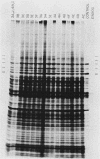
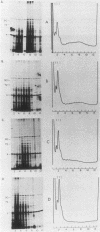
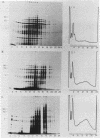
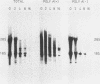
Abstract
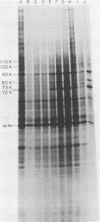
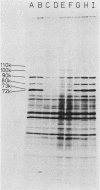
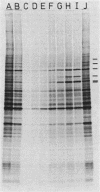
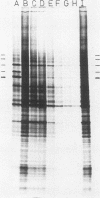
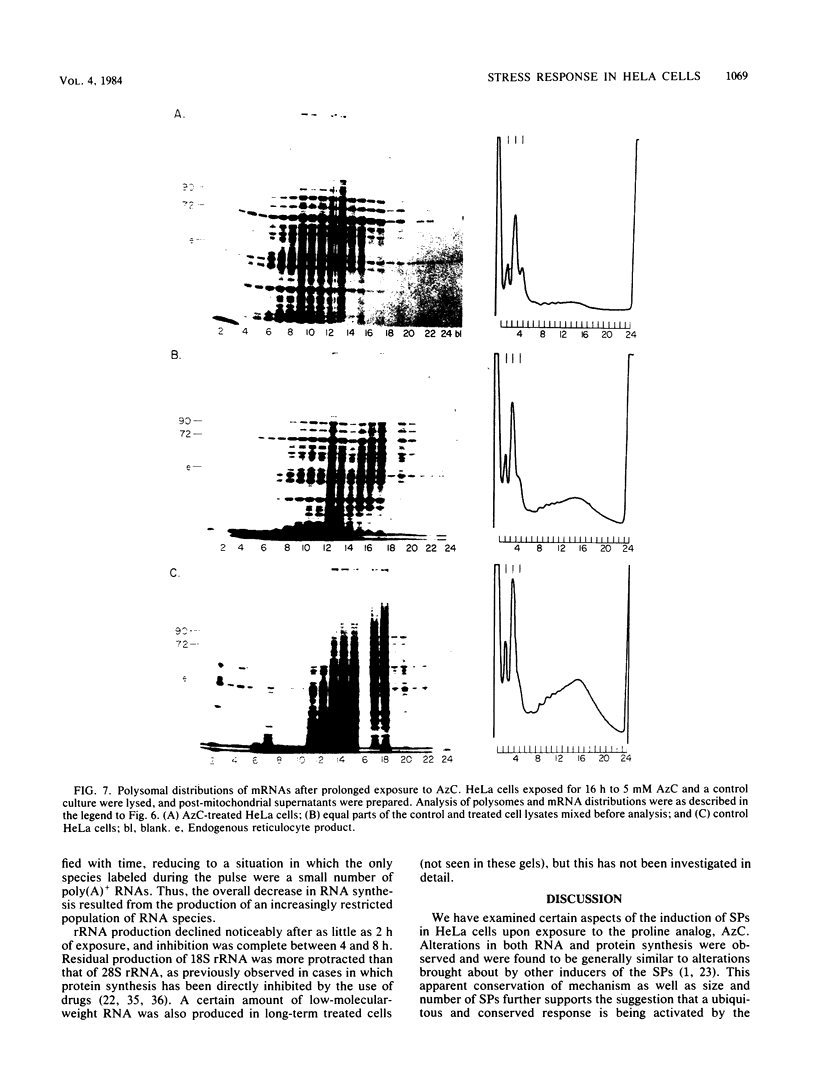
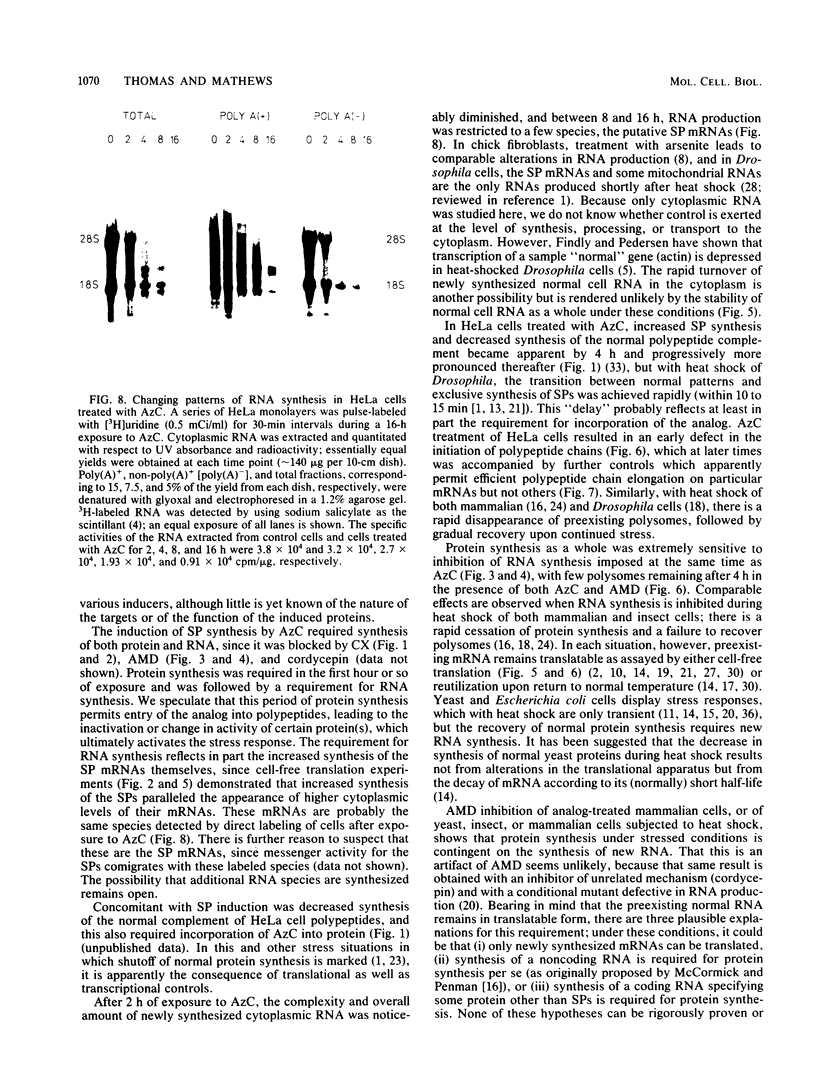
Amino acid analogs, like other effectors of the stress response, induce in mammalian cells the same gene products that are induced upon heat shock; incorporation of the analog into protein is required for induction. We show here that induction by analogs involves controls operating at the levels of both transcription and translation. The electrophoretic patterns of newly made mRNAs simplify with time such that the putative stress protein mRNAs are the only species transported from the nucleus. Concomitantly, the patterns of protein synthesis simplify such that the stress proteins become nearly exclusive polypeptide products. Although the normal mRNAs are either not used or used with greatly reduced efficiency, they are not degraded and retain translatability when transferred to cell-free systems. Soon after the stress response has been induced, there follows a defect in the initiation of polypeptide chains, as evidenced by examination of polysome profiles. Upon prolonged exposure, polysomes are recovered, and although they give rise to stress proteins almost exclusively, the normal mRNAs are still present in these structures. Thus, in addition to the initiation defect, a lesion in elongation may also be involved. The extreme sensitivity of protein synthesis to the inhibition of RNA synthesis, together with the parallel simplifications in the patterns of newly made mRNAs and polypeptides, may imply that only newly made mRNAs are efficiently translated in analog-treated cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Findly R. C., Pederson T. Regulated transcription of the genes for actin and heat-shock proteins in cultured Drosophila cells. J Cell Biol. 1981 Feb;88(2):323–328. doi: 10.1083/jcb.88.