Abstract
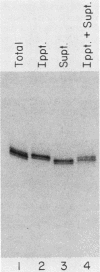
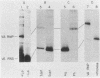
The La antigen is a cellular protein which interacts with many RNA species that are products of RNA polymerase III, including the adenovirus virus-associated (VA) RNAs. We demonstrate that the efficiency of antigen binding in vitro is determined by the number of U residues at the RNA 3' terminus. Forms of VA RNAI with more than two terminal U residues are fully bound, forms with two U residues are partially bound, and forms with fewer than two U residues are not bound at all. The antigen can be covalently linked to VA RNA by UV irradiation, and the site of cross-linking is shown to contain the 3' terminus of the RNA. We conclude that the antigen recognizes the U-rich 3' tail of VA RNA, and presumably that of other polymerase III products, and that it binds at or close to this site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celma M. L., Pan J., Weissman S. M. Studies of low molecular weight RNA from cells infected with adenovirus 2. I. The sequences at the 3' end of VA-RNA I. J Biol Chem. 1977 Dec 25;252(24):9032–9042. [PubMed] [Google Scholar]
- Celma M. L., Pan J., Weissman S. M. Studies of low molecular weight RNA from cells infected with adenovirus 2. II. Heterogeneity at the 5' end of VA-RNA I. J Biol Chem. 1977 Dec 25;252(24):9043–9046. [PubMed] [Google Scholar]
- Chambers J. C., Kurilla M. G., Keene J. D. Association between the 7 S RNA and the lupus La protein varies among cell types. J Biol Chem. 1983 Oct 10;258(19):11438–11441. [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. Binding of adenovirus VA RNA to mRNA: a possible role in splicing? Nature. 1980 Jun 19;285(5766):575–577. doi: 10.1038/285575a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Pettersson U. The low molecular weight of RNAs of adenovirus 2-infected cells. J Mol Biol. 1978 Feb 25;119(2):293–328. doi: 10.1016/0022-2836(78)90439-4. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Deng J. S., Stenberg R. M., Tan E. M. Characterization of a phosphoprotein associated with the SS-B/La nuclear antigen in adenovirus-infected and uninfected KB cells. Mol Cell Biol. 1983 Jul;3(7):1235–1245. doi: 10.1128/mcb.3.7.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin M. Current perspectives on serological reactions in SLE patients. Clin Exp Immunol. 1981 Apr;44(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Rosa M. D., Gottlieb E., Lerner M. R., Steitz J. A. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981 Sep;1(9):785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. The organization, structure, and in vitro transcription of Alu family RNA polymerase III transcription units in the human alpha-like globin gene cluster: precipitation of in vitro transcripts by lupus anti-La antibodies. J Mol Appl Genet. 1982;1(4):343–360. [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Vennström B., Pettersson U., Philipson L. Initiation of transcription in nuclei isolated from adenovirus infected cells. Nucleic Acids Res. 1978 Jan;5(1):205–219. doi: 10.1093/nar/5.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Segall J., Harris B., Ng S. Y., Roeder R. G. Faithful transcription of eukaryotic genes by RNA polymerase III in systems reconstituted with purified DNA templates. J Biol Chem. 1979 Jul 10;254(13):6163–6173. [PubMed] [Google Scholar]
- van Eekelen C., Buijtels H., Linné T., Ohlsson R., Philipson L., van Venrooij W. Detection of a cellular polypeptide associated with adenovirus-coded VA RNA using in vitro labeling of proteins cross-linked to RNA. Nucleic Acids Res. 1982 May 25;10(10):3039–3052. doi: 10.1093/nar/10.10.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]