Abstract
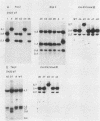
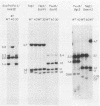
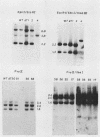
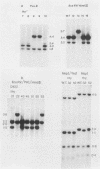
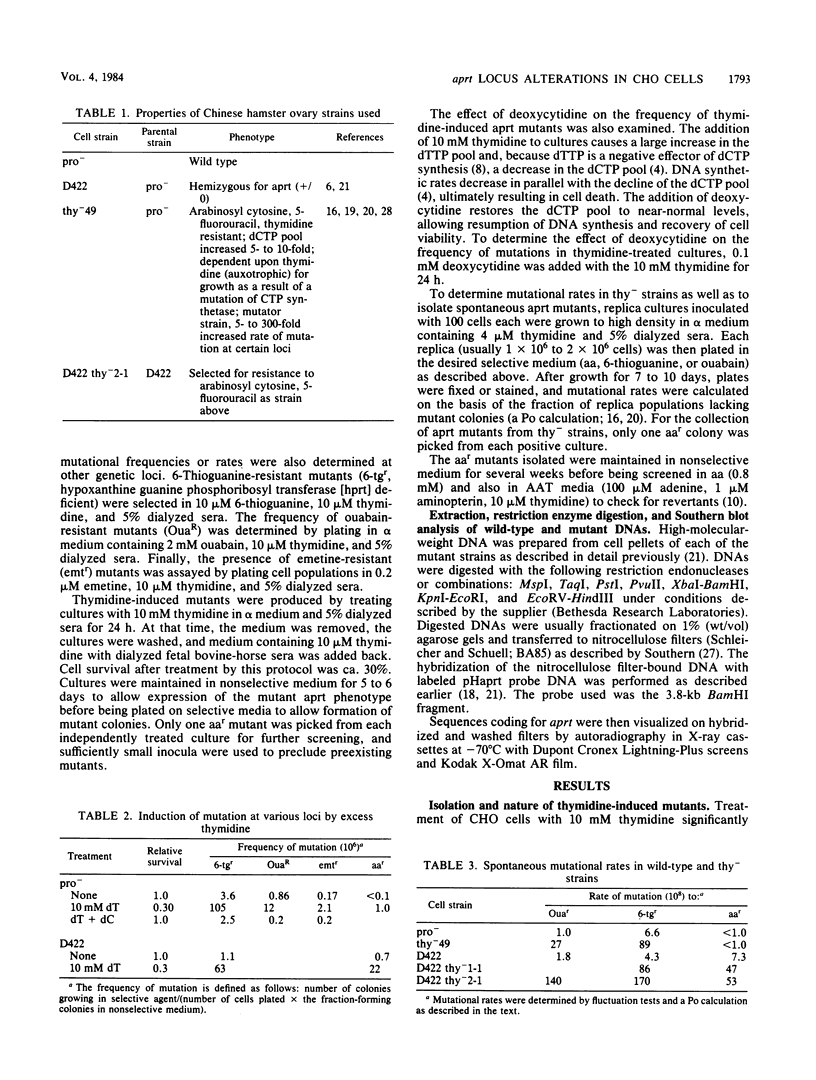
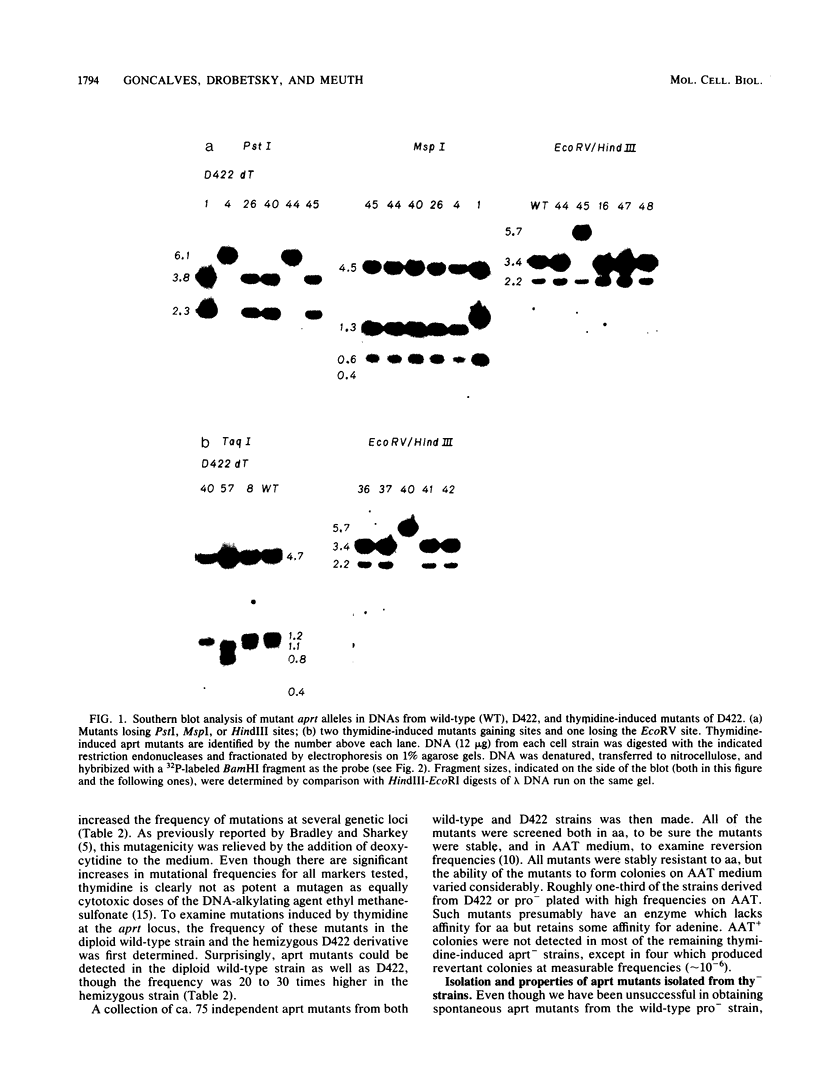
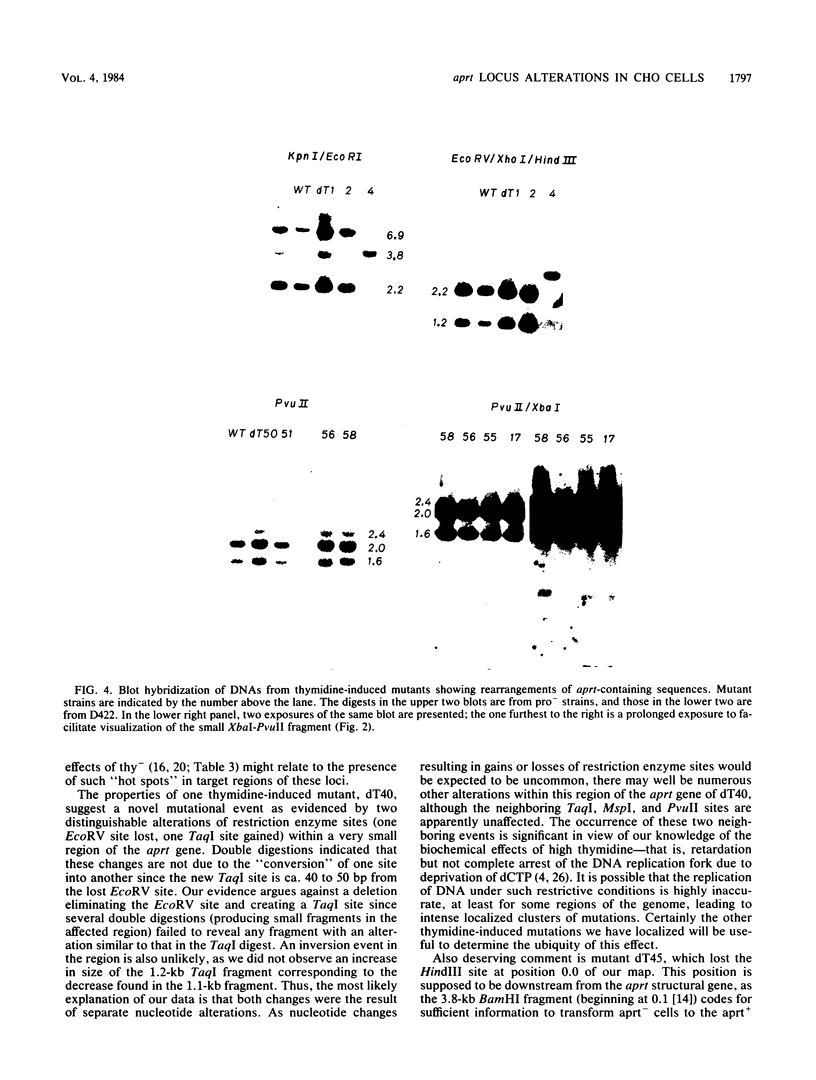
Mutants induced at the adenine phosphoribosyl transferase (aprt) locus by dTTP or dCTP pool imbalances were examined for alterations in genomic DNA sequences. No observable changes were detected by Southern blot analysis of most mutant DNAs, suggesting induction of base pair alterations or other events below our level of detection (approximately 30 base pairs). However, in a few strains (11 from a total collection of 125 mutant cell strains), we were able to localize these events to restriction endonuclease recognition sequences when the mutations resulted in the loss or gain of a particular site. The distribution of lost or gained sites in aprt-deficient mutants induced by the two types of pool imbalances clearly varied, with those occurring in a mutator strain with increased dCTP clustering at one end of the aprt gene. Mutants induced by dTTP also revealed novel events: multiple restriction site modifications in a small region of the aprt gene in one mutant and a small (approximately 50 base pairs) insertion or duplication of DNA sequences. As in previous studies, very few deletion or insertion mutants were detected at the aprt locus. The significance of these findings in terms of the known biochemical and genetic consequences of these pool imbalances is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D., Richardson C. R., Davies P. J. The genotoxic potential of bases and nucleosides. Mutat Res. 1981 May;91(3):265–272. doi: 10.1016/0165-7992(81)90044-0. [DOI] [PubMed] [Google Scholar]
