Abstract
The potential of growth factors to stimulate tissue healing through the enhancement of cell proliferation, migration, and differentiation is undeniable. However, critical parameters on the design of adequate carriers, such as uncontrolled spatiotemporal presence of bioactive factors, inadequate release profiles, and supraphysiological dosages of growth factors, have impaired the translation of these systems onto clinical practice. This review describes the healing cascades for bone, cartilage, and osteochondral interface, highlighting the role of specific growth factors for triggering the reactions leading to tissue regeneration. Critical criteria on the design of carriers for controlled release of bioactive factors are also reported, focusing on the need to provide a spatiotemporal control over the delivery and presentation of these molecules.
Introduction
The recreation and maintenance of functionally viable tissues using tissue engineering (TE) approaches incorporating controlled release functionalities is quite challenging due to the fact that cells respond to a wide array of structural, biochemical, and temporal cues in a microenvironment, which is difficult to simulate.1 These stimuli work in cooperation to assemble and organize cells and their respective matrix into tissues. The concept of biomimicry relies on the development of nature-inspired biomaterials aiming for the generation of new tissues and organs. Biomimetic approaches can be applied through different perspectives, including tissue functionality, materials/composition, and biological mechanisms, intervening in tissue formation, remodeling, and healing.2
This review will address the increasing level of complexity and functionality in the design of TE approaches through the spatiotemporal controlled delivery of bioactive factors from three-dimensional (3D) constructs and their effect on the skeletal tissue healing. It comprises the recapitulation of native bone, cartilage, and osteochondral interface morphogenesis and healing; specifically, the sequence of events leading to tissue regeneration and the key signaling molecules involved in those processes. Moreover, the variables involved in the design of controlled delivery systems for the desired targeting tissues are also highlighted; specifically, the presentation of the appropriate sequence, rate, and dosage of bioactive factors in a spatiotemporal controlled manner.
Brief Recapitulation of Native Skeletal Tissue Morphogenesis and Healing
There has been considerable interest in understanding the signaling interplay in bone and cartilage due to their limited ability to heal upon serious fracture or trauma. Bone is comprised of a variety of cell populations, extracellular matrix (ECM), and other proteins as well as inorganic components that work synergistically to sustain physical forces, molecular signals, and systemic hormone networks.3 On the other hand, articular cartilage is a highly resilient connective tissue that covers the surfaces at the ends of long bones.4 The osteochondral tissue is a gradual transition from cartilage to bone in which the key constituents of each tissue undergo an exchange in predominance.5,6 Therefore, the treatment of osteochondral lesions is even more problematic because tissue damage involves two different tissues with different intrinsic healing capabilities.7
Bone healing
Bone is distinguished from other tissues by the presence of inorganic hydroxyapatite8 and a wide range of organic components, mostly collagen type I. The mineral part constitutes 65%–70% of the matrix, whereas the organic phase comprises the remaining 25%–30% of the matrix. From a materials science perspective, bone can be considered as a truly composite material.9 The mineralized collagen-based ECM is the defining feature of bone, which provides its unique biomechanical properties.10–13 Collagen type I can initiate and orientate the growth of carbonated apatite mineral, controlling its size and 3D distribution.14–16 The complex hierarchical structure, material properties of the constituents, cellular organization, and molecular cues work in concert to perform the function of bone.17 The hierarchical geometrical structure of bone is critical, not only for the macroscopic mechanical properties, but also for cells, which respond to these by converting mechanical and architectural cues into intracellular signals, which drive activities, such as gene expression, protein production, and general phenotypic behavior.17–22
Upon injury, bone tissue presents the ability to self-repair, in contrast to soft tissue that heals by forming scar tissue.23,24 The vast majority of defects heals on their own or recovers after standard orthopedic procedures.25,26 Surgical treatments of bone defects typically fall into two groups: the Ilizarov method27 and the bone graft transplant.28 Although the Ilizarov method—osteotomy followed by bone distraction—takes advantage of the regeneration potential of bone, it is a highly inconvenient procedure for the patient.29 Over the last decades, the medical field has advanced dramatically in the understanding of tissue and organ healing and repair.30 Transplantation of organs or tissues is still a common accepted methodology to treat patients and tissue replacements, such as autografts,31–33 allografts,34–36 xenografts,37–39 and graft substitutes,40–42 are clinically available therapies to restore the tissue structure and function.43 However, the current situation is suboptimal at best44,45 with the current grafting methodologies presenting several and obvious limitations, including lack of donors,46 donor-site morbidity,47 complicated surgical procedures,48 immune rejection,49 chronic inflammation,50 and lack of clinical predictability.51 However, extreme situations, such as compromised wound environment,44,52 biomechanical instability,45,53 or insufficient surgical techniques,54,55 might lead to large defects with limited intrinsic regeneration potential, often designated as critical size defects. In these cases, complete regeneration cannot occur.25,56
Bone metabolism is a complex process regulated by a large number of bioactive molecules, and bone repair is, to a large extent, a recapitulation of developmental events.57,58 During the fracture repair process, cells progress through stages of differentiation reminiscent of those that cells undergo during normal fetal bone development.26 Four stages of bone repair have been described, as shown in Figure 1: (1) immediate postfracture (day 1–2). Trauma leads to the activation of the local host response, with the activation and influx of inflammatory cells and secretion of various mediators, leading to the formation of hematoma. This process is necessary for the initiation of tissue repair and wound healing59; (2) intramembranous ossification (days 3–5). Immune cells stimulate cell division of osteochondrogenitors and fibroblast-like cells in the cambium layer of periosteum60; (3) chondrogenesis (days 6–9), through the proliferation of chondrocytes and production of cartilaginous matrix61 and; (4) endochondral ossification (days 10–20), through the production of mineralized ECM and vascularization of the tissue.62,63 Although overlapping occurs, there is a temporal sequence of maximum levels of specific growth factors (GFs) and cytokines.64 One of the critical aspects for bone formation is the formation of an extensive network of blood vessels as both intramembranous and endochondral bone ossification occur in close proximity to vascular ingrowth.8,65 Endochondral ossification is the process by which mesenchymal stem cells (MSCs) differentiate toward chondrocytes, producing a cartilaginous template, which contributes to longitudinal growth of the majority of bones and is gradually replaced by bone and marrow. During this process, chondrocytes proliferate, undergo hypertrophy, and die. The deposited cartilage ECM is invaded by blood vessels, osteoclasts, bone marrow MSCs (BMSCs) and osteoblasts and deposition of mineralized ECM is initiated.66,67 On the other hand, intramembranous ossification occurs in the absence of a cartilage template, whereby bone develops directly from mesenchymal progenitors.67,68
FIG. 1.
Stages of bone healing upon fracture/injury, which can be divided in five main steps: inflammation, intramembranous ossification, chondrogenesis, endochondral ossification, and remodeling. In more detail, after injury (i), bone undergoes an induction stage where there is an influx of inflammatory cells and occurs the formation of hematoma, as shown in (ii). Gray areas represent necrotic bone tissue. Afterward, during inflammation, occurs the formation of a cartilage intermediate, as it can be observed by the gray area in (iii). Stage (iv) represents the formation of the soft callus, in which a chondrogenic matrix unites the defect area. From this moment, ossification occurs and woven bone replaces the temporary cartilaginous template through the invasion of blood vessels (stage (v)) and finally, remodeling occurs in step (vi) with lamellar bone promoting the union of the defects and the medullar cavity being restored. Adapted from refs.68,69 Color images available online at www.liebertpub.com/teb
Bone formation is comprised of series of cellular events, which include (1) chemotaxis of osteoblast precursors to the sites of resorption defects69,70; (2) proliferation of these precursors to form a team of osteoblasts capable of filling in the resorption defect71,72 followed by (3) differentiation of the preosteoblasts to form mature cells, which are responsible for expressing the structural proteins of bone, such as collagen type I and other functional proteins.67,73–75
Bone biochemical environment permits and promotes cellular functions that lead to matrix production and ossification.76 The matrix is an active and dynamic biochemical system, including important regulatory cues to nearby cells, affecting gene expression and changes at the cytostructural level.76 There are several mobile (GFs, transcription factors, and cytokines)77–83 and immobilized (collagen type I, fibronectin, decorin, and biglycan)84–89 macromolecules in the bone ECM directing bone cells behavior. Some of the most important soluble mobile macromolecules include the platelet-derived growth factor (PDGF),90 Bone morphogenetic protein (BMP),91 Insulin growth factor (IGF),92 and transfoming growth factor (TGF).93 In addition to mineralization, osteoblasts produce a matrix of proteins that not only serve to structurally support cells, but also to provide a variety of chemical cues, which regulate functionality, namely, the ability to promote mineralization.17,94–96 The most abundant protein is collagen type I, which besides playing the main role in the tensile properties of bone, also contains peptides, which cue bone cells.97 The most abundant noncollagenous bone ECM protein is osteonectin, known for its multiple calcium and collagen binding sites. Moreover, it has been shown to be a potential nucleator of hydroxyapatite.17,98,99 The second most abundant noncollagenous protein in bone ECM is osteocalcin, which presents affinity for calcium/hydroxyapatite and has also been involved in the osteoclast migration process.17,100
Inflammation is also a key component of the early response to bone injury.101,102 Inflammatory cells are recruited to the damaged bone and release cytokines, chemokines, and GFs that amplify the process.103 Inflammation in the early phase of fracture repair is associated with enhanced healing, while chronic inflammation has a deleterious effect on healing.3 Following implantation of materials, inflammatory responses are also expected. The host initiates a physiological healing reaction, consisting first of an acute inflammatory response followed by repair processes.104,105 This inflammatory stage provides the appropriate signals for the shift from inflammation to repair and remodeling of the tissue.106–108 The extent or degree of the inflammatory response is controlled by the extent of injury in the implantation procedure, by the tissue or organ into which the device is implanted and the extent of provisional matrix formation.107 The initial week-long inflammatory phase of fracture healing is characterized by the influx of inflammatory cells, that is, neutrophils, lymphocytes, and macrophages and the release of various cytokines and GFs.109 The inflammation process constitutes the fundamental difference between development and regeneration.26 Therefore, modulation of inflammation has been an increasingly used strategy to control tissue regeneration.110 When delivering cytokines, precise spatial and temporal control over the delivery profile is required because both prolonging and obliterating signaling might impair bone healing.109
There is a close inter-relationship between bone healing and formation of the vascular compartment. The clinical significance of angiogenesis in bone regeneration is of uttermost importance as an appropriate blood supply has been recognized as an essential component of normal fracture healing.111,112 Angiogenesis, the growth of new capillary blood vessels from pre-existing host vasculature,113 is also involved in the initiation of fracture healing and promotion of endochondral and intramembranous ossification in bone development, through blood vessel invasion of avascular cartilage114,115 and ingress of osteoblast progenitors,111,116,117 respectively. It involves the formation of capillary networks by endothelial cells, thereby enabling the transport of oxygen, nutrients, and waste throughout the tissue.118 The sufficient supply of nutrients and oxygen to the cells transplanted into the body is indispensable for cell survival and consequent maintenance of their biological function.119,120 Angiogenesis is a complex process involving endothelial cell activation,121 recruitment,122 and migration 123 to sprout the neovessels to the mural cells (pericytes or smooth muscle cells) making up the surrounding vessel wall for stabilization.8 All vascularization processes involve a series of interactions among cytokines, GFs, various types of cells, and enzymes.124,125 Osteoblasts and endothelial cells cross-talk and act synergistically toward the formation of a mature vascular network and for the differentiation of osteoprogenitor cells into osteoblast and formation of bone ECM.3,126,127
Cartilage healing
Articular cartilage is composed by a unique type of cell, the chondrocyte, embedded within a dense ECM consisting of 80% water, a collagen type II network, and proteoglycans.128,129 Although cartilage appears to be a simple aneural, avascular, connective tissue, there are many levels of complexity in its composition and structure.130,131 Both cells and matrix distribute within successive cartilage layers identified as superficial, transitional, radial (deep zone), and calcified zones.29 Cartilage tissue demonstrates significant differences in cell phenotype, composition, and matrix organization along the depth of the tissue, reflecting different biomechanical and functional requirements of different zones.132–134
Hyaline cartilage is characterized by its high content on proteoglycan aggrecan, which exists in the form of proteoglycan aggregates in association with hyaluronan (HA) and link protein (LP). These aggregates are responsible for the turgid nature of cartilage, providing the osmotic properties necessary to resist compressive loads.135,136 A variety of small leucine-rich repeat proteoglycans (SLRPs), such as decorin,137 biglycan,137 fibromodulin,138 and lumican,139 are also present and contribute for the maintenance of integrity of the tissue and to modulate its metabolism.135
The most basic functions of cartilage include providing near frictionless surface between load bearing in the joints to allow for pain-free motion, shock-absorbance, and load distribution.140 The avascularity of articular cartilage has led to the assertion that the tissue is immunoprivileged, whereby the body's immune system is limited in its ability to detect and reject implanted tissue.141
Numerous GFs work in concert to regulate development and homeostasis of articular cartilage throughout life, in particular bioactive factors from the TGF-β superfamily,142–145 Fibroblast growth factor (FGF) family,146 and IGF-I.147–149 Architectural design for regenerative medicine and surgery is also an adaptation of the phenomenon observed during development and morphogenesis.150 To properly restore the zonal structure of cartilage, phenotypical differences between chondrocyte populations and the response of chondrocyte subpopulations to GFs and external stimulus should be fully understood.151
Typically, chondrocytes respond to injury caused by mechanical insult, joint instability, and inflammatory cytokines through matrix activation, cell proliferation, apoptosis, and matrix destruction.152,153 The activation of chondrocytes can result in modulation of gene expression, resulting in different patterns of protein synthesis, fibroblast dedifferentiation, hypertrophy, or regeneration of mature cartilage.152 The lack of vascular supply in cartilage limits early repair responses upon injury.150 Consequently, injury to cartilage usually heals through a scar tissue formation mainly composed of fibrocartilage with inferior mechanical properties and a gradually degrading nature.4 Moreover, cartilage has a low cell:matrix ratio and chondrocytes have a relatively low metabolic activity limiting the tissue remodeling activity.154 Only when the injury reaches the subchondral bone, self-healing processes are initiated by the release of mesenchymal progenitor cells from bone marrow into the wound site.2 This observation forms the basis of surgical repair techniques, such as abrasion arthroplasty,155,156 drilling,157 and microfracture158 to penetrate the subchondral bone. Periosteum is also a source of cells that can differentiate into chondrocytes and autogenous or allogeneic cell/tissue transfer via periosteal grafts159,160 has been used, while other techniques have used chondrocytes or cartilage directly. Autologous chondrocyte transplantation involves harvesting cells from a noninvolved area of the joint, to expand them in culture, and then transplanting them to the area of involvement.161–163 However, these techniques present several limitations particularly on cell and tissue availability, unwanted fibrocartilage formation and inadequate graft integration.164–166 Another strategy for the regeneration of articular cartilage is mosaicplasty and it is based on the creation of multiple small osteochondral grafts.167–170 The limiting factor of mosaicplasty resides in the donor-site availability of grafts.
