Abstract
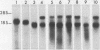
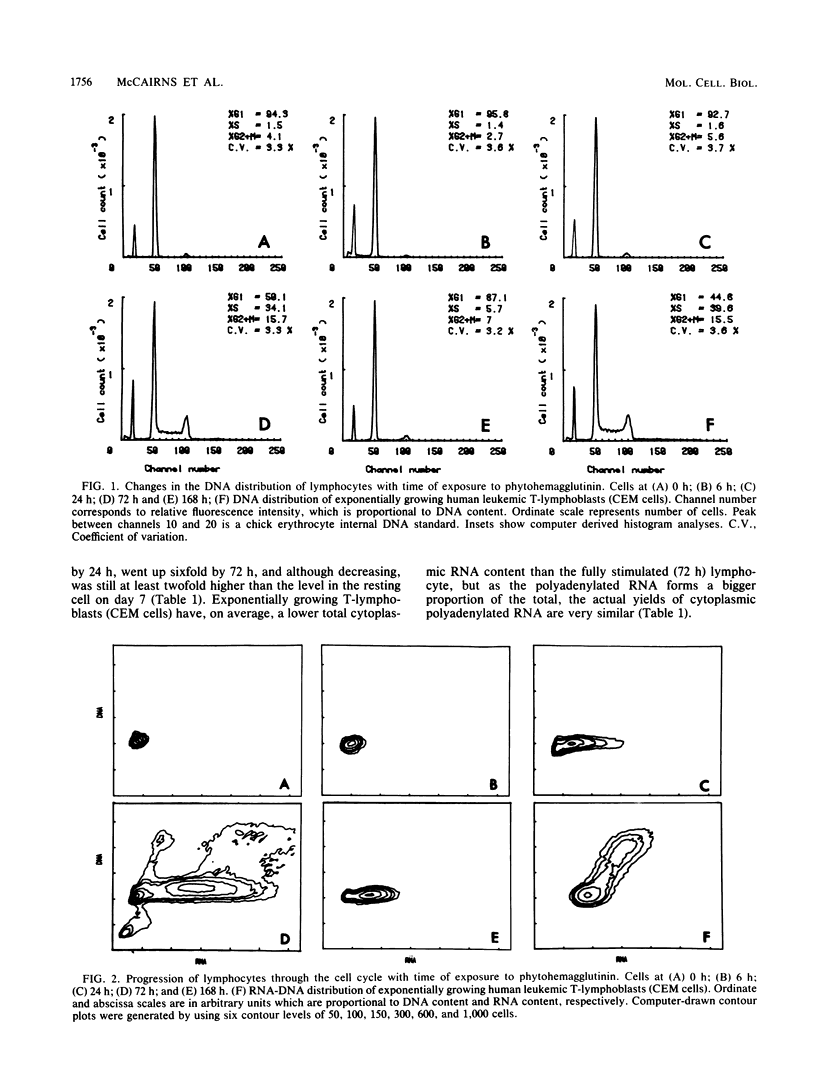
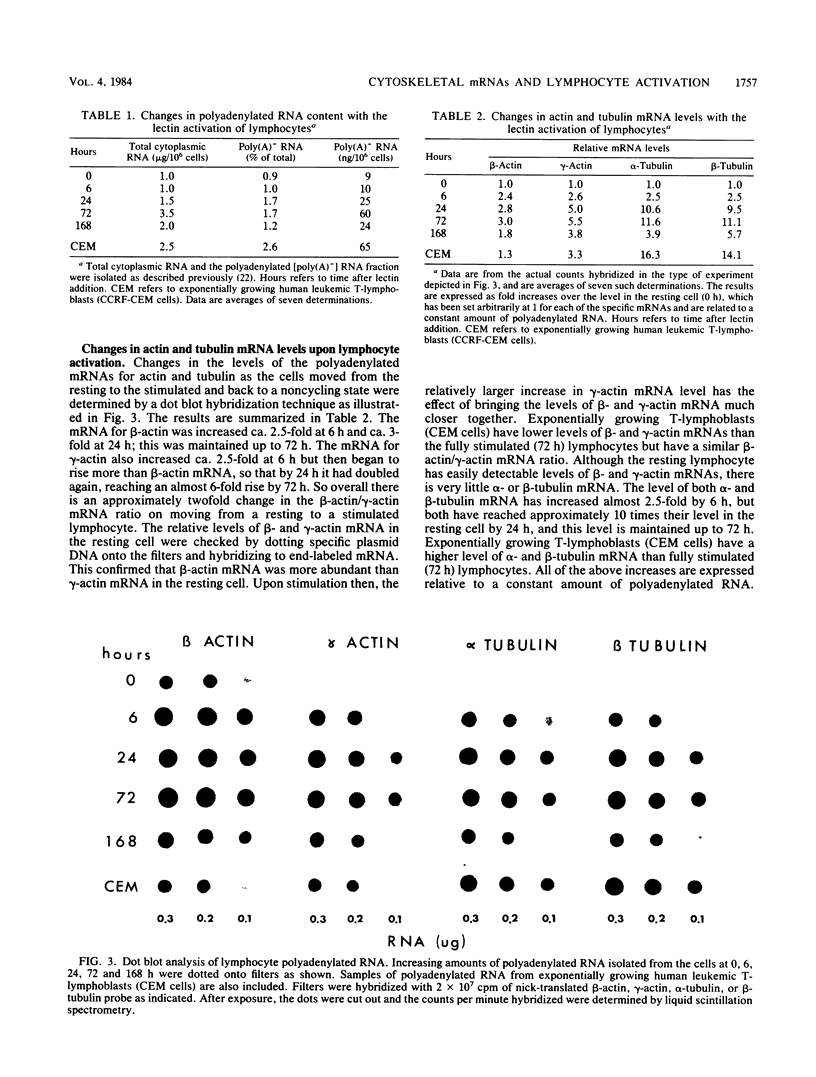
The expression of beta-actin, gamma-actin, alpha-tubulin, and beta-tubulin mRNA during the lectin activation of human peripheral blood lymphocytes was examined with specific cDNA clones. The resting lymphocyte has a low level of both alpha- and beta-tubulin mRNAs, and these increase 10-fold after 72 h of lectin stimulation in which maximum cell transformation is achieved. Although there is a slight increase in tubulin mRNA during the first 6 h, most of the increase occurs between 6 and 24 h as the cells start to increase their RNA content and progress from G0 into G1. Both beta- and gamma-actin mRNAs are more abundant than the tubulin mRNAs in resting cells, with beta-actin mRNA being the major species. Upon activation, beta-actin mRNA increases threefold, whereas gamma-actin mRNA increases almost sixfold. Both beta- and gamma-actin mRNA are elevated 2.5-fold as early as 6 h, the gamma-actin mRNA level then increasing more than beta-actin between 6 and 24 h, resulting in the reduced beta-actin/gamma-actin mRNA ratio. The lectin-stimulated lymphocyte has a similar beta-actin/gamma-actin mRNA ratio as that of the human leukemic T-lymphoblast cell line CCRF-CEM. These increases are over and above the general increase in polyadenylated RNA content upon lectin activation. On returning to a noncycling state, the levels of these cytoskeletal mRNAs decrease. There were two beta-tubulin mRNAs present in lymphocyte cytoplasm, one of 1.8 kilobases and one of 2.8 kilobases in length. The nongrowing lymphocytes had relatively lower levels of the larger sized mRNA. Upon stimulation, the relative level of the larger mRNA was increased, and at 72 h the cells had approximately equal levels of both mRNAs as did the leukemic lymphoblasts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R., Ben-Ze'av A., Benecke B. J., Penman S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell. 1978 Oct;15(2):627–637. doi: 10.1016/0092-8674(78)90031-4. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurrell J. G., Black P. H., Haber E., Zurawski V. R., Jr A rapid and efficient procedure for the removal of nonviable cells from suspension culture of lymphoid cell lines. Aust J Exp Biol Med Sci. 1980 Aug;58(4):373–376. doi: 10.1038/icb.1980.36. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kecskemethy N., Schäfer K. P. Lectin-induced changes among polyadenylated and non-polyadenylated mRNA in lymphocytes. mRNAs for actin, tubulin and calmodulin respond differently. Eur J Biochem. 1982 Sep 1;126(3):573–582. doi: 10.1111/j.1432-1033.1982.tb06819.x. [DOI] [PubMed] [Google Scholar]