- Bernstein C., Bernstein H., Mufti S., Strom B. Stimulation of mutation in phage T 4 by lesions in gene 32 and by thymidine imbalance. Mutat Res. 1972 Oct;16(2):113–119. doi: 10.1016/0027-5107(72)90171-6. [DOI] [PubMed] [Google Scholar]
- Bjursell G., Reichard P. Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J Biol Chem. 1973 Jun 10;248(11):3904–3909. [PubMed] [Google Scholar]
- Bradley M. O., Sharkey N. A. Mutagenicity of thymidine to cultured Chinese hamster cells. Nature. 1978 Aug 10;274(5671):607–608. doi: 10.1038/274607a0. [DOI] [PubMed] [Google Scholar]
- Bradley W. E., Letovanec D. High-frequency nonrandom mutational event at the adenine phosphoribosyltransferase (aprt) locus of sib-selected CHO variants heterozygous for aprt. Somatic Cell Genet. 1982 Jan;8(1):51–66. doi: 10.1007/BF01538650. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Law J., Munson R. J. Mutagenesis in Escherichia coli. II. Evidence for a common pathway for mutagenesis by ultraviolet light, ionizing radiation and thymine deprivation. Mol Gen Genet. 1968;103(3):266–273. doi: 10.1007/BF00273698. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Thelander L., Akerman M. Allosteric regulation of calf thymus ribonucleoside diphosphate reductase. Biochemistry. 1979 Jul 10;18(14):2948–2952. doi: 10.1021/bi00581a005. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Fidelity of replication of phage phi X174 DNA by DNA polymerase III holoenzyme: spontaneous mutation by misincorporation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4946–4950. doi: 10.1073/pnas.76.10.