In degenerative diseases, such as osteoporosis or rheumatoid arthritis, the remodeling equilibrium of cartilage is disrupted and the rate of collagen and proteoglycans loss from the matrix exceeds the rate of deposition of newly synthesized molecules.152,171,172 The upregulation of cartilage-degrading enzymes can be caused by several factors, such as chemokines, other inflammatory mediators, and mechanical loading.173
Although current approaches are reasonably effective in achieving clinical endpoints of symptomatic relief, they have not been successful at preventing future degeneration of the repaired tissue and surrounding host environment.174 Hence, TE is a promising approach for the regeneration of articular cartilage as it might provide the tools to overcome the limitations observed with the current treatments.175
Osteochondral regeneration
Cartilage and bone arise from a common progenitor and exist in apposition at articular surfaces of synovial joints as well as in the epiphyseal growth plate.176 Structurally, the osteochondral interface is the connection between a layer of hyaline cartilage and underlying bone and it is crucial for load transfer between bone and cartilage.6 Moreover, it has been shown that the osteochondral interface is critical to maintain the integrity of cartilage by simultaneously limiting vascular ingrowth from the subchondral bed and preventing ectopic mineralization.177 The interface typically is characterized by a decreased amount of water content and collagen type II in comparison with the more superficial layers of cartilage. Moreover, the collagen fiber diameter is also increased and perpendicular in direction with respect to the articulating surface.178
Osteochondral tissue is comprised of osteoblasts, osteoclasts, and chondrocytes, but as these phases merge, there is an overlap in cellular function. Hence, this interface is composed of hypertrophic chondrocytes embedded in a mineralized cartilage matrix.5,177 While the chondral repair mechanism relies on the intrinsic healing capabilities of chondrocytes, osteochondral defects allow the recruitment of progenitor cells from bone marrow to assist regeneration of cartilage and underlying bone structure.179
Osteochondral defects penetrate through the vascularized subchondral bone and spontaneous repair occurs as mesenchymal chondroprogenitor cells invade the lesion and form cartilage. However, full-thickness defect repair is only transient. The neo-formed tissue is fibrous and enriched in collagen type I and fibronectin and does not have the functional properties of native hyaline cartilage.4,29,180
For the treatment of large osteochondral defects, one of the options is autologous osteochondral grafts such as those used in mosaicplasty.170,181,182 However, donor-site morbidity and lack of integration can compromise long-term graft outcomes.183 Presently, a significant barrier to clinical translation is how to achieve functional integration of tissue-engineered orthopedic grafts.184
The concept of osteochondral TE, a hybrid of bone and cartilage regeneration, has attracted considerable attention, particularly as a technique for promoting superior cartilage integration and as a treatment for osteochondral defects.179,185–196 For osteochondral scaffolds, additional design criteria should be considered to achieve the best possible simultaneous growth of the two independent tissues involved. This may require the use of biphasic constructs with gradients of mechanical, structural, and molecular properties.154,177,197–200 The most common approach consists in the development of independent layers for each zone, because chondrocytes and bone cells show distinct metabolic and structural functionality, yet communicating and interacting in a unique culturing system.201 The junction of two layers has been achieved by fibrin sealant, simple press-fitting, suturing, or external fixation.179 These scaffolds constitute the first generation of stratified scaffolds.183 A scaffold with a predesigned inhomogeneity can better sustain and transmit the distribution of complex loads inherent at the multitissue interface and several studies have reported new designs to fulfill this request.202–206 Constructs to be used for regeneration of osteochondral defects may also benefit from the application of hierarchically and structurally organized drug delivery systems. One area of special importance in osteochondral graft design is the regeneration of the bone to cartilage interface or a calcified cartilage layer between bone and cartilage regions, which is critical for graft integration and for establishing long-term functionality.183
Signaling molecules on natural cascades of bone, cartilage, and osteochondral formation/healing
Bone
During bone formation, mobile cues directing cell behavior in bone can be produced by local osteoblasts or delivery via blood stream. These GFs have indisputable roles in osteoblast proliferation, differentiation, and subsequent bone formation and regulation.17,207–209 These molecules act through autocrine and paracrine signaling mechanisms to induce the migration, proliferation, and differentiation of osteoprogenitor cells and/or synthesis of collagen type I and matrix apposition by mature osteoblasts.210 Upon matrix destruction, either caused by natural bone remodeling process or bone fracture, these factors are released to initiate osseous healing and to maintain the cyclic anabolic and catabolic processes that continuously remodel bone.210
The local concentration of chemical cues that can influence bone cells increases substantially in the event of an injury. Bone tissue injury initiates a cascade of events leading to the migration of neutrophils, macrophages, and fibroblasts, which subsequently express and secrete a variety of cytokines and transcriptional factors, which direct migration of MSCs, neovascularization, and remodeling/healing.17,211
Moreover, immobilized bone ECM macromolecules act as primary chemical effectors in cell signaling and functionality. Several bone ECM proteins contain progenitor and osteoblast integrin-binding sites and GF-binding sites, presenting an obvious selection for developing scaffolds for bone.17
Many GFs, such as BMPs,212 basic FGF (bFGF),213 IGF,214 TGF-β,215 PDGF,216 and vascular endothelial growth factor (VEGF),217 have been found to induce new bone through their effects on the recruitment, proliferation, and differentiation of bone-forming cells.218 Their role in bone morphogenesis and regeneration is described in Table 1 and their expression profile is shown in Figure 2. It is likely that multiple factors regulating distinct aspects of the regenerative process can be used in parallel to affect the regeneration of functional tissues.219
Table 1.
Growth Factors Roles on Bone and Cartilage Formation and Healing
| Growth factor | Role on bone formation/healing | Role on cartilage formation/healing |
|---|---|---|
| BMPs | Most osteoinductive GFs223 | Induction of Sox9 expression |
| Promotion of migration, proliferation, and osteogenic differentiation of MSCs57,221–223 | Initiation and regulation of embryonic development and morphogenesis of cartilage | |
| Influence on skeletal pattern formation.222 | Proliferation and maturation of chondrocytes | |
| Strong induction of chondrogenic differentiation of MSCs by isoform 2 | ||
| TGF-βs | Chemoattractant for osteoprogenitor cells57,73,221,223 Promotion of collagen, osteonectin, and osteopontin production221,223 Stimulation of undifferentiated MSCs proliferation73,222 Inhibition of mature and progenitor osteoclast cells proliferation221 Inhibition of matrix metalloproteinases, known for their role on ECM digestion Stabilization of new blood vessels Direction of BMSCs toward resorption sites |
Induction of Sox9 expression Initiation of matrix proteins aggrecan and COMP expression Enhancement of chondrocyte proliferation by isoforms 2 and 3 Stimulation of chondrogenic differentiation of MSCs by isoforms 1 and 3 Inhibition of the activity of MMPs by isoform 1147 Isoform 3 is imperative for Sox9 expression, but it is not required for continued expression of chondrogenic markers at later stages of chondrogenesis |
| IGF-I | Stimulation of osteoprogenitors proliferation73 and bone matrix production221,222 Stimulatory effects on osteoblast activity and chemotaxis and antiapoptotic effect on preosteoblasts57 Upregulation of collagen type I Inflammation mediator |
Stimulation of anabolic activity and proliferation of chondrocytes147,151 Chondrogenic differentiation of MSCs Stimulation of ECM production151 Protection of ECM from IL-1 and TNF-α-mediated degradation during cartilage injury147 |
| FGF-2 | Stimulation of migration, proliferation, and differentiation of mature and progenitor osteoblasts57,73,111,221 Differentiation stage-specific effect Stimulation of angiogenesis through activation of capillary endothelial cells and fibroblasts223 |
Activation of pathways ultimately leading to the upregulation of Sox9 Promotion of cell proliferation4,147 and inhibition of chondrogenic differentiation |
| PDGF | Chemotactic and mitogenic effects (precursor and mature endothelial cells and inflammatory cells)57,73,111,222,223 Maturation of blood vessels by the recruitment of SMCs to the endothelial lining Upregulation of VEGF expression. |
Maintenance of hyaline-like cartilage phenotype Enhancement of chondrocyte proliferation and proteoglycan synthesis147 |
| VEGF | Essential for endochondral bone formation due to its ability to induce migration and differentiation of osteoblasts116 | |
| Initiatior of angiogenesis. Formation and maintenance of blood vessels57,111,223 | ||
| Recruitment of circulating endothelial progenitor cells and inhibition of endothelial cells apoptosis111,223 |
BMSCs, bone marrow mesenchymal stem cells; BMP, bone morphogenetic protein; COMP, cartilage oligomeric matrix protein; ECM, extracellular matrix; FGF, fibroblast growth factor; GFs, growth factors; IL, interleukin; IGF, insulin growth factor; MMPs, matrix metalloproteinases; MSCs, mesenchymal stem cells; SMC, smooth muscle cells; TGF, transfoming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
FIG. 2.