- Kefford R. F., Fox R. M., McCairns E., Fahey D., Muscat G. E., Rowe P. B. Terminal incorporation of 2'-deoxyadenosine into polyadenylate segments of polyadenylated RNA in G1-phase-arrested human T-lymphoblasts. Cancer Res. 1983 May;43(5):2252–2257. [PubMed] [Google Scholar]
- Leavitt J., Leavitt A., Attallah A. M. Dissimilar modes of expression of beta- and gamma-actin in normal and leukemic human T lymphocytes. J Biol Chem. 1980 Jun 10;255(11):4984–4987. [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Ling N. R., Holt P. J. The activation and reactivation of peripheral lymphocytes in culture. J Cell Sci. 1967 Mar;2(1):57–70. doi: 10.1242/jcs.2.1.57. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairns E., Fahey D., Muscat G. E., Rowe P. B. The effects of lectin transformation on cytoplasmic polyadenylated RNA from human lymphocytes. Mol Cell Biochem. 1983;56(2):165–175. doi: 10.1007/BF00227217. [DOI] [PubMed] [Google Scholar]
- McCairns E., Fahey D., Sauer D., Rowe P. B. De novo purine synthesis in human lymphocytes. Partial co-purification of the enzymes and some properties of the pathway. J Biol Chem. 1983 Feb 10;258(3):1851–1856. [PubMed] [Google Scholar]
- Murphy D. B., Wallis K. T. Brain and erythrocyte microtubules from chicken contain different beta-tubulin polypeptides. J Biol Chem. 1983 Jun 25;258(12):7870–7875. [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle V. G., Dubrow R., Pardee A. B. Changes in the synthesis of actin and other cell proteins after stimulation of serum-arrested cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1298–1302. doi: 10.1073/pnas.76.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle V. G., Pardee A. B. Quiescent cells but not cycling cells exhibit enhanced actin synthesis before they synthesize DNA. J Cell Physiol. 1980 Apr;103(1):11–15. doi: 10.1002/jcp.1041030103. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Stark R., Liebes L. F., Nevrla D., Conklyn M., Silber R. Decreased actin content of lymphocytes from patients with chronic lymphocytic leukemia. Blood. 1982 Mar;59(3):536–541. [PubMed] [Google Scholar]
- Taylor I. W. A rapid single step staining technique for DNA analysis by flow microfluorimetry. J Histochem Cytochem. 1980 Sep;28(9):1021–1024. doi: 10.1177/28.9.6157714. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Sharpless T., Melamed M. R. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977 Jan;25(1):46–56. doi: 10.1177/25.1.64567. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]