepatic cholangiocarcinoma tumors, the subset of patients with tumors where the mTOR pathway was activated had increased tumor depth invasion and staging, leading to decreased survival50. Another similarly conducted study of 77 intrahepatic cholangiocarcinomas found that high expression of phospho-4E-BP1 (which would result in release of eIF4E and increased translation of oncogenes such as VEGF and c-myc) was independently predictive of a poor prognosis51. Thus, one can deduce that the mTOR pathway is involved either directly or indirectly in cholangiocarcinoma tumorigenesis, although its biological significance in initiating and promoting tumor progression remains ambiguous. Additionally, sirolimus inhibited growth of cholangiocarcinoma cell lines in a dose-dependent manner in vitro52. In a prospective pilot study with a single treatment arm with 9 patients having advanced cholangiocarcinoma, sirolimus induced temporary partial remission or stabilization of disease53. Therefore, the data on the mTOR pathway in cholangiocarcinoma, although valuable, is relatively sparse and merits further investigation.
mTOR pathway in Hepatoblastoma
Hepatoblastoma is the most common primary malignant liver tumor in childhood. Immunohistochemical staining of hepatoblastoma tumors demonstrated upregulation of the mTOR pathway, with phosphorylated Akt and mTOR being highly expressed54. Inhibition of PI3K upstream of mTOR affected hepatoblastoma cell growth in vitro through induction of apoptosis. Sirolimus has also been tested in vitro and in vivo in hepatoblastoma with a marked dose-dependent reduction in tumor growth55. These data suggest that the mTOR pathway could serve as a therapeutic target in hepatoblastoma, although this has not been established in clinical practice as yet.
Conclusion
The mTOR pathway governs a multitude of metabolic processes essential for cell proliferation and survival, and is upregulated in hepatobiliary malignancies. There is accumulating evidence of the efficacy of mTOR inhibition as a chemotherapeutic strategy for hepatocellular carcinoma. mTOR inhibition is a plausible strategy in cholangiocarcinoma and hepatoblastoma, given preliminary evidence of mTOR upregulation. Future chemotherapeutic strategies in hepatic malignancies will likely involve the combination of an mTOR inhibitor with agents targeting complementary pathways in order to extend recurrence-free survival.
Acknowledgements
The authors would like to thank Ms. Courtney Hoover for her able secretarial assistance.
This work was supported by Fonds de recherche du Québec – Santé and Canadian Association for Study of Liver Disease fellowship support to MB, NIH grants DK41876 to GJG and the Mayo Foundation.
REFERENCES
- 1.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nature Reviews Drug Discovery. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 3.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E–from translation to transformation. Oncogene. 2004;23(18):3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 4.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23(18):3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 5.Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell cycle (Georgetown, Tex.) 2011;10(14) doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawahara T, Asthana S, Kneteman NM. M-TOR Inhibitors: What Role in Liver Transplantation? J Hepatol. 2011;55(6):1441–1451. doi: 10.1016/j.jhep.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laplante M, Sabatini DM. mTOR signaling at a glance. Journal of cell science. 2009;122(20):3589. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell metabolism. 2008;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JE, Chen J. Regulation of Peroxisome Proliferator–Activated Receptor-γ Activity by Mammalian Target of Rapamycin and Amino Acids in Adipogenesis. Diabetes. 2004;53(11):2748–2756. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- 11.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102(23):8204. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nature Reviews Cancer. 2008;8(11):851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 13.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 14.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature reviews. Cancer. 2009;9(8):563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hepatocellular carcinoma: molecular pathways and new therapeutic targets. New York: Thieme-Stratton; 2005. p. c1981. [Google Scholar]