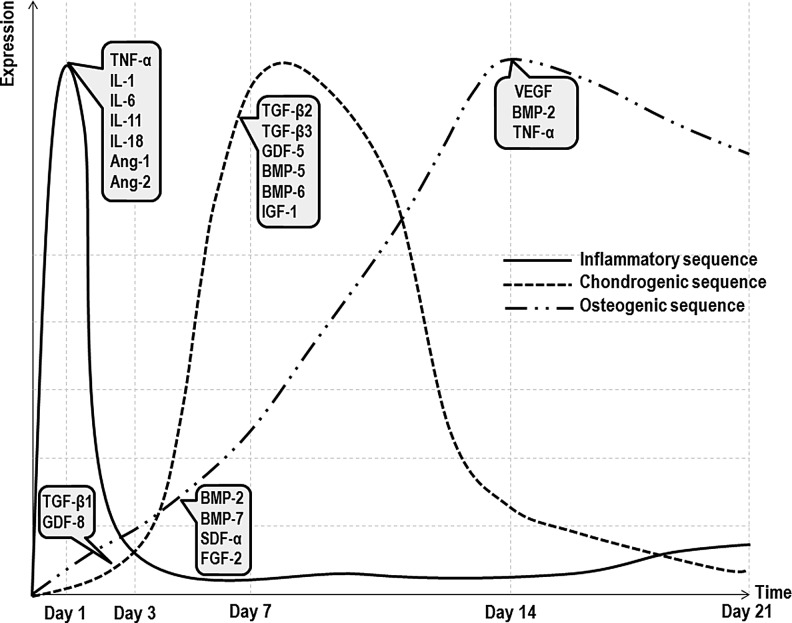
Temporal sequence of growth factors (GFs) and cytokines expression during bone regeneration. The solid dash line represents the inflammatory sequence of events, peaking early upon the injury with high expression of cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, -6, -11, and -18, and angiogenic molecules Angiopoietin-1 (Ang-1) and Ang-2. These molecules are involved in the stimulation of cell migration, production of cartilaginous callus, and vascular endothelial growth factor (VEGF) production. The square dot line represents the chondrogenic route that leads to the generation of a cartilaginous callus peaking between 7 and 14 days after injury. Transfoming growth factor (TGF)-β1 and Growth differentiation factor-8 (GDF-8) act early in this step, with other isoforms responsible for chondrogenesis and endochondral ossification peaking later in the cycle. The long dash double dot line represents the osteogenic sequence of molecules acting on the formation of a bony callus. Simultaneously to the expression of chondrogenic markers, bone morphogenetic protein (BMP)-2, BMP-7, and stromal cell-derived factor-α (SDF-α) are responsible for inducing the migration of mesenchymal stem cells (MSCs) with fibroblast growth factor (FGF)-2 stimulating their proliferation. As callus chondrocytes proliferate, they become hypertrophic and express high levels of VEGF, promoting the invasion of blood vessels, leading to the tissue vascularization. During this step, TNF-α initiates chondrocyte apoptosis and promotes the recruitment of MSCs with osteogenic potential. Moreover, this cytokine is also involved in bone remodeling, the last step in which the hard callus undergoes a resorptive phase to form the typical lamellar bone structure with a central medullar cavity.201–203
Cytokines are included in this group of factors and they are mainly involved in systemic processes, such as host defense and homeostasis, including interleukins (ILs), which are proinflammatory molecules involved in bone resorption and remodeling.76 Cytokines encompass a large family of immunomodulating agents playing key roles in the cross talk between the immune and skeletal systems.57 Bone fracture is an injury that initiates an inflammatory response that peaks 24 h following the injury and is complete by the first week.110 During this time, a complex cascade of proinflammatory signals and GFs are released in a temporally and spatially controlled manner.110
Bone fracture stimulates expression of several dozen inflammatory cytokines, including several isoforms of ILs. IL-1, IL-6, as well as tumor necrosis factor-alpha (TNF-α) are shown to play a key role in initiating the repair cascade. They have a chemotactic effect on other inflammatory cells and on the recruitment of MSCs.220
Levels of most inflammatory mediators return to baseline after the week-long acute inflammatory phase.109 Other relevant bioactive factors acting on the morphogenesis of these tissues include hormones known for controlling serum calcium concentrations and stimulating osteoblast proliferation and differentiation.76
During bone regeneration there is a temporal sequence of GFs and cytokines expression.63,234,235 Angiogenic GFs are predominantly expressed during the early phases to re-establish vascularity, whereas osteogenic GFs are continuously expressed during bone formation and remodeling.219 Numerous GFs are involved in angiogenesis, including VEGF,236 FGF-2,237 PDGF,238 Ang-1,239 and -2,240 IGF,241 among others.124
During normal bone healing, VEGF expression was shown to peak in early days with high expression from days 5 to 14, while BMP expression peaked at a later time point. Since establishment of a vascular bed is an early event that precedes the formation of bone, a similar temporal release profile should be designed to promote bone regeneration.242,243 VEGF is likely produced by inflammatory cells as well as mesenchymal progenitors that are recruited to the site of bone injury. VEGF expression can also be driven to hypoxia as VEGF represents a target gene of hypoxia-inducible factor.111
Despite extensive studies on the biology and delivery of GFs, only two formulations are currently approved by the Food and Drug Administration (FDA) for clinical use in USA. BMP2 (Infuse™, Medtronic Sofamor Danek, Inc.) and BMP7 (OP-1™, Stryker Biotech) repair injuries by mediating spinal fusion or fracture healing.244,245
During fracture repair, BMPs are produced by MSCs, osteoblasts, and chondroblasts, and trigger a cascade of events, such as proliferation and differentiation of MSCs, angiogenesis, and synthesis of the ECM.246 BMP-2 acts on global cellular mobilization and it is also present during the later stages of osteogenesis and chondrogenesis, whereas BMP-7 acts on osteogenic differentiation.246 Given that BMPs are not tissue specific, their localized, targeted, and controlled delivery is required to prevent any undesired and uncontrolled bone formation in inadequate tissues of the body.247
In human clinical treatments, large doses of BMPs (2–12 mg) have been used,248–251 which exceed by far the normal physiological concentrations of these proteins (18.8–22 pg/mL)252 in bone defect areas. As an example, the concentration of BMP-2 approved for application in spine fusion is 1.5 mg/mL.251 Therefore, it is clear that the collagen carriers used in the FDA-approved formulations mentioned above are less effective in providing structural integrity, effective mechanotransduction in large nonunion sites, and control over release kinetics.253 Furthermore, BMP-2 is a well-known chemoattractant for lymphocytes, monocytes, and macrophages and it has also been reported that rapid release of BMP-2 in high doses can induce transient osteoclast-mediated resorption before new bone formation occurs in metaphyseal defects.251,254 The role of the carrier on the outcome of the delivery therapy has been evaluated and the effective and therapeutic doses change according to the carrier, thus highlighting its relevant function.255,256
While the current landscape of GFs used for bone regeneration is dominated by BMPs, a number of other GFs are being investigated for their potential to regenerate bone.245 It has been reported that TGF-β1 promotes osteogenic differentiation in the early and late stages of ectopic bone formation despite its inhibitory effects in vitro.257 FGF-2 displays a dual role, acting both on the stimulation of angiogenesis and osteogenesis.111 It is suggested that (FGF/FGF receptor) signaling pathways coordinates a bone anabolic effect by simultaneously activating Runx2 and BMP-2 pathways.231 The sequential supplementation of FGF-2 followed by BMP-2 tends to enhance bone phenotype markers.258 While BMP-2 acts mainly on the osteoblastic differentiation, FGF-2 promotes cell proliferation, increasing the cell population that will be influenced by the action of BMP-2.259
Factors that drive mobilization of BMSCs have been unclear, but one of the earliest consequences of a bone fracture or injury is local tissue hypoxia. Multipotential MSCs are mobilized into peripheral blood and the Stromal-Derived Factor-α (SDF-α) is known for its role in stem cell homing, mobilization of endothelial progenitor cells, and hematopoietic stem cells to the injury site.260
Cartilage
In cartilage repair, the most investigated GFs include TGF-β, BMPs, and IGF-I.147,148,261 Further details on the role of the most significant GFs acting on cartilage can be found further in Table 1 and Figure 3. Despite the amount of publications reporting the effect of specific GFs on chondrocytes and chondrogenic differentiation of stem cells, little is known about GFs regulating cartilage wound healing and, specifically, the spatial and temporal expression of GFs in acute cartilage wound healing.262 Most of the information regarding TE of cartilage was provided by the study of growth plate of long bones. However, it is admitted that the knowledge of fetal development can provide relevant insights for regenerative medicine purposes as regeneration partially recapitulates several developmental steps.263,264
FIG. 3.
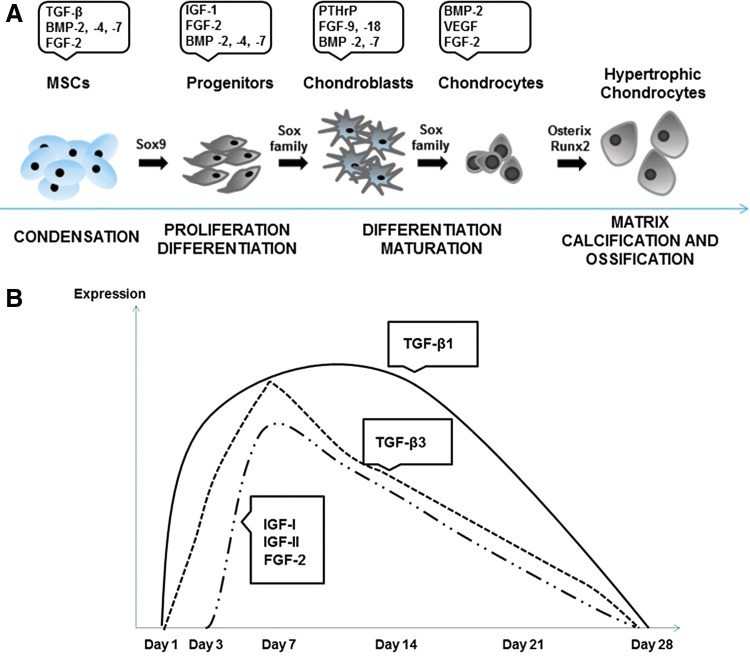
(A) Chondrogenic differentiation of MSCs, which involves three main stages influenced mainly by the Sox family transcription factors, followed by the ossification process. Once chondrocytes reach their mature phenotypic state, osteogenic transcription factors Osterix and Runx 2 are expressed and chondrocytes evolve to a hypertrophic state. Different GFs are expressed by cells, depending on their differentiation state. BMP-2 is expressed throughout the process, while TGF-β family is highly expressed during the MSCs condensation step. The mechanism here described occurs in the growth plate of long bones and it is responsible for the endochondralossification for bone turn-over. Adapted from 236,243 (B). Cartilage layers expressing TGF-β1 and TGF-β3 increased between day 1 and 3 and TGF-β1 maintained approximately those levels until day 14. An increase of insulin growth factor (IGF)-I and FGF-2 was observed between day 3 and 7. IGF-I, FGF-2, and TGF-β3 reached a new peak level at day 7 and showed gradual decrease afterward. At day 28, GF expression returned to basal levels. Color images available online at www.liebertpub.com/teb
In early embryological development, Sox9 is required for the aggregation of MSCs. Moreover, this transcription factor is also required for the expression of collagen II and aggrecan, two of the most important ECM components in hyaline cartilage.261
Active TGF-β1, 2, and 3 are generally considered to be potent stimulators of proteoglycans and collagen type II synthesis.265 During acute cartilage wound healing (Fig. 3B), TGF-β1 and TGF-β3 are highly expressed, particularly during the first 2 weeks upon injury.262 Despite the powerful ability of TGF-β1 to repair damaged cartilage, high dose of intra-articular injections of the GF resulted in chemotaxis and activation of inflammatory cells, promoting fibrosis. Additionally, TGF-β1 has been shown to regulate the autocrine/paracrine axis of IGF-I.266 Hence, drug delivery systems with fine tuning over dosage and rate of delivery are required to fulfill the potential of these bioactive agents.267
In fact, intra-articular injections of specific drugs have failed to produce the desired therapeutical outcomes. The high reactivity of synovium and rapid efflux of drugs from the joint cavity have impaired the effect of bioactive agents injected directly into the articular cartilage.268
Numerous GFs are needed to properly sequence chondrogenesis and it is unlikely that any single GF will lead to complete cartilage repair or affect the arthritic milieu, but rather a combination in a synergistic approach will be required.147 There is considerable cross talk between the TGF and BMP signaling pathways and simultaneous activation of both seems to promote chondrogenic maturation.227
Combined treatments of TGF-β1 with BMP-2 and IGF-I have led to the enhancement of glycosaminoglycan deposition and mechanical properties.175 However, the most serious limitations of the use of MSCs for chondrogenic differentiation through the supplementation with bioactive factors reside on the fact that cell differentiation do not stop at the prehypertrophic stage.263
Osteochondral interface
Hypertrophic chondrocytes are the resident cell population identified at the native osteochondral interface.206 As described in Figure 3, after differentiation and maturation of cells toward the chondrocyte phenotype, Osterix and Runx2 become upregulated and chondrocytes achieve their hypertrophic state. At this stage, collagen type X is highly expressed. The osteochondral interface is also composed by a mineralized and vascularized matrix, in which angiogenic factors, such as VEGF and FGF-2, are upregulated.269,270 However, much interest has been centered on BMPs to promote osteochondral tissue formation as these molecules have a powerful effect in stimulating both new bone and cartilage formation.271
In conclusion, natural processes are multifactorial and skeletal morphogenesis and regeneration are typically driven by the concomitant action of multiple factors that can work synergistically on the same process. Release kinetics are dependent on the type of tissue and defect.210,272
Besides the presentation of the appropriate factor or combination of factors, the concentration and duration of function are critical parameters involved in promoting neotissue formation. Concentrations of GFs should be used within a therapeutic range, whereas crossing the dosage limits would result either in an inefficient strategy or in the production of an abnormal tissue.272
Controlled Release Strategies: The Need for Spatiotemporal Control
The delivery of multiple GFs has been pursued through application of different methodologies, making use of the great versatility of delivery systems developed over the last decades. However, biological mechanisms of tissue regeneration are more complex and, thus, require more than a particular temporal sequence of therapeutic agents, with some regenerative tasks demanding tight coordination of the spatial and temporal presentation of multiple factors.219
GF signaling in tissue healing involves precise regulation of the concentration, temporal gradient, and spatial gradient of factors, which ultimately determines the outcome of the regenerative therapy.124 Controlling the location of release can create concentration gradients by diffusion of the factors from the release site.272 The osteochondral interface is a paramount example of this scenario. The regulation of concentration and delivery rates is also particularly relevant as a suboptimal delivery system does not exploit the full potential of the released bioactive factor, thus requiring higher dosages to provide the desired effect.254
Systems releasing drugs acting synergistically provide a highly inductive therapeutic option, replicating more accurately developmental osteogeonic and chondrogenic cascades, which will ultimately result in a superior clinical performance using considerably reduced doses of GFs. However, this will be achieved at the cost of greatly increased complexity.273 Some of the materials used to produce the carriers for GFs may provide additional favorable properties by themselves, such as calcium phosphates or other ions for bone regeneration, which might not be enhanced by the addition of specific bioactive agents.274 The combination of stimulus is not straightforward and the outcomes of the combination are most of the times unexpected. Hierarchical systems,275–277 multiple layer systems,278,279 and intelligent hydrogels280,281 have been developed aiming for the simultaneous and/or sequential release of multiple signaling molecules.
