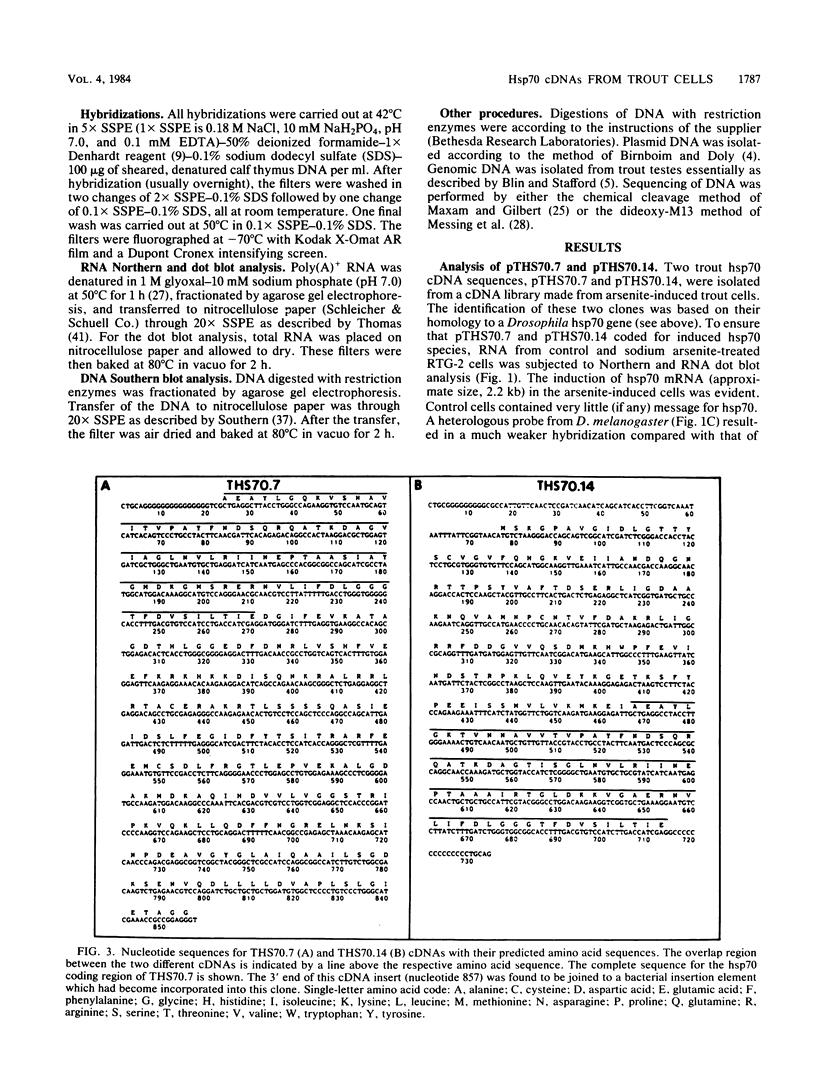
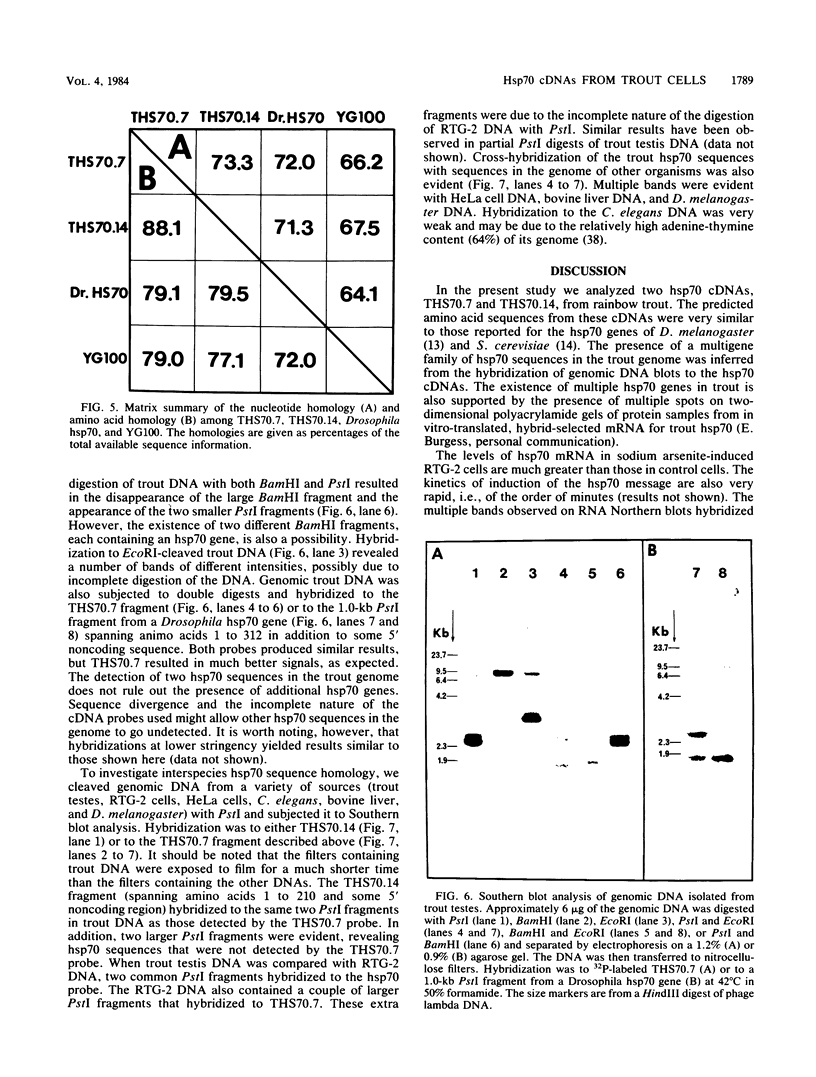
Abstract
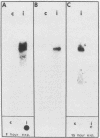
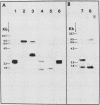
RTG-2 cells, a line of fibroblasts from rainbow trout (Salmo gairdnerii), are induced to synthesize a distinct set of heat-shock polypeptides after exposure to elevated temperature or to low concentrations of sodium arsenite. We isolated and characterized two cDNA sequences, THS70.7 and THS70.14, encoding partial information for two distinct species of 70-kilodalton heat shock polypeptide (hsp70) from these cells. These sequences are identical at 73.3% of the nucleotide positions in their regions of overlap, and their degree of sequence conservation at the polypeptide level is 88.1%. The two derived trout hsp70 polypeptide sequences show extensive homology with derived amino acid sequences for hsp70 polypeptides from Drosophila melanogaster and Saccharomyces cerevisiae. Northern blot analysis of RNA from arsenite-induced RTG-2 cells, with the trout hsp70 cDNAs as probes, revealed the presence of three hsp70 mRNA species. Southern blot analysis of trout testis DNA cleaved with various restriction endonucleases revealed a small number of bands hybridizing to the hsp70 cDNAs, suggesting the existence of a small family of hsp70 genes in this species. Finally, trout hsp70 cDNA sequences cross-hybridized with restriction fragments in genomic DNA from HeLa cells, bovine liver, Caenorhabditis elegans, and D. melanogaster.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Fakan S., Tissières A. Localization of the heat shock-induced proteins in Drosophila melanogaster tissue culture cells. Dev Biol. 1980 Jul;78(1):86–103. doi: 10.1016/0012-1606(80)90320-6. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Deng G., Wu R. An improved procedure for utilizing terminal transferase to add homopolymers to the 3' termini of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4173–4188. doi: 10.1093/nar/9.16.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Heikkila J. J., Schultz G. A., Iatrou K., Gedamu L. Expression of a set of fish genes following heat or metal ion exposure. J Biol Chem. 1982 Oct 25;257(20):12000–12005. [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Drosophila gene related to the major heat shock-induced gene is transcribed at normal temperatures and not induced by heat shock. Proc Natl Acad Sci U S A. 1982 Jan;79(2):525–529. doi: 10.1073/pnas.79.