- 16.Gonzalez-Rodriguez A, Alba J, Zimmerman V, Kozma SC, Valverde AM. S6K1 deficiency protects against apoptosis in hepatocytes. Hepatology. 2009;50(1):216–229. doi: 10.1002/hep.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haga S, Ozaki M, Inoue H, Okamoto Y, Ogawa W, Takeda K, et al. The survival pathways phosphatidylinositol-3 kinase (PI3-K)/phosphoinositide-dependent protein kinase 1 (PDK1)/Akt modulate liver regeneration through hepatocyte size rather than proliferation. Hepatology. 2009;49(1):204–214. doi: 10.1002/hep.22583. [DOI] [PubMed] [Google Scholar]
- 18.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135(6):1972–1983. doi: 10.1053/j.gastro.2008.08.008. 83, e1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon S, Yecies JL, Zhang HH, Howell JJ, Nicholatos J, Harputlugil E, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5(217):ra24. doi: 10.1126/scisignal.2002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe S, Horie Y, Suzuki A. Hepatocyte-specific Pten-deficient mice as a novel model for nonalcoholic steatohepatitis and hepatocellular carcinoma. Hepatol Res. 2005;33(2):161–166. doi: 10.1016/j.hepres.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140(3):1071–1083. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez?López N, Varela?Rey M, Fernández?Ramos D, Woodhoo A, Vázquez?Chantada M, Embade N, et al. Activation of LKB1?Akt pathway independent of phosphoinositide 3?kinase plays a critical role in the proliferation of hepatocellular carcinoma from nonalcoholic steatohepatitis. Hepatology. 2010;52(5):1621–1631. doi: 10.1002/hep.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakanishi K, Sakamoto M, Yamasaki S, Todo S, Hirohashi S. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer. 2005;103(2):307–312. doi: 10.1002/cncr.20774. [DOI] [PubMed] [Google Scholar]
- 24.Guba M, Von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nature medicine. 2002;8(2):128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 25.Schumacher G, Oidtmann M, Rueggeberg A, Jacob D, Jonas S, Langrehr JM, et al. Sirolimus inhibits growth of human hepatoma cells alone or combined with tacrolimus, while tacrolimus promotes cell growth. World J Gastroenterol. 2005;11(10):1420–1425. doi: 10.3748/wjg.v11.i10.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buitrago?Molina LE, Pothiraju D, Lamlé J, Marhenke S, Kossatz U, Breuhahn K, et al. Rapamycin delays tumor development in murine livers by inhibiting proliferation of hepatocytes with DNA damage. Hepatology. 2009;50(2):500–509. doi: 10.1002/hep.23014. [DOI] [PubMed] [Google Scholar]
- 27.Zhu AX, Abrams TA, Miksad R, Blaszkowsky LS, Meyerhardt JA, Zheng H, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117(22):5094. doi: 10.1002/cncr.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley RK, Nimeiri HS, Vergo MT, Bergsland EK, Ko AH, Munster PN, Reinert A, Mulcahy MF, Benson AB, Venook AP. A phase I trial of the combination of temsirolimus (TEM) and sorafenib (SOR) in advanced hepatocellular carcinoma (HCC): 2010. J Clin Oncol 28:15s. 2010 (suppl; abstr TPS213) [Google Scholar]
- 29.Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Evers BM. PKI-587 and Sorafenib Targeting PI3K/AKT/mTOR and Ras/Raf/MAPK Pathways Synergistically Inhibit HCC Cell Proliferation. J Surg Res. 2012 Aug;176(2):542–548. doi: 10.1016/j.jss.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 30.Alain T, Morita M, Fonseca BD, Yanagiya A, Siddiqui N, Bhat M, et al. eIF4E/4E-BP Ratio Predicts the Efficacy of mTOR Targeted Therapies. Cancer Res. 2012;72(24):6468–6476. doi: 10.1158/0008-5472.CAN-12-2395. [DOI] [PubMed] [Google Scholar]