The spatial organization of the GFs in the matrix is critical because the retention of the molecular bioactivity is also affected by several parameters, including interactions between the biomaterial and the GF, the influence of pH and temperature and porosity.207,282
Spatial gradients of factors in scaffolds can be controlled by varying the positioning of polymer vehicles, immobilizing ligands on polymer networks to attract target cell populations, or designing delivery systems to provide spatially distinct cues.124 Current knowledge of the signals within the microenvironment that regulate cell fate has led to the development of increasingly sophisticated constructs. Scaffolds with precisely controlled architectures regulating spatiotemporal release of GFs and morphogens and responding dynamically to environmental cues have been increasingly developed.283
Spatial patterns in tissues are controlled by both the architectural features of the ECM and concentration profiles and gradients of diffusible bioactive factors.284 The ability to combine topographical and biochemical cues within a single scaffold presents a valuable opportunity to evaluate their synergistic impact.285
Angiogenesis is paramount of the relevance of a temporal sequence of bioactive factors expression. Certain factors initiate angiogenesis, while others induce maturation of newly formed vessels.286 Later on, a third group of molecules act on the maintenance of the integrity of the established vasculature.287 If the appropriate presence and sequence of bioactive factors are not achieved, poor vascularization occurs.283
Scaffolds can perform the dual roles of biomechanical and biochemical support by presenting the appropriate mediators to the surrounding tissue. The ultimate goal is to develop a multifunctional support performing two main roles: (1) acting as a temporary structure for cell attachment and colonization; (2) acting as a delivery platform for multiple GFs to stimulate tissue regeneration.
Many techniques have been developed to regulate the kinetics and distribution of soluble factors, including multiple levels of encapsulation and noncovalent bonding of bioactive factors to peptides with a range of dissociation constants mimicking ECM immobilization of GFs.283 One of the most used approaches involves the use of the scaffold as a controlled release platform by the simple dispersion of bioactive agents in the matrix or via immobilization to the scaffold by electrostatic interactions or covalent bonding.284 These monolithic scaffolds, even when combined with GFs and cells, are still far from leading to successful tissue reconstruction in clinical settings, mainly due to the limited control exerted over biodegradation and drug delivery.288
Incorporation of GFs into preformed scaffolds has the advantage that the optimized conditions for scaffold processing are not substantially affected by the presence of proteins. Moreover, the biological activity of the protein can be preserved.43 However, typically, only small amounts of proteins can be attached and their release profile becomes unpredictable. Therefore, scaffold bioactivation is increasingly being accomplished through the incorporation of preformed GF-loaded delivery systems, such as polymeric particles instead of through direct incorporation, as demonstrated in Figure 4.43
FIG. 4.
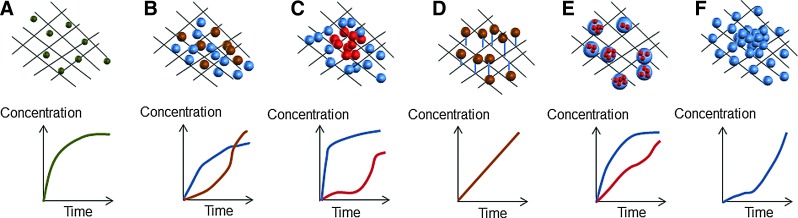
Strategies to promote the release of multiple bioactive factors from a matrix. Molecules can be physically dispersed inside the matrix by dispersion of the factor before the carrier processing or by impregnation postcarrier preparation (A). Typically, this leads to uncontrolled and fast drug delivery kinetics. One strategy to incorporate multiple bioactive signaling molecules into a matrix is developing hierarchical multiscale systems by the incorporation of nano- and microscale carriers into the constructs. The entrapment of the drugs in these carriers might offer an enhanced protection for degradation and high control over the delivery kinetics. These particulate carriers can be tailored to release bioactive factors in specific kinetics, allowing either a simultaneous (B) or sequential (C) presentation of signaling molecules. The spatiotemporal controlled release profiles can also be achieved through the conjugation of the drugs with ligands. Bioactive factors or particulate carriers can be covalently bound to the matrix, resulting in a more controlled mechanism of incorporation (D). Affinity-bound systems such as binding through heparin domains are a common approach. These mechanisms can be combined to design even more complex systems. Moreover, the development of multiscale systems is highly promising as nanoparticles can be incorporated inside microparticles within the constructs (E), promoting dual or multiple release systems with distinct delivery rates. The development of gradients of bioactive factors (F) is also an increasingly used approach. Besides promoting cell migration and inducing specific cell responses according to the concentration gradients, these systems allow a precise tailoring over the availability of the desired factor, following a biomimetic approach. Color images available online at www.liebertpub.com/teb
Microspheres/nanospheres have been widely used as tools for controlled drug delivery due to their small dimensions and the corresponding high surface area, high drug loading efficiency, high reactivity toward surrounding tissues, and high diffusibility and mobility.288 Moreover, their size allows them to respond quickly to environmental stimulus. Generally, microspheres can be processed into macroscopic constructs as (1) a dispersed phase surrounded by a continuous matrix289,290; or as (2) building blocks to establish integral scaffolds without a surrounding matrix by a bottom-up approach.288,291,292
When included in a scaffold, they can also act as reinforcement phase, providing higher mechanical strength and protecting the drug from in vivo degradation.288 Moreover, the incorporation of preformed delivery systems allows the combination of carriers with different release rates, entrapping different drugs, thus showing the potential for tailoring the availability of multiple signaling molecules at different preprogrammed rates (Fig. 4).288–290
Some of the advantages of entrapping preformed microparticulate delivery systems in TE constructs include the extra protection of the entrapped drugs to the physical and chemical adversities inherent to scaffold processing techniques and the fact that it avoids a new scaffold design for optimization of processing parameters.43 Delivery from scaffolds loaded with particulate carriers allows to retain the bioactive factor for an extended time, overcoming the disadvantages of the direct immobilization of GF in scaffolds that have poor control over release rates due to an open pore structure and exposure of the drug to the medium.43,293 When molded into 3D constructs, the drug delivery capacity of the carriers is coupled with the structural support provided by the scaffold. Although in these cases the release is mostly controlled by the properties of the carriers, the entrapment within the hydrogel/scaffold also influences the delivery.272
The integration of controlled release systems such as micro- and nanoparticles within scaffolds leads to the development of hierarchically organized and multifunctional constructs with enhanced ability to control and guide neotissue formation through the recapitulation of spatial and temporal microenvironments presented by the ECM.284,294 However, it should be noted that the addition of those carriers by themselves into the scaffolding structure might have a surprisingly large impact on the cell response and should be considered when designing these structures.295
Following a bottom-up approach, some studies have shown the possibility of designing highly defined geometries by the assembly of micro- and nanocarriers.296,297 The bottom-up approach for the generation of new materials has become increasingly attractive for developing novel engineering scaffolds with precise combination of cells, biomolecules, and synthetic biomaterials. These particles act as building blocks and can assemble by random packing, while simultaneously entrapping signaling biomolecules, bioactive minerals, or cells seeded on the surface.288 However, one drawback is their poor integrity resulting from weak particle interactions. To preserve the agglomeration of these formulations, glues and crosslinkers have been used or even multilayered films prepared by layer by layer of polyelectrolytes.298 Moreover, thermal fusion of the particles into integrated scaffolds has also been used as an alternative. Nanoparticles can also assemble by an electrostatic interaction between oppositely charged spheres—colloidal gels.299–301
Summary
There are several GFs and other bioactive molecules involved in the process of bone, cartilage, and osteochondral interface regeneration. These signaling molecules are presented in situ with a specific dosage, spatial distribution, and temporal sequence and TE strategies are increasingly trying to mimic these native healing cascades. As expected, this is not an easy task and mixed results have been obtained in this field. Part II of this review will report some of the most relevant studies on the use of single, dual, and multiple GF delivery in direct and indirect approaches for bone, cartilage, and osteochondral TE strategies.
Acknowledgments
The authors thank Fundação para a Ciência e Tecnologia for V.E.Santo's PhD grant (SFRH/BD/39486/2007). This work was carried out under the scope of the European FP7 Project Find and Bind (NMP4-SL-2009–229292) and Project MIT/ECE/0047/2009.
Disclosure Statement
No competing financial interests exist.
