Abstract
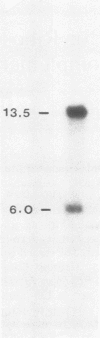
We cloned and sequenced the second gene coding for yeast ribosomal protein 51 (RP51B). When the DNA sequence of this gene was compared with the DNA sequence of RP51A (J.L. Teem and M. Rosbash, Proc. Natl. Acad. Sci. U.S.A. 80:4403--4407, 1983), the following conclusions emerged: both genes code for a protein of 135 amino acids; both open reading frames are interrupted by a single intron which occurs directly after the initiating methionine; the open reading frames are 96% homologous and code for the same protein with the exception of the carboxy-terminal amino acid; DNA sequence homology outside of the coding region is extremely limited. The cloned genes, in combination with the one-step gene disruption techniques of Rothstein (R. J. Rothstein, Methods Enzymol. 101:202-211, 1983), were used to generate haploid strains containing mutations in the RP51A or RP51B genes or in both. Strains missing a normal RP51A gene grew poorly (180-min generation time versus 130 min for the wild type), whereas strains carrying a mutant RP51B were relatively normal. Strains carrying mutations in the two genes grew extremely poorly (6 to 9 h), which led us to conclude that RP51A and RP51B were both expressed. The results of Northern blot and primer extension experiments indicate that strains with a wild-type copy of the RP51B gene and a mutant (or deleted) RP51A gene grow slowly because of an insufficient amount of RP51 mRNA. The growth defect was completely rescued with additional copies of RP51B. The data suggest that RP51A contributes more RP51 mRNA (and more RP51 protein) than does RP51B and that intergenic dosage compensation, sufficient to rescue the growth defect of strains missing a wild-type RP51A gene, does not take place.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollen G. H., Cohen L. H., Mager W. H., Klaassen A. W., Planta R. J. Isolation of cloned ribosomal protein genes from the yeast Saccharomyces carlsbergensis. Gene. 1981 Sep;14(4):279–287. doi: 10.1016/0378-1119(81)90160-8. [DOI] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Bozzoni I., Beccari E., Luo Z. X., Amaldi F. Xenopus laevis ribosomal protein genes: isolation of recombinant cDNA clones and study of the genomic organization. Nucleic Acids Res. 1981 Mar 11;9(5):1069–1086. doi: 10.1093/nar/9.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot H. V., Rosbash M. Behavior of individual maternal pA+ RNAs during embryogenesis of Xenopus laevis. Dev Biol. 1982 Nov;94(1):79–86. doi: 10.1016/0012-1606(82)90070-7. [DOI] [PubMed] [Google Scholar]
- Fabijanski S., Pellegrini M. Isolation of a cloned DNA segment containing a ribosomal protein gene of Drosophila melanogaster. Gene. 1982 Jun;18(3):267–276. doi: 10.1016/0378-1119(82)90165-2. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Pearson N. J., Kim C. H., Warner J. R. The genes for fifteen ribosomal proteins of Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 10;256(19):10176–10183. [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S., Warner J. R. Identification of ten genes that control ribosome formation in yeast. Mol Gen Genet. 1970;109(1):42–56. doi: 10.1007/BF00334045. [DOI] [PubMed] [Google Scholar]
- Hereford L., Fahrner K., Woolford J., Jr, Rosbash M., Kaback D. B. Isolation of yeast histone genes H2A and H2B. Cell. 1979 Dec;18(4):1261–1271. doi: 10.1016/0092-8674(79)90237-x. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. H., Warner J. R. Messenger RNA for ribosomal proteins in yeast. J Mol Biol. 1983 Mar 25;165(1):79–89. doi: 10.1016/s0022-2836(83)80243-5. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meyuhas O., Perry R. P. Construction and identification of cDNA clones for mouse ribosomal proteins: application for the study of r-protein gene expression. Gene. 1980 Jul;10(2):113–129. doi: 10.1016/0378-1119(80)90129-8. [DOI] [PubMed] [Google Scholar]
- Monk R. J., Meyuhas O., Perry R. P. Mammals have multiple genes for individual ribosomal proteins. Cell. 1981 May;24(2):301–306. doi: 10.1016/0092-8674(81)90319-6. [DOI] [PubMed] [Google Scholar]
- Pearson N. J., Fried H. M., Warner J. R. Yeast use translational control to compensate for extra copies of a ribosomal protein gene. Cell. 1982 Jun;29(2):347–355. doi: 10.1016/0092-8674(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Petes T. D. Unequal meiotic recombination within tandem arrays of yeast ribosomal DNA genes. Cell. 1980 Mar;19(3):765–774. doi: 10.1016/s0092-8674(80)80052-3. [DOI] [PubMed] [Google Scholar]
- Pikielny C. W., Teem J. L., Rosbash M. Evidence for the biochemical role of an internal sequence in yeast nuclear mRNA introns: implications for U1 RNA and metazoan mRNA splicing. Cell. 1983 Sep;34(2):395–403. doi: 10.1016/0092-8674(83)90373-2. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A high speed, high capacity homology matrix: zooming through SV40 and polyoma. Nucleic Acids Res. 1982 Aug 11;10(15):4765–4782. doi: 10.1093/nar/10.15.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M., Harris P. K., Woolford J. L., Jr, Teem J. L. The effect of temperature-sensitive RNA mutants on the transcription products from cloned ribosomal protein genes of yeast. Cell. 1981 Jun;24(3):679–686. doi: 10.1016/0092-8674(81)90094-5. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rykowski M. C., Wallis J. W., Choe J., Grunstein M. Histone H2B subtypes are dispensable during the yeast cell cycle. Cell. 1981 Aug;25(2):477–487. doi: 10.1016/0092-8674(81)90066-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teem J. L., Rosbash M. Expression of a beta-galactosidase gene containing the ribosomal protein 51 intron is sensitive to the rna2 mutation of yeast. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4403–4407. doi: 10.1073/pnas.80.14.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaslet C. A., O'Connell P., Izquierdo M., Rosbash M. Isolation and mapping of a cloned ribosomal protein gene of Drosophila melanogaster. Nature. 1980 Jun 26;285(5767):674–676. doi: 10.1038/285674a0. [DOI] [PubMed] [Google Scholar]
- Wallis J. W., Hereford L., Grunstein M. Histone H2B genes of yeast encode two different proteins. Cell. 1980 Dec;22(3):799–805. doi: 10.1016/0092-8674(80)90556-5. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Hereford L. M., Rosbash M. Isolation of cloned DNA sequences containing ribosomal protein genes from Saccharomyces cerevisiae. Cell. 1979 Dec;18(4):1247–1259. doi: 10.1016/0092-8674(79)90236-8. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Rosbash M. Ribosomal protein genes rp 39(10 - 78), rp 39(11 - 40), rp 51, and rp 52 are not contiguous to other ribosomal protein genes in the Saccharomyces cerevisiae genome. Nucleic Acids Res. 1981 Oct 10;9(19):5021–5036. doi: 10.1093/nar/9.19.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]