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. E., Sargent P. A. Mutants of cultured chinese hamster cells deficient in adenine phosphoribosyl transferase. Cell. 1974 May;2(1):43–54. doi: 10.1016/0092-8674(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. Effect of divalent metal ion activators and deoxyrionucleoside triphosphate pools on in vitro mutagenesis. J Biol Chem. 1979 Jul 10;254(13):5718–5725. [PubMed] [Google Scholar]
- Kunz B. A., Barclay B. J., Game J. C., Little J. G., Haynes R. H. Induction of mitotic recombination in yeast by starvation for thymine nucleotides. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6057–6061. doi: 10.1073/pnas.77.10.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A. Genetic effects of deoxyribonucleotide pool imbalances. Environ Mutagen. 1982;4(6):695–725. doi: 10.1002/em.2860040609. [DOI] [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- Meuth M., Arrand J. E. Alterations of gene structure in ethyl methane sulfonate-induced mutants of mammalian cells. Mol Cell Biol. 1982 Nov;2(11):1459–1462. doi: 10.1128/mcb.2.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth M., Gonçalves O., Thom P. A selection system specific for the Thy mutator phenotype. Somatic Cell Genet. 1982 Jul;8(4):423–432. doi: 10.1007/BF01538705. [DOI] [PubMed] [Google Scholar]
- Meuth M., L'Heureux-Huard N., Trudel M. Characterization of a mutator gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6505–6509. doi: 10.1073/pnas.76.12.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth M. Role of deoxynucleoside triphosphate pools in the cytotoxic and mutagenic effects of DNA alkylating agents. Somatic Cell Genet. 1981 Jan;7(1):89–102. doi: 10.1007/BF01544750. [DOI] [PubMed] [Google Scholar]