References
- 1.Dutta R.C. Dutta A.K. Comprehension of ECM-cell dynamics: a prerequisite for tissue regeneration. Biotechnol Adv. 2010;28:764. doi: 10.1016/j.biotechadv.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Barrère F. Mahmood T.A. de Groot K. van Blitterswijk C.A. Advanced biomaterials for skeletal tissue regeneration: instructive and smart functions. Mater Sci Eng R. 2008;59:38. [Google Scholar]
- 3.O'Keefe R.J. Mao J. Bone tissue engineering and regeneration: from discovery to the clinic-an overview. Tissue Eng Part B Rev. 2011;17:389. doi: 10.1089/ten.teb.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed T.A. Hincke M.T. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16:305. doi: 10.1089/ten.TEB.2009.0590. [DOI] [PubMed] [Google Scholar]
- 5.Keeney M. Pandit A. The osteochondral junction and its repair via bi-phasic tissue engineering scaffolds. Tissue Eng Part B Rev. 2009;15:55. doi: 10.1089/ten.teb.2008.0388. [DOI] [PubMed] [Google Scholar]
- 6.Yang P.J. Temenoff J.S. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009;15:127. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kon E. Delcogliano M. Filardo G. Fini M. Giavaresi G. Francioli S., et al. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J Orthop Res. 2010;28:116. doi: 10.1002/jor.20958. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S. Uludag H. Nanoparticulate systems for growth factor delivery. Pharm Res. 2009;26:1561. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 9.Salgado A.J. Coutinho O.P. Reis R.L. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 10.Muschler G.F. Raut V.P. Patterson T.E. Wenke J.C. Hollinger J.O. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2010;16:123. doi: 10.1089/ten.TEB.2009.0658. [DOI] [PubMed] [Google Scholar]
- 11.Wallace J.M. Chen Q. Fang M. Erickson B. Orr B.G. Banaszak Holl M.M. Type I collagen exists as a distribution of nanoscale morphologies in teeth, bones, and tendons. Langmuir. 2010;26:7349. doi: 10.1021/la100006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viguet-Carrin S. Garnero P. Delmas P. The role of collagen in bone strength. Osteoporos Int. 2006;17:319. doi: 10.1007/s00198-005-2035-9. [DOI] [PubMed] [Google Scholar]
- 13.Moro L. Romanello M. Favia A. Lamanna M.P. Lozupone E. Posttranslational modifications of bone collagen type I are related to the function of rat femoral regions. Calcif Tissue Int. 2000;66:151. doi: 10.1007/s002230010030. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y. Azaïs T. Robin M. Vallée A. Catania C. Legriel P., et al. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat Mater. 2012;11:724. doi: 10.1038/nmat3362. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira A.M. Gentile P. Chiono V. Ciardelli G. Collagen for bone tissue regeneration. Acta Biomaterialia. 2012;8:3191. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y. Kim Y.K. Dai L. Li N. Khan S.O. Pashley D.H., et al. Hierarchical and non-hierarchical mineralisation of collagen. Biomaterials. 2011;32:1291. doi: 10.1016/j.biomaterials.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter J.R. Ruckh T.T. Popat K.C. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25:1539. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 18.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 19.Galbraith C.G. Sheetz M.P. Cell traction. Curr Protoc Cell Biol. 2001;12:123. doi: 10.1002/0471143030.cb1203s00. [DOI] [PubMed] [Google Scholar]
- 20.Galbraith C.G. Yamada K.M. Sheetz M.P. The relationship between force and focal complex development. J Cell Biol. 2002;159:695. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheetz M.P. Felsenfeld D.P. Galbraith C.G. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998;8:51. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 22.Arnsdorf E.J. Tummala P. Kwon R.Y. Jacobs C.R. Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122:546. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos M.I. Reis R.L. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci. 2010;10:12. doi: 10.1002/mabi.200900107. [DOI] [PubMed] [Google Scholar]
- 24.Uthgenannt B.A. Kramer M.H. Hwu J.A. Wopenka B. Silva M.J. Skeletal self-repair: stress fracture healing by rapid formation and densification of woven bone. J Bone Miner Res. 2007;22:1548. doi: 10.1359/jbmr.0070614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelled G. Ben-Arav A. Hock C. Reynolds D.G. Yazici C. Zilberman Y., et al. Direct gene therapy for bone regeneration: gene delivery, animal models, and outcome measures. Tissue Eng Part B Rev. 2010;16:13. doi: 10.1089/ten.teb.2009.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alman B.A. Kelley S.P. Nam D. Heal thyself: using endogenous regeneration to repair bone. Tissue Eng Part B Rev. 2011;17:431. doi: 10.1089/ten.TEB.2011.0189. [DOI] [PubMed] [Google Scholar]
- 27.Ilizarov G.A. The principles of the Ilizarov method. Bull Hosp Jt Dis Orthop Inst. 1988;48:1. [PubMed] [Google Scholar]
- 28.Donos N. Kostopoulos L. Tonetti M. Karring T. Long-term stability of autogenous bone grafts following combined application with guided bone regeneration. Clin Oral Implant Res. 2005;16:133. doi: 10.1111/j.1600-0501.2004.01104.x. [DOI] [PubMed] [Google Scholar]
- 29.Cancedda R. Dozin B. Giannoni P. Quarto R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003;22:81. doi: 10.1016/s0945-053x(03)00012-x. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder J.E. Mosheiff R. Tissue engineering approaches for bone repair: concepts and evidence. Injury. 2011;42:609. doi: 10.1016/j.injury.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Salkeld S.L. Patron L.P. Barrack R.L. Cook S.D. The effect of osteogenic protein-1 on the healing of segmental bone defects treated with autograft or allograft bone. J Bone Joint Surg Am. 2001;83-A:803. doi: 10.2106/00004623-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Floyd T. Ohnmeiss D. A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J. 2000;9:398. doi: 10.1007/s005860000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane J.G. Tontz W.L. Ball S.T. Massie J.B. Chen A.C. Bae W.C., et al. A morphologic, biochemical, and biomechanical assessment of short-term effects of osteochondral autograft plug transfer in an animal model. Arthroscopy. 2001;17:856. doi: 10.1016/s0749-8063(01)90010-6. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler D.L. Enneking W.F. Allograft bone decreases in strength in vivo over time. Clin Orthop Rel Res. 2005;435:36. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 35.Aro H.T. Aho A.J. Clinical use of bone allografts. Ann Med. 1993;25:403. doi: 10.3109/07853899309147303. [DOI] [PubMed] [Google Scholar]
- 36.Kelly B.T. Potter H.G. Deng X.H. Pearle A.D. Turner A.S. Warren R.F., et al. Meniscal allograft transplantation in the sheep knee - Evaluation of chondroprotective effects. Am J Sports Med. 2006;34:1464. doi: 10.1177/0363546506287365. [DOI] [PubMed] [Google Scholar]
- 37.Homminga G.N. Bulstra S.K. Kuijer R. Vanderlinden A.J. Repair of sheep articular-cartilage defects with a rabbit costal perichondrial graft. Acta Orthop Scand. 1991;62:415. doi: 10.3109/17453679108996635. [DOI] [PubMed] [Google Scholar]
- 38.Poumarat G. Squire P. Comparison of mechanical properties of human, bovine bone and a new processed bone xenograft. Biomaterials. 1993;14:337. doi: 10.1016/0142-9612(93)90051-3. [DOI] [PubMed] [Google Scholar]
- 39.Shahgaldi B.F. Amis A.A. Heatley F.W. McDowell J. Bentley G. Repair of cartilage lesions using biological implants - a comparative histological and biomechanical study in goats. J Bone Joint Surg Br. 1991;73:57. doi: 10.1302/0301-620X.73B1.1991776. [DOI] [PubMed] [Google Scholar]
- 40.Sgaglione N.A. Florence A.S. Bone graft substitute plug failure with giant cell reaction in the treatment of osteochondral lesions of the distal femur: a report of 2 cases with operative revision. Arthroscopy. 2009;25:815. doi: 10.1016/j.arthro.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 41.Zdeblick T.A. Cooke M.E. Kunz D.N. Wilson D. McCabe R.P. Anterior cervical diskectomy and fusion using a porous hydroxyapatite bone grat substitute. Spine. 1994;19:2348. doi: 10.1097/00007632-199410150-00017. [DOI] [PubMed] [Google Scholar]
- 42.Walsh W.R. Chapman-Sheath P.J. Cain S. Debes J. Bruce WJM. Svehla M.J., et al. A resorbable porous ceramic composite bone graft substitute in a rabbit metaphyseal defect model. J Orthop Res. 2003;21:655. doi: 10.1016/S0736-0266(03)00012-3. [DOI] [PubMed] [Google Scholar]
- 43.Chen F.M. An Y. Zhang R. Zhang M. New insights into and novel applications of release technology for periodontal reconstructive therapies. J Control Release. 2011;149:92. doi: 10.1016/j.jconrel.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Hu W-W. Ward B.B. Wang Z. Krebsbach P.H. Bone regeneration in defects compromised by radiotherapy. J Dental Res. 2010;89:77. doi: 10.1177/0022034509352151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strube P. Sentuerk U. Riha T. Kaspar K. Mueller M. Kasper G., et al. Influence of age and mechanical stability on bone defect healing: age reverses mechanical effects. Bone. 2008;42:758. doi: 10.1016/j.bone.2007.12.223. [DOI] [PubMed] [Google Scholar]
- 46.Lane J.G. Healey R.M. Chen A.C. Sah R.L. Amiel D. Can osteochondral grafting be augmented with microfracture in an extended-size lesion of articular cartilage? Am J Sports Med. 2010;38:1316. doi: 10.1177/0363546510363433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laurie S.W. Kaban L.B. Mulliken J.B. Murray J.E. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73:933. doi: 10.1097/00006534-198406000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Nehrer S. Domayer S. Dorotka R. Schatz K. Bindreiter U. Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57:3. doi: 10.1016/j.ejrad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Hamlet W. Liu S.H. Yang R. Destruction of a cyropreserved meniscal allograft: a case for acute rejection. Arthroscopy. 1997;13:517. doi: 10.1016/s0749-8063(97)90135-3. [DOI] [PubMed] [Google Scholar]
- 50.Glassman S.D. Dimar J.R. Puno R.M. Johnson J.R. Salvage of instrumented lumbar fusions complicated by surgical wound infection. Spine. 1996;21:2163. doi: 10.1097/00007632-199609150-00021. [DOI] [PubMed] [Google Scholar]
- 51.Roychoudhury A. Parkash H. Trikha A. Functional restoration by gap arthroplasty in temporomandibular joint ankylosis: A report of 50 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:166. doi: 10.1016/s1079-2104(99)70267-2. [DOI] [PubMed] [Google Scholar]
- 52.David L.A. Sandor G.K. Evans A.W. Brown D.H. Hyperbaric oxygen therapy and mandibular osteoradionecrosis: a retrospective study and analysis of treatment outcomes. J Can Dent Assoc. 2001;67:384. [PubMed] [Google Scholar]
- 53.Drosse I. Volkmer E. Seitz S. Seitz H. Penzkofer R. Zahn K., et al. Validation of a femoral critical size defect model for orthotopic evaluation of bone healing: a biomechanical, veterinary and trauma surgical perspective. Tissue Eng Part C Methods. 2008;14:79. doi: 10.1089/tec.2007.0234. [DOI] [PubMed] [Google Scholar]
- 54.Perry C.R. Bone repair techniques, bone graft, and bone graft substitutes. Clin Orthop Relat Res. 1999;360:71. doi: 10.1097/00003086-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Aronson J. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J Bone Joint Surg Am. 1997;79:1243. doi: 10.2106/00004623-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 56.Reichert J.C. Epari D.R. Wullschleger M.E. Saifzadeh S. Steck R. Lienau J., et al. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng Part B Rev. 2010;16:93. doi: 10.1089/ten.TEB.2009.0455. [DOI] [PubMed] [Google Scholar]
- 57.Kempen D.H. Creemers L.B. Alblas J. Lu L. Verbout A.J. Yaszemski M.J., et al. Growth factor interactions in bone regeneration. Tissue Eng Part B Rev. 2010;16:551. doi: 10.1089/ten.teb.2010.0176. [DOI] [PubMed] [Google Scholar]
- 58.Dawson J.I. Oreffo R.O. Bridging the regeneration gap: stem cells, biomaterials and clinical translation in bone tissue engineering. Arch Biochem Biophys. 2008;473:124. doi: 10.1016/j.abb.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 59.Lenz A. Franklin G.A. Cheadle W.G. Systemic inflammation after trauma. Injury. 2007;38:1336. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15:53. doi: 10.22203/ecm.v015a05. [DOI] [PubMed] [Google Scholar]
- 61.Marzona L. Pavolini B. Play and players in bone fracture healing match. Clin Cases Miner Bone Metab. 2009;6:159. [PMC free article] [PubMed] [Google Scholar]
- 62.Raiche A.T. Puleo D.A. Cell responses to BMP-2 and IGF-I released with different time-dependent profiles. J Biomed Mater Res A. 2004;69:342. doi: 10.1002/jbm.a.30006. [DOI] [PubMed] [Google Scholar]