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Johnston D., Oppermann H., Jackson J., Levinson W. Induction of four proteins in chick embryo cells by sodium arsenite. J Biol Chem. 1980 Jul 25;255(14):6975–6980. [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koninkx J. F. Protein synthesis in salivary glands of Drosophila hydei after experimental gene induction. Biochem J. 1976 Sep 15;158(3):623–628. doi: 10.1042/bj1580623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary R. K., Candido E. P. Induction of a novel set of polypeptides by heat shock or sodium arsenite in cultured cells of rainbow trout, Salmo gairdnerii. Can J Biochem. 1982 Mar;60(3):347–355. doi: 10.1139/o82-041. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders H. J., Berendes H. D. The effect of changes in the respiratory metabolism upon genome activity in Drosophila. I. The induction of gene activity. Chromosoma. 1972;37(4):433–444. doi: 10.1007/BF00284892. [DOI] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. Heat-shock proteins of Drosophila are associated with nuclease-resistant, high-salt-resistant nuclear structures. J Cell Biol. 1981 Sep;90(3):793–796. doi: 10.1083/jcb.90.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Oppermann H., Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606(1):170–180. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. G., Fulford W. D., Moran L. A. Mouse and Drosophila genes encoding the major heat shock protein (hsp70) are highly conserved. Mol Cell Biol. 1983 Aug;3(8):1540–1543. doi: 10.1128/mcb.3.8.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran L. A., Chauvin M., Kennedy M. E., Korri M., Lowe D. G., Nicholson R. C., Perry M. D. The major heat-shock protein (hsp70) gene family: related sequences in mouse, Drosophila, and yeast. Can J Biochem Cell Biol. 1983 Jun;61(6):488–499. doi: 10.1139/o83-065. [DOI] [PubMed] [Google Scholar]
