Abstract
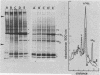
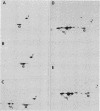
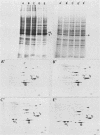
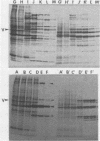
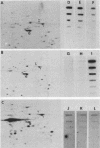
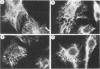
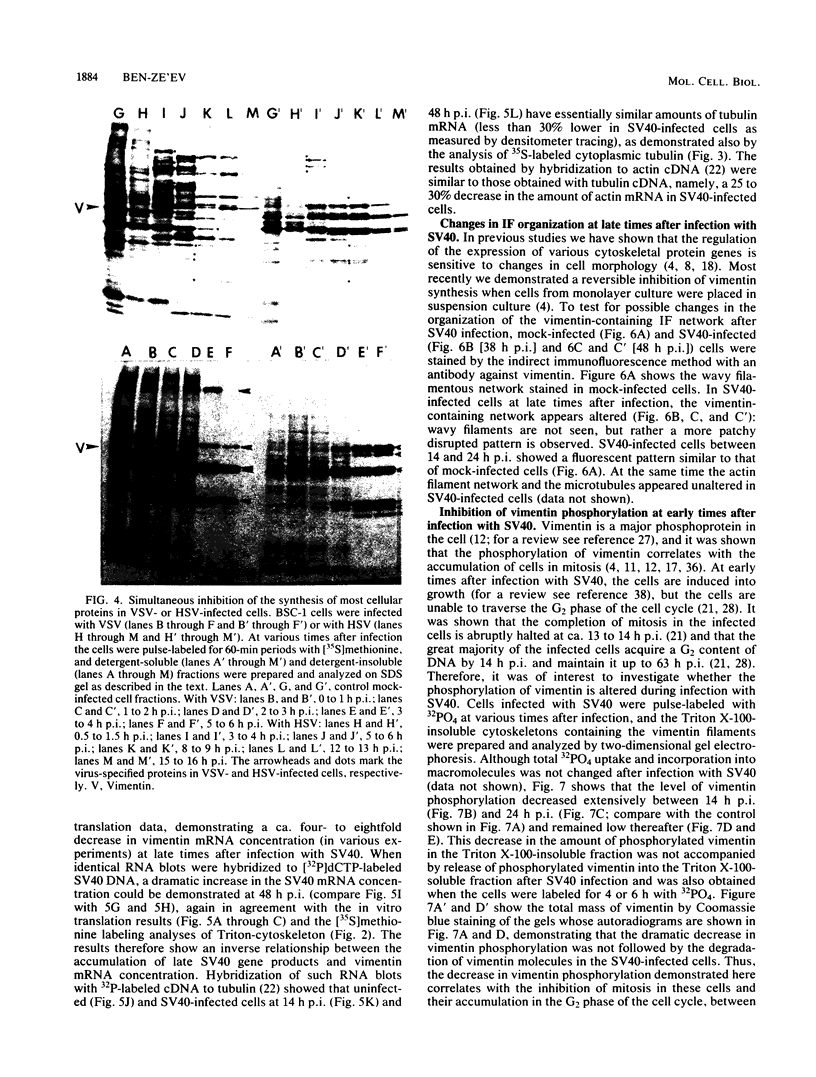
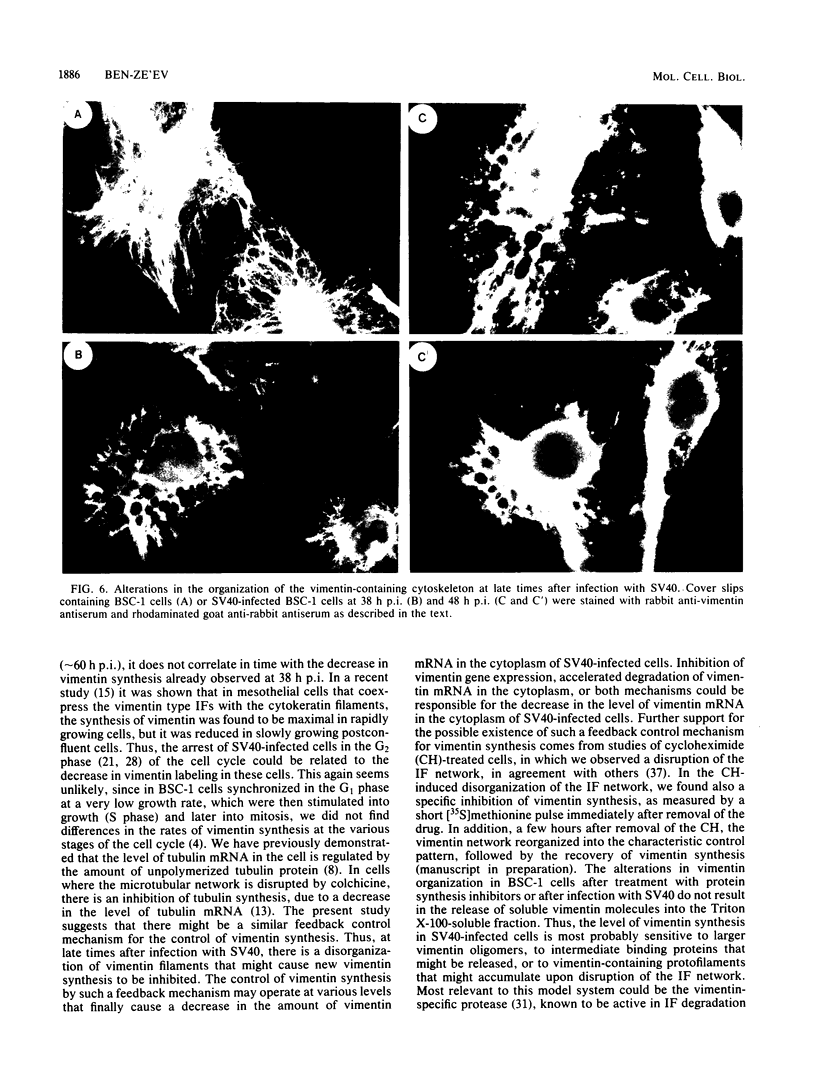
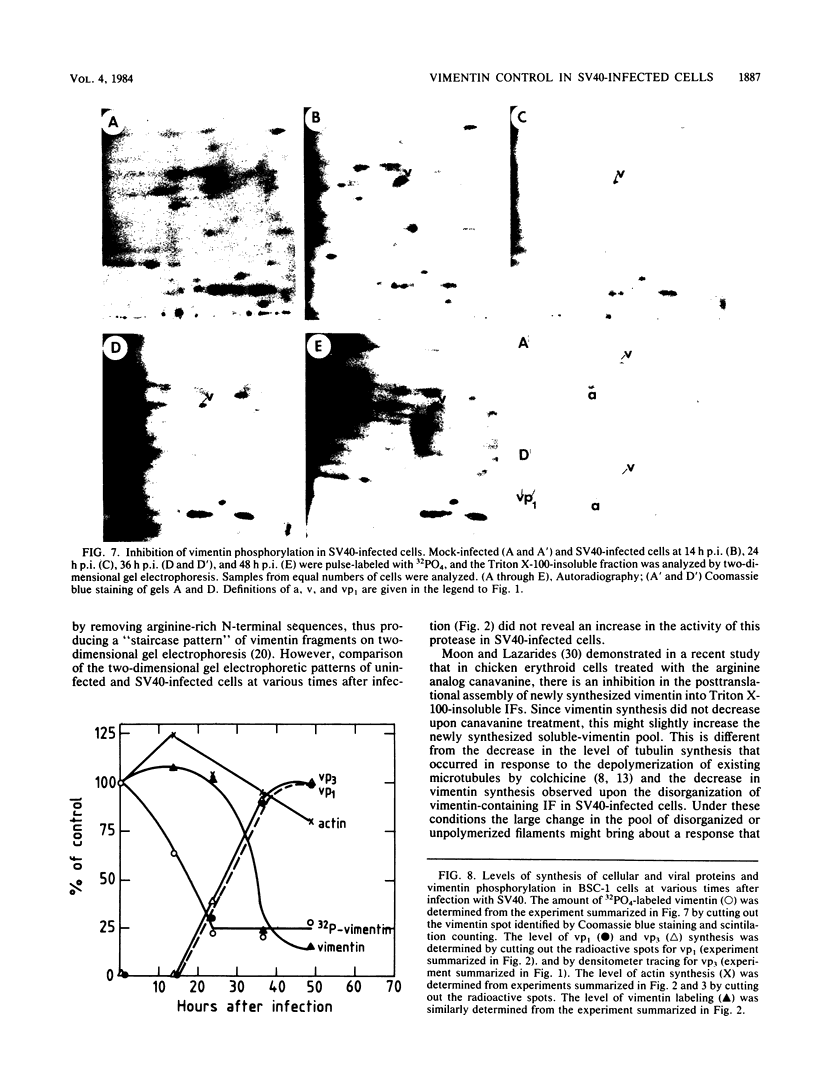
The organization, synthesis, and phosphorylation of vimentin were studied at various times after infection of monkey kidney cells with simian virus 40. Late after infection (between 36 and 48 h postinfection) there is a dramatic reduction in vimentin synthesis that is paralleled by a specific disruption of the intermediate filament network. At the same time there is no apparent alteration of the organization or the synthesis of the actin-containing filaments and of the microtubules. The inhibition of vimentin synthesis is also reflected by the level of vimentin mRNA activity in the infected cells, as assayed in a cell-free in vitro translation system, and vimentin mRNA concentration as revealed by RNA blot hybridization to cloned vimentin cDNA. The level of vimentin phosphorylation also decreases dramatically but at a much earlier time after infection (between 14 and 24 h postinfection), when mitosis in the infected cells is blocked. Although the decrease in vimentin synthesis in simian virus 40-infected cells is paralleled by the alterations in the organization of the intermediate filament network, the phosphorylation of vimentin correlates with the cell cycle, as it does in other systems. A possible feedback control mechanism of vimentin synthesis by alterations in the organization of the intermediate filament network is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abulafia R., Ben-Ze'ev A., Hay N., Aloni Y. Control of late simian virus 40 transcription by the attenuation mechanism and transcriptionally active ternary complexes are associated with the nuclear matrix. J Mol Biol. 1984 Feb 5;172(4):467–487. doi: 10.1016/s0022-2836(84)80018-2. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Abulafia R., Aloni Y. SV40 virions and viral RNA metabolism are associated with cellular substructures. EMBO J. 1982;1(10):1225–1231. doi: 10.1002/j.1460-2075.1982.tb00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Abulafia R., Bratosin S. Herpes simplex virus and protein transport are associated with the cytoskeletal framework and the nuclear matrix in infected BSC-1 cells. Virology. 1983 Sep;129(2):501–507. doi: 10.1016/0042-6822(83)90190-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Aloni Y. Processing of SV40 RNA is associated with the nuclear matrix and is not followed by the accumulation of low-molecular-weight RNA products. Virology. 1983 Mar;125(2):475–479. doi: 10.1016/0042-6822(83)90218-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cell configuration-related control of vimentin biosynthesis and phosphorylation in cultured mammalian cells. J Cell Biol. 1983 Sep;97(3):858–865. doi: 10.1083/jcb.97.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980 Sep;21(2):365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Horowitz M., Skolnik H., Abulafia R., Laub O., Aloni Y. The metabolism of SV40 RNA is associated with the cytoskeletal framework. Virology. 1981 Jun;111(2):475–487. doi: 10.1016/0042-6822(81)90350-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Virus replication in infected epithelial cells is coupled to cell shape-responsive metabolic controls. J Cell Physiol. 1983 Feb;114(2):145–152. doi: 10.1002/jcp.1041140202. [DOI] [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Cabral F., Gottesman M. M. Phosphorylation of the 10-nm filament protein from Chinese hamster ovary cells. J Biol Chem. 1979 Jul 25;254(14):6203–6206. [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Connell N. D., Rheinwald J. G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983 Aug;34(1):245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Dodemont H. J., Soriano