- 31.Masuda M, Shimomura M, Kobayashi K, Kojima S, Nakatsura T. Growth inhibition by NVP-BEZ235, a dual PI3K/mTOR inhibitor, in hepatocellular carcinoma cell lines. Oncology reports. 2011;26(5):1273–1279. doi: 10.3892/or.2011.1370. [DOI] [PubMed] [Google Scholar]
- 32.Thomas HE, Mercer CA, Carnevalli LS, Park J, Andersen JB, Conner EA, et al. mTOR inhibitors synergize on regression, reversal of gene expression, and autophagy in hepatocellular carcinoma. Science translational medicine. 2012;4(139):139ra84. doi: 10.1126/scitranslmed.3003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell metabolism. 2006;3(6):393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Soefje SA, Karnad A, Brenner AJ. Common toxicities of mammalian target of rapamycin inhibitors. Targeted oncology. 2011:1–5. doi: 10.1007/s11523-011-0174-9. [DOI] [PubMed] [Google Scholar]
- 35.Toso C, Mentha G, Majno P. Liver Transplantation for Hepatocellular Carcinoma: Five Steps to Prevent Recurrence. American Journal of Transplantation. 2011;11(10):2031–2035. doi: 10.1111/j.1600-6143.2011.03689.x. [DOI] [PubMed] [Google Scholar]
- 36.Liang W, Wang D, Ling X, Cao AA, Kong Y, Shang Y, et al. Sirolimus?based immunosuppression in liver transplantation for hepatocellular carcinoma: A Meta?analysis. Liver transplantation. 2012 Jan;8(1):62–69. doi: 10.1002/lt.22441. [DOI] [PubMed] [Google Scholar]
- 37.Gomez?Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transplantation. 2012;18(1):45–52. doi: 10.1002/lt.22434. [DOI] [PubMed] [Google Scholar]
- 38.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nature reviews. Cancer. 2012;12(3):159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 39.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver International. 2010;30(5):750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2012 Jul 7; doi: 10.1136/gutjnl-2011-301708. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 2012;5(4):544–552. doi: 10.1158/1940-6207.CAPR-11-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu Z, Zhang Y, Liao M, Chen Y, Zhao J, Pan Y. In vitro and in vivo antitumoral action of metformin on hepatocellular carcinoma. Hepatology research : the official journal of the Japan Society of Hepatology. 2012;42(9):922–933. doi: 10.1111/j.1872-034X.2012.01007.x. [DOI] [PubMed] [Google Scholar]
- 43.Xiong Y, Lu QJ, Zhao J, Wu GY. Metformin inhibits growth of hepatocellular carcinoma cells by inducing apoptosis via mitochondrion-mediated pathway. Asian Pacific journal of cancer prevention : APJCP. 2012;13(7):3275–3279. doi: 10.7314/apjcp.2012.13.7.3275. [DOI] [PubMed] [Google Scholar]
- 44.Cui L, Zhou H, Zhao H, Zhou Y, Xu R, Xu X, et al. MicroRNA-99a induces G1-phase cell cycle arrest and suppresses tumorigenicity in renal cell carcinoma. BMC cancer. 2012;12:546. doi: 10.1186/1471-2407-12-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, et al. miR-221 overexpression contributes to liver tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(1):264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Totary-Jain H, Sanoudou D, Ben-Dov IZ, Dautriche CN, Guarnieri P, Marx SO, et al. Reprogramming of the microRNA transcriptome mediates resistance to rapamycin. The Journal of biological chemistry. 2013 Jan; doi: 10.1074/jbc.M112.416446. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKay SC, Unger K, Pericleous S, Stamp G, Thomas G, Hutchins RR, et al. Array comparative genomic hybridization identifies novel potential therapeutic targets in cholangiocarcinoma. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2011;13(5):309–319. doi: 10.1111/j.1477-2574.2010.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X, Kobayashi S, Qiao W, Li C, Xiao C, Radaeva S, et al. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. The Journal of clinical investigation. 2006;116(7):1843–1852. doi: 10.1172/JCI27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee D, Do IG, Choi K, Sung CO, Jang KT, Choi D, et al. The expression of phospho-AKT1 and phospho-MTOR is associated with a favorable prognosis independent of PTEN expression in intrahepatic cholangiocarcinomas. Mod Pathol. 2012 Jan;25(1):131–139. doi: 10.1038/modpathol.2011.133. [DOI] [PubMed] [Google Scholar]