- 63.Bourque W.T. Gross M. Hall B.K. Expression of four growth factors during fracture repair. Int J Dev Biol. 1993;37:573. [PubMed] [Google Scholar]
- 64.Cho T.J. Gerstenfeld L.C. Einhorn T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 65.Cenni E. Perut F. Baldini N. In vitro models for the evaluation of angiogenic potential in bone engineering. Acta Pharmacol Sin. 2011;32:21. doi: 10.1038/aps.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mackie E.J. Ahmed Y.A. Tatarczuch L. Chen K.S. Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Ortega N. Behonick D.J. Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004;14:86. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mackie E.J. Tatarczuch L. Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011;211:109. doi: 10.1530/JOE-11-0048. [DOI] [PubMed] [Google Scholar]
- 69.Garrett I.R. Chen D. Gutierrez G. Zhao M. Escobedo A. Rossini G., et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lalani Z. Wong M. Brey E.M. Mikos A.G. Duke P.J. Spatial and temporal localization of transforming growth factor-β1, bone morphogenetic protein-2, and platelet-derived growth factor-A in healing tooth extraction sockets in a rabbit model. J Oral Maxillofac Surg. 2003;61:1061. doi: 10.1016/s0278-2391(03)00319-7. [DOI] [PubMed] [Google Scholar]
- 71.Harris S.E. Bonewald L.F. Harris M.A. Sabatini M. Dallas S. Feng J.Q., et al. Effects of transforming growth factor β on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res. 1994;9:855. doi: 10.1002/jbmr.5650090611. [DOI] [PubMed] [Google Scholar]
- 72.Hong L. Tabata Y. Miyamoto S. Yamada K. Aoyama I. Tamura M., et al. Promoted bone healing at a rabbit skull gap between autologous bone fragment and the surrounding intact bone with biodegradable microspheres containing transforming growth factor-beta1. Tissue Eng. 2000;6:331. doi: 10.1089/107632700418056. [DOI] [PubMed] [Google Scholar]
- 73.Mundy G.R. Chen D. Zhao M. Dallas S. Xu C. Harris S. Growth regulatory factors and bone. Rev Endocr Metab Disord. 2001;2:105. doi: 10.1023/a:1010015309973. [DOI] [PubMed] [Google Scholar]
- 74.Bennett J.H. Moffatt S. Horton M. Cell adhesion molecules in human osteoblasts: structure and function. Histol Histopathol. 2001;16:603. doi: 10.14670/HH-16.603. [DOI] [PubMed] [Google Scholar]
- 75.Chai Y.C. Roberts S.J. Desmet E. Kerckhofs G. van Gastel N. Geris L., et al. Mechanisms of ectopic bone formation by human osteoprogenitor cells on CaP biomaterial carriers. Biomaterials. 2012;33:3127. doi: 10.1016/j.biomaterials.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 76.Allori A.C. Sailon A.M. Pan J.H. Warren S.M. Biological basis of bone formation, remodeling, and repair-part III: biomechanical forces. Tissue Eng Part B Rev. 2008;14:285. doi: 10.1089/ten.teb.2008.0084. [DOI] [PubMed] [Google Scholar]
- 77.Maurer T. Zimmermann G. Maurer S. Stegmaier S. Wagner C. Hansch G.M. Inhibition of osteoclast generation: a novel function of the bone morphogenetic protein 7/osteogenic protein 1. Mediators Inflamm. 2012;2012:171209. doi: 10.1155/2012/171209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alliston T. Chang J. TGFβ and Runx2 calibration of bone extracellular matrix quality for tissue-specific function. IBMS BoneKEy. 2011;8:370. [Google Scholar]
- 79.Pitaru S. Kotev-Emeth S. Noff D. Kaffuler S. Savion N. Effect of basic fibroblast growth factor on the growth and differentiation of adult stromal bone marrow cells: Enhanced development of mineralized bone-like tissue in culture. J Bone Miner Res. 1993;8:919. doi: 10.1002/jbmr.5650080804. [DOI] [PubMed] [Google Scholar]
- 80.Komori T. Yagi H. Nomura S. Yamaguchi A. Sasaki K. Deguchi K., et al. Targeted disruption of cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 81.Kim Y-J. Kim H-N. Park E-K. Lee B-H. Ryoo H-M. Kim S-Y, et al. The bone-related Zn finger transcription factor Osterix promotes proliferation of mesenchymal cells. Gene. 2006;366:145. doi: 10.1016/j.gene.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 82.Suzawa M. Takeuchi Y. Fukumoto S. Kato S. Ueno N. Miyazono K., et al. Extracellular matrix-associated bone morphogenetic proteins are essential for differentiation of murine osteoblastic cells in vitro. Endocrinology. 1999;140:2125. doi: 10.1210/endo.140.5.6704. [DOI] [PubMed] [Google Scholar]
- 83.Einhorn T.A. Majeska R.J. Rush E.B. Levine P.M. Horowitz M.C. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- 84.Lamoureux F. Baud'huin M. Duplomb L. Heymann D. Rédini F. Proteoglycans: key partners in bone cell biology. Bio Essays. 2007;29:758. doi: 10.1002/bies.20612. [DOI] [PubMed] [Google Scholar]
- 85.Zhu J.X. Sasano Y. Takahashi I. Mizoguchi I. Kagayama M. Temporal and spatial gene expression of major bone extracellular matrix molecules during embryonic mandibular osteogenesis in rats. Histochem J. 2001;33:25. doi: 10.1023/a:1017587712914. [DOI] [PubMed] [Google Scholar]
- 86.Nordahl J. Mengarelliwidholm S. Hultenby K. Reinholt F.P. Ultrastructural immunolocalization of fibronectin in epiphyseal and metaphyseal bone of young rats. Calcif Tissue Int. 1995;57:442. doi: 10.1007/BF00301948. [DOI] [PubMed] [Google Scholar]
- 87.Takeuchi Y. Kodama Y. Matsumoto T. Bone matrix decorin binds transfoming growth factor beta and enhances its bioactivity. J Biol Chem. 1994;269:32634. [PubMed] [Google Scholar]
- 88.Chen X.D. Allen M.R. Bloomfield S. Xu T. Young M. Biglycan-deficient mice have delayed osteogenesis after marrow ablation. Calcif Tissue Int. 2003;72:577. doi: 10.1007/s00223-002-1101-y. [DOI] [PubMed] [Google Scholar]
- 89.Wang X. Shen X. Li X. Agrawal C.M. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 90.Horner A. Bord S. Kemp P. Grainger D. Compston J.E. Distribution of platelet-derived growth factor (PDGF) a chain mRNA, protein, and PDGF-alpha receptor in rapidly forming human bone. Bone. 1996;19:353. doi: 10.1016/s8756-3282(96)00217-7. [DOI] [PubMed] [Google Scholar]
- 91.Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 92.Yakar S. Rosen C.J. Beamer W.G. Ackert-Bicknell C.L. Wu Y.P. Liu J.L., et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Noda M. Camilliere J.J. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989;124:2991. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- 94.Toma C.D. Ashkar S. Gray M.L. Schaffer J.L. Gerstenfeld L.C. Signal transduction of mechanical stimuli is dependent on microfilament integrity: identification of osteopontin as a mechanically induced gene in osteoblasts. J Bone Miner Res. 1997;12:1626. doi: 10.1359/jbmr.1997.12.10.1626. [DOI] [PubMed] [Google Scholar]
- 95.McKee M.D. Nanci A. Osteopontin: an interfacial extracellular matrix protein in mineralized tissues. Connect Tissue Res. 1996;35:197. doi: 10.3109/03008209609029192. [DOI] [PubMed] [Google Scholar]
- 96.Baht G.S. Hunter G.K. Goldberg H.A. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008;27:600. doi: 10.1016/j.matbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 97.Zhu X. Eibl O. Scheideler L. Geis-Gerstorfer J. Characterization of nano hydroxyapatite/collagen surfaces and cellular behaviors. J Biomed Mater Res Part A. 2006;79A:114. doi: 10.1002/jbm.a.30706. [DOI] [PubMed] [Google Scholar]
- 98.Young M.F. Kerr J.M. Ibaraki K. Heegaard A.M. Robey P.G. Structure, Expression, and Regulation of the major noncollagenous matrix proteins of bone. Clin Orthop Rel Res. 1992;281:275. [PubMed] [Google Scholar]
- 99.Hunter G.K. Hauschka P.V. Poole A.R. Rosenberg L.C. Goldberg H.A. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317(Pt 1):59. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chenu C. Colucci S. Grano M. Zigrino P. Barattolo R. Zambonin G., et al. Osteocalcin induces chemotaxis, secretion of matrix proteins, and calcium-mediated intracellular signaling in human osteoclast-like cells. J Cell Biol. 1994;127:1149. doi: 10.1083/jcb.127.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nam D. Mau E. Wang Y. Wright D. Silkstone D. Whetstone H., et al. T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair. PLoS One. 2012;7:e40044. doi: 10.1371/journal.pone.0040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rundle C.H. Wang H. Yu H. Chadwick R.B. Davis E.I. Wergedal J.E., et al. Microarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repair. Bone. 2006;38:521. doi: 10.1016/j.bone.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 103.Rappolee D.A. Mark D. Banda M.J. Werb Z. Wound macrophages express TGF-α and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988;241:708. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- 104.Hanna I. Recent advances in mechanobiological modeling of bone regeneration. Mech Res Commun. 2012;42:22. [Google Scholar]
- 105.Kenneth J. Koval M.D. 1st. New York: Amer Acad of Orthopaedic Surgeons; 2002. Orthopaedic Knowledge Update 7: Home Study Syllabus. [Google Scholar]
- 106.Weber B. Emmert M.Y. Schoenauer R. Brokopp C. Baumgartner L. Hoerstrup S.P. Tissue engineering on matrix: future of autologous tissue replacement. Semin Immunopathol. 2011;33:307. doi: 10.1007/s00281-011-0258-8. [DOI] [PubMed] [Google Scholar]
- 107.Anderson J.M. Rodriguez A. Chang D.T. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bischoff D.S. Sakamoto T. Ishida K. Makhijani N.S. Gruber H.E. Yamaguchi D.T. CXC receptor knockout mice: Characterization of skeletal features and membranous bone healing in the adult mouse. Bone. 2011;48:267. doi: 10.1016/j.bone.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 109.Mountziaris P.M. Spicer P.P. Kasper F.K. Mikos A.G. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17:393. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mountziaris P.M. Mikos A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hankenson K.D. Dishowitz M. Gray C. Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42:556. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rhinelander F.W. Tibial blood supply in relation to fracture healing. Clin Orthop Relat Res. 1974;105:34. [PubMed] [Google Scholar]
- 113.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 114.Zelzer E. Olsen B.R. Multiple Roles of Vascular Endothelial Growth Factor (VEGF) in Skeletal Development, Growth, and Repair. Current Topics in Developmental Biology. 2005;65:169. doi: 10.1016/S0070-2153(04)65006-X. [DOI] [PubMed] [Google Scholar]
- 115.Lieu S. Hansen E. Dedini R. Behonick D. Werb Z. Miclau T., et al. Impaired remodeling phase of fracture repair in the absence of matrix metalloproteinase-2. Dis Models Mech. 2011;4:203. doi: 10.1242/dmm.006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang Y.C. Kaigler D. Rice K.G. Krebsbach P.H. Mooney D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 117.Chen Y-J. Wurtz T. Wang C-J. Kuo Y-R. Yang K.D. Huang H-C, et al. Recruitment of mesenchymal stem cells and expression of TGF-β1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J Orthop Res. 2004;22:526. doi: 10.1016/j.orthres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 118.Masters K.S. Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromol Biosci. 2011;11:1149. doi: 10.1002/mabi.201000505. [DOI] [PubMed] [Google Scholar]
- 119.Tabata Y. Regenerative inductive therapy based on DDS technology of protein and gene. J Drug Target. 2006;14:483. doi: 10.1080/10611860600844879. [DOI] [PubMed] [Google Scholar]
- 120.Peng H. Wright V. Usas A. Gearhart B. Shen H.C. Cummins J., et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holderfield M.T. Hughes C.C.W. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102:637. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 122.Matsumoto T. Mifune Y. Kawamoto A. Kuroda R. Shoji T. Iwasaki H., et al. Fracture induced mobilization and incorporation of bone marrow-derived endothelial progenitor cells for bone healing. J Cell Physiol. 2008;215:234. doi: 10.1002/jcp.21309. [DOI] [PubMed] [Google Scholar]
- 123.Lamalice L. Le Boeuf F. Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 124.Cao L. Mooney D.J. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv Drug Deliv Rev. 2007;59:1340. doi: 10.1016/j.addr.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Freeman I. Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 126.Santos M.I. Unger R.E. Sousa R.A. Reis R.L. Kirkpatrick C.J. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone–starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30:4407. doi: 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 127.Buschmann J. Welti M. Hemmi S. Neuenschwander P. Baltes C. Giovanoli P., et al. Three-dimensional co-cultures of osteoblasts and endothelial cells in DegraPol foam: histological and high-field magnetic resonance imaging analyses of pre-engineered capillary networks in bone grafts. Tissue Eng Part A. 2011;17:291. doi: 10.1089/ten.TEA.2010.0278. [DOI] [PubMed] [Google Scholar]