- Meuth M. Sensitivity of a mutator gene in Chinese hamster ovary cell to deoxynucleoside triphosphate pool alterations. Mol Cell Biol. 1981 Jul;1(7):652–660. doi: 10.1128/mcb.1.7.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth M. The genetic consequences of nucleotide precursor pool imbalance in mammalian cells. Mutat Res. 1984 Apr;126(2):107–112. doi: 10.1016/0027-5107(84)90051-4. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J., Goncalves O., Meuth M. Structure of mutant alleles at the aprt locus of Chinese hamster ovary cells. J Mol Biol. 1983 Jul 5;167(3):575–594. doi: 10.1016/s0022-2836(83)80099-0. [DOI] [PubMed] [Google Scholar]
- Perry P. E. Induction of sister-chromatid exchanges (SCEs) by thymidine and the potentiation of mutagen-induced SCEs in Chinese hamster ovary cells. Mutat Res. 1983 May;109(2):219–229. doi: 10.1016/0027-5107(83)90048-9. [DOI] [PubMed] [Google Scholar]
- Peterson A. R., Landolph J. R., Peterson H., Heidelberger C. Mutagenesis of Chinese hamster cells is facilitated by thymidine and deoxycytidine. Nature. 1978 Nov 30;276(5687):508–510. doi: 10.1038/276508a0. [DOI] [PubMed] [Google Scholar]
- Potter C. G. Induction of polyploidy by concentrated thymidine. Exp Cell Res. 1971 Oct;68(2):442–448. doi: 10.1016/0014-4827(71)90171-6. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Reichard P. From deoxynucleotides to DNA synthesis. Fed Proc. 1978 Jan;37(1):9–14. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Trudel M., Van Genechten T., Meuth M. Biochemical characterization of the hamster thy mutator gene and its revertants. J Biol Chem. 1984 Feb 25;259(4):2355–2359. [PubMed] [Google Scholar]
- Weinberg G., Ullman B., Martin D. W., Jr Mutator phenotypes in mammalian cell mutants with distinct biochemical defects and abnormal deoxyribonucleoside triphosphate pools. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2447–2451. doi: 10.1073/pnas.78.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]