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Johnston D., Oppermann H., Jackson J., Levinson W. Induction of four proteins in chick embryo cells by sodium arsenite. J Biol Chem. 1980 Jul 25;255(14):6975–6980. [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Krüger C., Benecke B. J. In vitro translation of Drosophila heat-shock and non--heat-shock mRNAs in heterologous and homologous cell-free systems. Cell. 1981 Feb;23(2):595–603. doi: 10.1016/0092-8674(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Lemaux P. G., Herendeen S. L., Bloch P. L., Neidhardt F. C. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell. 1978 Mar;13(3):427–434. doi: 10.1016/0092-8674(78)90317-3. [DOI] [PubMed] [Google Scholar]
- Levis R., Penman S. The metabolism of poly (A)+ and poly(A)-hnRNA in cultured Drosophila cells studied with a rapid uridine pulse-chase. Cell. 1977 May;11(1):105–113. doi: 10.1016/0092-8674(77)90321-x. [DOI] [PubMed] [Google Scholar]
- Lewis M., Helmsing P. J., Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- McCormick W., Penman S. Regulation of protein synthesis in HeLa cells: translation at elevated temperatures. J Mol Biol. 1969 Jan;39(2):315–333. doi: 10.1016/0022-2836(69)90320-9. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Meselson M. Translation in vitro of Drosophila heat-shock messages. J Mol Biol. 1977 Nov 25;117(1):279–283. doi: 10.1016/0022-2836(77)90035-3. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Xuong N. H., Geiduschek E. P. A response of protein synthesis to temperature shift in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5222–5225. doi: 10.1073/pnas.76.10.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirault M. E., Goldschmidt-Clermont M., Moran L., Arrigo A. P., Tissières A. The effect of heat shock on gene expression in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):819–827. doi: 10.1101/sqb.1978.042.01.082. [DOI] [PubMed] [Google Scholar]