- 128.Gerwin N. Hops C. Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58:226. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 129.Maroudas A. Bayliss M.T. Venn M.F. Further studies on the composition of human femoral head cartilage. Ann Rheum Dis. 1980;39:514. doi: 10.1136/ard.39.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Steward A. Liu Y. Wagner D. Engineering cell attachments to scaffolds in cartilage tissue engineering. JOM. 2011;63:74. [Google Scholar]
- 131.Klein T.J. Malda J. Sah R.L. Hutmacher D.W. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev. 2009;15:143. doi: 10.1089/ten.teb.2008.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keeney M. Lai J.H. Yang F. Recent progress in cartilage tissue engineering. Curr Opin Biotechnol. 2011;22:734. doi: 10.1016/j.copbio.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 133.Kim T.K. Sharma B. Williams C.G. Ruffner M.A. Malik A. McFarland E.G., et al. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11:653. doi: 10.1016/s1063-4584(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 134.Sharma B. Williams C.G. Kim T.K. Sun D.N. Malik A. Khan M., et al. Designing zonal organization into tissue-engineered cartilage. Tissue Eng. 2007;13:405. doi: 10.1089/ten.2006.0068. [DOI] [PubMed] [Google Scholar]
- 135.Roughley P.J. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- 136.Horkay F. Basser P.J. Hecht A.M. Geissler E. Gel-like behavior in aggrecan assemblies. J Chem Phys. 2008;128:135103. doi: 10.1063/1.2884350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roughley P.J. White R.J. Mort J.S. Presence of pro-forms of decorin and biglycan in human articular cartilage. Biochem J. 1996;318:779. doi: 10.1042/bj3180779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hedlund H. Mengarelliwidholm S. Heinegard D. Reinholt F.P. Svensson O. Fibromodulin distribution and association with collagen. Matrix Biol. 1994;14:227. doi: 10.1016/0945-053x(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 139.Grover J. Chen X.N. Korenberg J.R. Roughley P.J. The human lumican gene - organization, chromosomal, location and expression in articular cartilage. J Biol Chem. 1995;270:21942. doi: 10.1074/jbc.270.37.21942. [DOI] [PubMed] [Google Scholar]
- 140.Hendriks J. Riesle J. van Blitterswijk C.A. Co-culture in cartilage tissue engineering. J Tissue Eng Regen Med. 2007;1:170. doi: 10.1002/term.19. [DOI] [PubMed] [Google Scholar]
- 141.Revell C.M. Athanasiou K.A. Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Eng Part B Rev. 2009;15:1. doi: 10.1089/ten.teb.2008.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morales T.I. Transforming growth factor-beta 1 stimulates synthesis of proteoglycan aggregates in calf articular cartilage organ cultures. Arch Biochem Biophys. 1991;286:99. doi: 10.1016/0003-9861(91)90013-9. [DOI] [PubMed] [Google Scholar]
- 143.van Beuningen H.M. van der Kraan P.M. Arntz O.J. van den Berg W.B. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: age-related differences. Ann Rheum Dis. 1994;53:593. doi: 10.1136/ard.53.9.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Glansbeek H.L. van Beuningen H.M. Vitters E.L. Morris E.A. van der Kraan P.M. van den Berg W.B. Bone morphogenetic protein 2 stimulates articular cartilage proteoglycan synthesis in vivo but does not counteract interleukin-1alpha effects on proteoglycan synthesis and content. Arthritis Rheum. 1997;40:1020. doi: 10.1002/art.1780400605. [DOI] [PubMed] [Google Scholar]
- 145.Glansbeek H.L. van Beuningen H.M. Vitters E.L. van der Kraan P.M. van den Berg W.B. Stimulation of articular cartilage repair in established arthritis by local administration of transforming growth factor-beta into murine knee joints. Lab Invest. 1998;78:133. [PubMed] [Google Scholar]
- 146.Martin I. Vunjak-Novakovic G. Yang J. Langer R. Freed L.E. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue. Exp Cell Res. 1999;253:681. doi: 10.1006/excr.1999.4708. [DOI] [PubMed] [Google Scholar]
- 147.Fortier L.A. Barker J.U. Strauss E.J. McCarrel T.M. Cole B.J. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goldring M.B. Tsuchimochi K. Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 149.Bhakta N.R. Garcia A.M. Frank E.H. Grodzinsky A.J. Morales T.I. The insulin-like growth factors (IGFs) I and II bind to articular cartilage via the IGF-binding proteins. J Biol Chem. 2000;275:5860. doi: 10.1074/jbc.275.8.5860. [DOI] [PubMed] [Google Scholar]
- 150.Becerra J. Andrades J.A. Guerado E. Zamora-Navas P. Lopez-Puertas J.M. Reddi A.H. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 151.Coates E. Fisher J.P. Gene expression of alginate-embedded chondrocyte subpopulations and their response to exogenous IGF-1 delivery. J Tissue Eng Regen Med. 2011;6:179. doi: 10.1002/term.411. [DOI] [PubMed] [Google Scholar]
- 152.Sandell L.J. Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martin J.A. Brown T. Heiner A. Buckwalter J.A. Post-traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology. 2004;41:479. [PubMed] [Google Scholar]
- 154.Mano J.F. Reis R.L. Osteochondral defects: present situation and tissue engineering approaches. J Tissue Eng Regen Med. 2007;1:261. doi: 10.1002/term.37. [DOI] [PubMed] [Google Scholar]
- 155.Akizuki S. Yasukawa Y. Takizawa T. A new method of hemostasis for cementless total knee arthroplasty. Bull Hosp Jt Dis. 1997;56:222. [PubMed] [Google Scholar]
- 156.Singh S. Lee C.C. Tay B.K. Results of arthroscopic abrasion arthroplasty in osteoarthritis of the knee joint. Singapore Med J. 1991;32:34. [PubMed] [Google Scholar]
- 157.Beiser I.H. Kanat I.O. Subchondral bone drilling: a treatment for cartilage defects. J Foot Surg. 1990;29:595. [PubMed] [Google Scholar]
- 158.Sledge S.L. Microfracture techniques in the treatment of osteochondral injuries. Clin Sports Med. 2001;20:365. doi: 10.1016/s0278-5919(05)70311-2. [DOI] [PubMed] [Google Scholar]
- 159.O'Driscoll S.W. Keeley F.W. Salter R.B. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68:1017. [PubMed] [Google Scholar]
- 160.O'Driscoll S.W. Salter R.B. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin Orthop Relat Res. 1986;208:131. [PubMed] [Google Scholar]
- 161.Peterson L. Minas T. Brittberg M. Nilsson A. Sjogren-Jansson E. Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 162.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 163.Chen A.C. Nagrampa J.P. Schinagl R.M. Lottman L.M. Sah R.L. Chondrocyte transplantation to articular cartilage explants in vitro. J Orthop Res. 1997;15:791. doi: 10.1002/jor.1100150602. [DOI] [PubMed] [Google Scholar]
- 164.Bedi A. Feeley B.T. Williams R.J., 3rd. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 165.Frisbie D.D. Trotter G.W. Powers B.E. Rodkey W.G. Steadman J.R. Howard R.D., et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28:242. doi: 10.1053/jvet.1999.0242. [DOI] [PubMed] [Google Scholar]
- 166.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 167.Chow J.C. Hantes M.E. Houle J.B. Zalavras C.G. Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2- to 5-year follow-up study. Arthroscopy. 2004;20:681. doi: 10.1016/j.arthro.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 168.Hangody L. Feczko P. Bartha L. Bodo G. Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res. 2001;391(Suppl):S328. doi: 10.1097/00003086-200110001-00030. [DOI] [PubMed] [Google Scholar]
- 169.Hangody L. Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(Suppl 2):25. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 170.Hangody L. Kish G. Karpati Z. Szerb I. Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:262. doi: 10.1007/s001670050061. [DOI] [PubMed] [Google Scholar]
- 171.Bi X. Yang X. Bostrom M.P. Camacho N.P. Fourier transform infrared imaging spectroscopy investigations in the pathogenesis and repair of cartilage. Biochim Biophys Acta. 2006;1758:934. doi: 10.1016/j.bbamem.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 172.David-Vaudey E. Burghardt A. Keshari K. Brouchet A. Ries M. Majumdar S. Fourier Transform Infrared Imaging of focal lesions in human osteoarthritic cartilage. Eur Cell Mater. 2005;10:51. doi: 10.22203/ecm.v010a06. discussion 60. [DOI] [PubMed] [Google Scholar]
- 173.Goldring M.B. Marcu K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fong E.L. Watson B.M. Kasper F.K. Mikos A.G. Building bridges: leveraging interdisciplinary collaborations in the development of biomaterials to meet clinical needs. Adv Mater. 2012;24:4995. doi: 10.1002/adma.201201762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Elder B.D. Athanasiou K.A. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Grayson W.L. Martens T.P. Eng G.M. Radisic M. Vunjak-Novakovic G. Biomimetic approach to tissue engineering. Semin Cell Dev Biol. 2009;20:665. doi: 10.1016/j.semcdb.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Khanarian N.T. Jiang J. Wan L.Q. Mow V.C. Lu H.H. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Part A. 2012;18:533. doi: 10.1089/ten.tea.2011.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Castro N.J. Hacking S.A. Zhang L.G. Recent progress in interfacial tissue engineering approaches for osteochondral defects. Ann Biomed Eng. 2012;40:1628. doi: 10.1007/s10439-012-0605-5. [DOI] [PubMed] [Google Scholar]
- 179.O'Shea T.M. Miao X. Bilayered scaffolds for osteochondral tissue engineering. Tissue Eng Part B Rev. 2008;14:447. doi: 10.1089/ten.teb.2008.0327. [DOI] [PubMed] [Google Scholar]
- 180.Madry H. van Dijk C.N. Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18:419. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- 181.Jackson D.W. Scheer M.J. Simon T.M. Cartilage substitutes: overview of basic science and treatment options. J Am Acad Orthop Surg. 2001;9:37. doi: 10.5435/00124635-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 182.Redman S.N. Oldfield S.F. Archer C.W. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23. doi: 10.22203/ecm.v009a04. discussion 23. [DOI] [PubMed] [Google Scholar]
- 183.Jiang J. Tang A. Ateshian G.A. Guo X.E. Hung C.T. Lu H.H. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng. 2010;38:2183. doi: 10.1007/s10439-010-0038-y. [DOI] [PubMed] [Google Scholar]
- 184.Lu H.H. Subramony S.D. Boushell M.K. Zhang X. Tissue engineering strategies for the regeneration of orthopedic interfaces. Ann Biomed Eng. 2010;38:2142. doi: 10.1007/s10439-010-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alhadlaq A. Mao J.J. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951. doi: 10.1177/154405910308201203. [DOI] [PubMed] [Google Scholar]
- 186.Alhadlaq A. Mao J.J. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87:936. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 187.Allan K.S. Pilliar R.M. Wang J. Grynpas M.D. Kandel R.A. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 2007;13:167. doi: 10.1089/ten.2006.0081. [DOI] [PubMed] [Google Scholar]
- 188.Angele P. Kujat R. Nerlich M. Yoo J. Goldberg V. Johnstone B. Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng. 1999;5:545. doi: 10.1089/ten.1999.5.545. [DOI] [PubMed] [Google Scholar]
- 189.Cao T. Ho K.H. Teoh S.H. Scaffold design and in vitro study of osteochondral coculture in a three-dimensional porous polycaprolactone scaffold fabricated by fused deposition modeling. Tissue Eng. 2003;9(Suppl 1):S103. doi: 10.1089/10763270360697012. [DOI] [PubMed] [Google Scholar]
- 190.Elisseeff J. Puleo C. Yang F. Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res. 2005;8:150. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 191.Frenkel S.R. Bradica G. Brekke J.H. Goldman S.M. Ieska K. Issack P., et al. Regeneration of articular cartilage—evaluation of osteochondral defect repair in the rabbit using multiphasic implants. Osteoarthritis Cartilage. 2005;13:798. doi: 10.1016/j.joca.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 192.Gao J. Dennis J.E. Solchaga L.A. Awadallah A.S. Goldberg V.M. Caplan A.I. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001;7:363. doi: 10.1089/10763270152436427. [DOI] [PubMed] [Google Scholar]
- 193.Gao J. Dennis J.E. Solchaga L.A. Goldberg V.M. Caplan A.I. Repair of osteochondral defect with tissue-engineered two-phase composite material of injectable calcium phosphate and hyaluronan sponge. Tissue Eng. 2002;8:827. doi: 10.1089/10763270260424187. [DOI] [PubMed] [Google Scholar]
- 194.Harley B.A. Lynn A.K. Wissner-Gross Z. Bonfield W. Yannas I.V. Gibson L.J. Design of a multiphase osteochondral scaffold III: Fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res A. 2010;92:1078. doi: 10.1002/jbm.a.32387. [DOI] [PubMed] [Google Scholar]