P., Quax W. J., Ramaekers F., Lenstra J. A., Groenen M. A., Bernardi G., Bloemendal H. The genes coding for the cytoskeletal proteins actin and vimentin in warm-blooded vertebrates. EMBO J. 1982;1(2):167–171. doi: 10.1002/j.1460-2075.1982.tb01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Fink L. M. An alteration in the phosphorylation of vimentin-type intermediate filaments is associated with mitosis in cultured mammalian cells. Cell. 1982 May;29(1):43–52. doi: 10.1016/0092-8674(82)90088-5. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Bell P. B., Lazarides E. Coexistence of desmin and the fibroblastic intermediate filament subunit in muscle and nonmuscle cells: identification and comparative peptide analysis. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3894–3898. doi: 10.1073/pnas.76.8.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershey E. L. Simian virus 40-host cell interaction during lytic infection. J Virol. 1979 Apr;30(1):76–83. doi: 10.1128/jvi.30.1.76-83.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg I., de Baetselier A., Walker M. D., Behar L., Lehrach H., Frischauf A. M., Littauer U. Z. Brain tubulin and actin cDNA sequences: isolation of recombinant plasmids. Nucleic Acids Res. 1980 Aug 25;8(16):3553–3564. doi: 10.1093/nar/8.16.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Lin W., Edens J., Revel J. P. Visualization of antigens attached to cytoskeletal framework in animal cells: colocalization of simian virus 40 Vp1 polypeptide and actin in TC7 cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4339–4343. doi: 10.1073/pnas.80.14.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laub O., Rall L., Bell G. I., Rutter W. J. Expression of the human insulin gene in an alternate mammalian cell and in cell extracts. J Biol Chem. 1983 May 25;258(10):6037–6042. [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Klein I. B., Cram L. S. Flow cytometry analysis of early DNA content changes in human and monkey cells following infection with Simian Virus 40. J Supramol Struct. 1979;11(1):25–31. doi: 10.1002/jss.400110104. [DOI] [PubMed] [Google Scholar]