- 50.Chung JY, Hong SM, Choi BY, Cho H, Yu E, Hewitt SM. The expression of phospho-AKT, phospho-mTOR, and PTEN in extrahepatic cholangiocarcinoma. Clin Cancer Res. 2009;15(2):660–667. doi: 10.1158/1078-0432.CCR-08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Zheng T, Wu Q, Wang J, Wu C. Immunohistochemical analysis of the mTOR pathway in intrahepatic cholangiocarcinoma. Neoplasma. 2012;59(2):137. doi: 10.4149/neo_2012_018. [DOI] [PubMed] [Google Scholar]
- 52.Okada T, Sawada T, Kubota K. Rapamycin inhibits growth of cholangiocarcinoma cells. Hepato-gastroenterology. 2009;56(89):6–10. [PubMed] [Google Scholar]
- 53.Rizell M, Andersson M, Cahlin C, Hafstrom L, Olausson M, Lindner P. Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. International journal of clinical oncology / Japan Society of Clinical Oncology. 2008;13(1):66–70. doi: 10.1007/s10147-007-0733-3. [DOI] [PubMed] [Google Scholar]
- 54.Hartmann W, Kuchler J, Koch A, Friedrichs N, Waha A, Endl E, et al. Activation of phosphatidylinositol-3'-kinase/AKT signaling is essential in hepatoblastoma survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(14):4538–4545. doi: 10.1158/1078-0432.CCR-08-2878. [DOI] [PubMed] [Google Scholar]
- 55.Wagner F, Henningsen B, Lederer C, Eichenmuller M, Godeke J, Muller-Hocker J, et al. Rapamycin blocks hepatoblastoma growth in vitro and in vivo implicating new treatment options in high-risk patients. Eur J Cancer. 2012;48(15):2442–2450. doi: 10.1016/j.ejca.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 56.Patsenker E, Schneider V, Ledermann M, Saegesser H, Dorn C, Hellerbrand C, et al. Potent antifibrotic activity of mTOR inhibitors sirolimus and everolimus but not of cyclosporine A and tacrolimus in experimental liver fibrosis. J Hepatol. 2011;55(2):388–398. doi: 10.1016/j.jhep.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 57.Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology. 2012;55(2):594–608. doi: 10.1002/hep.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reyes-Gordillo K, Shah R, Popratiloff A, Fu S, Hindle A, Brody F, et al. Thymosin-beta4 (Tbeta4) blunts PDGF-dependent phosphorylation and binding of AKT to actin in hepatic stellate cells. Am J Pathol. 2011;178(5):2100–2108. doi: 10.1016/j.ajpath.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osawa Y, Kanamori H, Seki E, Hoshi M, Ohtaki H, Yasuda Y, et al. L-tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J Biol Chem. 2011;286(40):34800–34808. doi: 10.1074/jbc.M111.235473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu K, Ding J, Chen C, Sun W, Ning BF, Wen W, et al. Hepatic transforming growth factor beta gives rise to tumor-initiating cells and promotes liver cancer development. Hepatology. 2012;56(6):2255–2267. doi: 10.1002/hep.26007. [DOI] [PubMed] [Google Scholar]
- 61.Decaens T, Luciani A, Itti E, Hulin A, Roudot-Thoraval F, Laurent A, et al. Phase II study of sirolimus in treatment-naive patients with advanced hepatocellular carcinoma. Dig Liver Dis. 2012;44(7):610–616. doi: 10.1016/j.dld.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, et al. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107(1):264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shao H, Gao C, Tang H, Zhang H, Roberts LR, Hylander BL, et al. Dual targeting of mTORC1/C2 complexes enhances histone deacetylase inhibitor-mediated anti-tumor efficacy in primary HCC cancer in vitro and in vivo. J Hepatol. 2012;56(1):176–183. doi: 10.1016/j.jhep.2011.07.013. [DOI] [PubMed] [Google Scholar]