- Moran L., Mirault M. E., Tissières A., Lis J., Schedl P., Artavanis-Tsakonas S., Gehring W. J. Physical map of two D. melanogaster DNA segments containing sequences coding for the 70,000 dalton heat shock protein. Cell. 1979 May;17(1):1–8. doi: 10.1016/0092-8674(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., Mitchell H. K. Recovery of protein synthesis after heat shock: prior heat treatment affects the ability of cells to translate mRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1708–1711. doi: 10.1073/pnas.78.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Russnak R. H., Jones D., Candido E. P. Cloning and analysis of cDNA sequences coding for two 16 kilodalton heat shock proteins (hsps) in Caenorhabditis elegans: homology with the small hsps of Drosophila. Nucleic Acids Res. 1983 May 25;11(10):3187–3205. doi: 10.1093/nar/11.10.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snutch T. P., Baillie D. L. Alterations in the pattern of gene expression following heat shock in the nematode Caenorhabditis elegans. Can J Biochem Cell Biol. 1983 Jun;61(6):480–487. doi: 10.1139/o83-064. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Brenner S. The DNA of Caenorhabditis elegans. Genetics. 1974 May;77(1):95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay R. M. Genetic regulation during heat shock and function of heat-shock proteins: a review. Can J Biochem Cell Biol. 1983 Jun;61(6):387–394. doi: 10.1139/o83-053. [DOI] [PubMed] [Google Scholar]
- Tanguay R. M., Vincent M. Intracellular translocation of cellular and heat shock induced proteins upon heat shock in Drosophila Kc cells. Can J Biochem. 1982 Mar;60(3):306–315. doi: 10.1139/o82-037. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török I., Karch F. Nucleotide sequences of heat shock activated genes in Drosophila melanogaster. I. Sequences in the regions of the 5' and 3' ends of the hsp 70 gene in the hybrid plasmid 56H8. Nucleic Acids Res. 1980 Jul 25;8(14):3105–3123. doi: 10.1093/nar/8.14.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez J. M., DiDomenico B. J., Lindquist S. Intracellular localization of heat shock proteins in Drosophila. Cell. 1980 Jul;20(3):679–689. doi: 10.1016/0092-8674(80)90314-1. [DOI] [PubMed] [Google Scholar]
- Vincent M., Tanguay R. M. Heat-shock induced proteins present in the cell nucleus of Chironomus tentans salivary gland. Nature. 1979 Oct 11;281(5731):501–503. doi: 10.1038/281501a0. [DOI] [PubMed] [Google Scholar]
- WOLF K., QUIMBY M. C. Established eurythermic line of fish cells in vitro. Science. 1962 Mar 23;135(3508):1065–1066. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]