- Pederson T., Kumar A. Relationship between protein synthesis and ribosome assembly in HeLa cells. J Mol Biol. 1971 Nov 14;61(3):655–668. doi: 10.1016/0022-2836(71)90070-2. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Perry R. P. Characterization of the messenger RNA released from L cell polyribosomes as a result of temperature shock. J Mol Biol. 1972 Feb 14;63(3):577–590. doi: 10.1016/0022-2836(72)90449-4. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Fostel J. M., Pardue M. L. A new type of virus from cultured Drosophila cells: characterization and use in studies of the heat-shock response. Cell. 1980 Dec;22(3):929–941. doi: 10.1016/0092-8674(80)90570-x. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermeijer P. J., Lubsen N. H. Heat-shock peptides in Drosophila hydei and their synthesis in vitro. Eur J Biochem. 1978 Aug 1;88(2):331–339. doi: 10.1111/j.1432-1033.1978.tb12454.x. [DOI] [PubMed] [Google Scholar]
- Spradling A., Pardue M. L., Penman S. Messenger RNA in heat-shocked Drosophila cells. J Mol Biol. 1977 Feb 5;109(4):559–587. doi: 10.1016/s0022-2836(77)80091-0. [DOI] [PubMed] [Google Scholar]
- Stanners C. P. The effect of cycloheximide on polyribosomes from hamster cells. Biochem Biophys Res Commun. 1966 Sep 8;24(5):758–764. doi: 10.1016/0006-291x(66)90390-1. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Welch W. J., Mathews M. B., Feramisco J. R. Molecular and cellular effects of heat-shock and related treatments of mammalian tissue-culture cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):985–996. doi: 10.1101/sqb.1982.046.01.092. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Girard M., Latham H., Darnell J. E. Ribosome formation in HeLa cells in the absence of protein synthesis. J Mol Biol. 1966 Aug;19(2):373–382. doi: 10.1016/s0022-2836(66)80011-6. [DOI] [PubMed] [Google Scholar]
- Willems M., Penman M., Penman S. The regulation of RNA synthesis and processing in the nucleolus during inhibition of protein synthesis. J Cell Biol. 1969 Apr;41(1):177–187. doi: 10.1083/jcb.41.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]