- Luftig R. B. Does the cytoskeleton play a significant role in animal virus replication? J Theor Biol. 1982 Nov 7;99(1):173–191. doi: 10.1016/0022-5193(82)90397-6. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Lazarides E. Canavanine inhibits vimentin assembly but not its synthesis in chicken embryo erythroid cells. J Cell Biol. 1983 Oct;97(4):1309–1314. doi: 10.1083/jcb.97.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M. Resolution of simian virus 40 proteins in whole cell extracts by two-dimensional electrophoresis: heterogeneity of the major capsid protein. Cell. 1976 Oct;9(2):289–298. doi: 10.1016/0092-8674(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Robbins A. K., Crawford L. V. Phophorylation of polyoma and SV40 virus proteins. J Gen Virol. 1977 Oct;37(1):75–83. doi: 10.1099/0022-1317-37-1-75. [DOI] [PubMed] [Google Scholar]
- Prives C. L., Shure H. Cell-free translation of simian virus 40 16S and 19S L-strand-specific mRNA classes to simian virus 40 major VP-1 and minor VP-2 and VP-3 capsid proteins. J Virol. 1979 Mar;29(3):1204–1212. doi: 10.1128/jvi.29.3.1204-1212.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B., Kaufmann S., Vogelstein B. Increased phosphorylation rate of intermediate filaments during mitotic arrest. Exp Cell Res. 1981 Jun;133(2):445–448. doi: 10.1016/0014-4827(81)90338-4. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Chen L. B., Murphy J. R., Fields B. N. Specific disruption of vimentin filament organization in monkey kidney CV-1 cells by diphtheria toxin, exotoxin A, and cycloheximide. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7267–7271. doi: 10.1073/pnas.77.12.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Robbins E. Histone synthesis in polyoma- and SV40-infected cells. Virology. 1970 Feb;40(2):307–315. doi: 10.1016/0042-6822(70)90406-x. [DOI] [PubMed] [Google Scholar]