- 195.Holland T.A. Bodde E.W. Baggett L.S. Tabata Y. Mikos A.G. Jansen J.A. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75:156. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 196.Hung C.T. Lima E.G. Mauck R.L. Takai E. LeRoux M.A. Lu H.H., et al. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 197.Chen J. Chen H. Li P. Diao H. Zhu S. Dong L., et al. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials. 2011;32:4793. doi: 10.1016/j.biomaterials.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 198.Nguyen L.H. Kudva A.K. Saxena N.S. Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32:6946. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 199.Dormer N.H. Singh M. Zhao L. Mohan N. Berkland C.J. Detamore M.S. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A. 2012;100:162. doi: 10.1002/jbm.a.33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mohan N. Dormer N.H. Caldwell K.L. Key V.H. Berkland C.J. Detamore M.S. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng Part A. 2011;17:2845. doi: 10.1089/ten.tea.2011.0135. [DOI] [PubMed] [Google Scholar]
- 201.Rodrigues M.T. Gomes M.E. Reis R.L. Current strategies for osteochondral regeneration: from stem cells to pre-clinical approaches. Curr Opin Biotechnol. 2011;22:726. doi: 10.1016/j.copbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 202.Kon E. Delcogliano M. Filardo G. Pressato D. Busacca M. Grigolo B., et al. A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury. 2010;41:693. doi: 10.1016/j.injury.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 203.Liu C. Han Z. Czernuszka J.T. Gradient collagen/nanohydroxyapatite composite scaffold: development and characterization. Acta Biomater. 2009;5:661. doi: 10.1016/j.actbio.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 204.Tampieri A. Sandri M. Landi E. Pressato D. Francioli S. Quarto R., et al. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials. 2008;29:3539. doi: 10.1016/j.biomaterials.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 205.Wahl D.A. Sachlos E. Liu C. Czernuszka J.T. Controlling the processing of collagen-hydroxyapatite scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2007;18:201. doi: 10.1007/s10856-006-0682-9. [DOI] [PubMed] [Google Scholar]
- 206.Khanarian N.T. Haney N.M. Burga R.A. Lu H.H. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials. 2012;33:5247. doi: 10.1016/j.biomaterials.2012.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bessa P.C. Casal M. Reis R.L. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 208.Kagiwada H. Yashiki T. Ohshima A. Tadokoro M. Nagaya N. Ohgushi H. Human mesenchymal stem cells as a stable source of VEGF-producing cells. J Tissue Eng Regen Med. 2008;2:184. doi: 10.1002/term.79. [DOI] [PubMed] [Google Scholar]
- 209.Xiao C. Zhou H. Liu G. Zhang P. Fu Y. Gu P., et al. Bone marrow stromal cells with a combined expression of BMP-2 and VEGF-165 enhanced bone regeneration. Biomed Mater. 2011;6:015013. doi: 10.1088/1748-6041/6/1/015013. [DOI] [PubMed] [Google Scholar]
- 210.Kofron M.D. Li X. Laurencin C.T. Protein- and gene-based tissue engineering in bone repair. Curr Opin Biotechnol. 2004;15:399. doi: 10.1016/j.copbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 211.Hoch A.I. Binder B.Y. Genetos D.C. Leach J.K. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7:e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Groeneveld EHJ. Burger E.H. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol. 2000;142:9. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- 213.Du X.L. Xie Y.L. Xian C.J. Chen L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J Cell Physiol. 2012;227:3731. doi: 10.1002/jcp.24083. [DOI] [PubMed] [Google Scholar]
- 214.Koch H. Jadlowiec J.A. Campbell P.G. Insulin-like growth factor-I induces early osteoblast gene expression in human mesenchymal stem cells. Stem Cells Dev. 2005;14:621. doi: 10.1089/scd.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 215.Hong L. Tabata Y. Miyamoto S. Yamamoto M. Yamada K. Hashimoto N., et al. Bone regeneration at rabbit skull defects treated with transforming growth factor-beta 1 incorporated into hydrogels with different levels of biodegradability. J Neurosurg. 2000;92:315. doi: 10.3171/jns.2000.92.2.0315. [DOI] [PubMed] [Google Scholar]
- 216.Yu X.H. Hsieh S.C. Bao W. Graves D.T. Temporal expression of PDGF receptors and PDGF regulatory effects on osteoblastic cells in mineralizing cultures. Am J Physiol Cell Physiol. 1997;272:C1709. doi: 10.1152/ajpcell.1997.272.5.C1709. [DOI] [PubMed] [Google Scholar]
- 217.Mayr-Wohlfart U. Waltenberger J. Hausser H. Kessler S. Gunther K.P. Dehio C., et al. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 218.Fischer J. Kolk A. Wolfart S. Pautke C. Warnke P.H. Plank C., et al. Future of local bone regeneration - Protein versus gene therapy. J Craniomaxillofac Surg. 2011;39:54. doi: 10.1016/j.jcms.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 219.Chen F.M. Zhang M. Wu Z.F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 220.Dimitriou R. Tsiridis E. Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 221.Bose S. Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2011;8:1401. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lieberman J.R. Daluiski A. Einhorn T.A. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 223.Allori A.C. Sailon A.M. Warren S.M. Biological basis of bone formation, remodeling, and repair-part I: biochemical signaling molecules. Tissue Eng Part B Rev. 2008;14:259. doi: 10.1089/ten.teb.2008.0082. [DOI] [PubMed] [Google Scholar]
- 224.Bessa P.C. Casal M. Reis R.L. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 225.Kaully T. Kaufman-Francis K. Lesman A. Levenberg S. Vascularization—the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 226.Kock L. van Donkelaar C.C. Ito K. Tissue engineering of functional articular cartilage: the current status. Cell Tissue Res. 2011;347:613. doi: 10.1007/s00441-011-1243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Quintana L. zur Nieden N.I. Semino C.E. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng Part B Rev. 2009;15:29. doi: 10.1089/ten.teb.2008.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fan H. Hu Y. Qin L. Li X. Wu H. Lv R. Porous gelatin-chondroitin-hyaluronate tri-copolymer scaffold containing microspheres loaded with TGF-beta1 induces differentiation of mesenchymal stem cells in vivo for enhancing cartilage repair. J Biomed Mater Res A. 2006;77:785. doi: 10.1002/jbm.a.30647. [DOI] [PubMed] [Google Scholar]
- 229.Bian L. Zhai D.Y. Tous E. Rai R. Mauck R.L. Burdick J.A. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mullen L.M. Best S.M. Brooks R.A. Ghose S. Gwynne J.H. Wardale J., et al. Binding and release characteristics of insulin-like growth factor-1 from a collagen-glycosaminoglycan scaffold. Tissue Eng Part C Methods. 2010;16:1439. doi: 10.1089/ten.tec.2009.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kodama N. Nagata M. Tabata Y. Ozeki M. Ninomiya T. Takagi R. A local bone anabolic effect of rhFGF2-impregnated gelatin hydrogel by promoting cell proliferation and coordinating osteoblastic differentiation. Bone. 2009;44:699. doi: 10.1016/j.bone.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 232.Meinel L. Zoidis E. Zapf J. Hassa P. Hottiger M.O. Auer J.A., et al. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33:660. doi: 10.1016/s8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 233.Bhattarai N. Gunn J. Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62:83. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 234.Marsell R. Einhorn T.A. The biology of fracture healing. Injury. 2011;42:551. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gerstenfeld L.C. Cullinane D.M. Barnes G.L. Graves D.T. Einhorn T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 236.Lyons J.M. Schwimer J.E. Anthony C.T. Thomson J.L. Cundiff J.D. Casey D.T., et al. The role of VEGF pathways in human physiologic and pathologic angiogenesis. J Surg Res. 2010;159:517. doi: 10.1016/j.jss.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 237.Friesel R.E. Maciag T. Molecular mechanisms of angiogenesis - fibroblast growth factor signal transduction. Faseb J. 1995;9:919. doi: 10.1096/fasebj.9.10.7542215. [DOI] [PubMed] [Google Scholar]
- 238.Battegay E.J. Rupp J. Iruelaarispe L. Sage E.H. Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125:917. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kobayashi K. Kondo T. Inoue N. Aoki M. Mizuno M. Komori K., et al. Combination of in vivo angiopoietin-1 gene transfer and autologous bone marrow cell implantation for functional therapeutic angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1465. doi: 10.1161/01.ATV.0000223865.64812.26. [DOI] [PubMed] [Google Scholar]
- 240.Felcht M. Luck R. Schering A. Seidel P. Srivastava K. Hu J.H., et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122:1991. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shigematsu S. Yamauchi K. Nakajima K. Iijima S. Aizawa T. Hashizume K. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocrine J. 1999;46(Suppl):S59. doi: 10.1507/endocrj.46.suppl_s59. [DOI] [PubMed] [Google Scholar]
- 242.Kempen D.H. Lu L. Heijink A. Hefferan T.E. Creemers L.B. Maran A., et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 243.Kanczler J.M. Ginty P.J. White L. Clarke N.M. Howdle S.M. Shakesheff K.M., et al. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31:1242. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 244.Senta H. Park H. Bergeron E. Drevelle O. Fong D. Leblanc E., et al. Cell responses to bone morphogenetic proteins and peptides derived from them: biomedical applications and limitations. Cytokine Growth Factor Rev. 2009;20:213. doi: 10.1016/j.cytogfr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 245.Nauth A. Ristevski B. Li R. Schemitsch E.H. Growth factors and bone regeneration: how much bone can we expect? Injury. 2011;42:574. doi: 10.1016/j.injury.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 246.Nair M.B. Kretlow J.D. Mikos A.G. Kasper F.K. Infection and tissue engineering in segmental bone defects—a mini review. Curr Opin Biotechnol. 2011;22:721. doi: 10.1016/j.copbio.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Haidar Z.S. Hamdy R.C. Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009;31:1825. doi: 10.1007/s10529-009-0100-8. [DOI] [PubMed] [Google Scholar]
- 248.Gautschi O.P. Frey S.P. Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 249.Shields L.B. Raque G.H. Glassman S.D. Campbell M. Vitaz T. Harpring J., et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 2006;31:542. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 250.Alonso N. Tanikawa D.Y. Freitas Rda S. Canan L., Jr. Ozawa T.O. Rocha D.L. Evaluation of maxillary alveolar reconstruction using a resorbable collagen sponge with recombinant human bone morphogenetic protein-2 in cleft lip and palate patients. Tissue Eng Part C Methods. 2010;16:1183. doi: 10.1089/ten.TEC.2009.0824. [DOI] [PubMed] [Google Scholar]
- 251.Zara J.N. Siu R.K. Zhang X. Shen J. Ngo R. Lee M., et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011;17:1389. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Glass G.E. Chan J.K. Freidin A. Feldmann M. Horwood N.J. Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bae J.H. Song H.R. Kim H.J. Lim H.C. Park J.H. Liu Y., et al. Discontinuous release of bone morphogenetic protein-2 loaded within interconnected pores of honeycomb-like polycaprolactone scaffold promotes bone healing in a large bone defect of rabbit ulna. Tissue Eng Part A. 2011;17:2389. doi: 10.1089/ten.tea.2011.0032. [DOI] [PubMed] [Google Scholar]
- 254.Brown K.V. Li B. Guda T. Perrien D.S. Guelcher S.A. Wenke J.C. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue Eng Part A. 2011;17:1735. doi: 10.1089/ten.TEA.2010.0446. [DOI] [PubMed] [Google Scholar]
- 255.Boerckel J.D. Kolambkar Y.M. Dupont K.M. Uhrig B.A. Phelps E.A. Stevens H.Y., et al. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011;32:5241. doi: 10.1016/j.biomaterials.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kempen D.H. Lu L. Hefferan T.E. Creemers L.B. Maran A. Classic K.L., et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tachi K. Takami M. Sato H. Mochizuki A. Zhao B. Miyamoto Y., et al. Enhancement of bone morphogenetic protein-2-induced ectopic bone formation by transforming growth factor-beta1. Tissue Eng Part A. 2011;17:597. doi: 10.1089/ten.TEA.2010.0094. [DOI] [PubMed] [Google Scholar]
- 258.Maegawa N. Kawamura K. Hirose M. Yajima H. Takakura Y. Ohgushi H. Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2) J Tissue Eng Regen Med. 2007;1:306. doi: 10.1002/term.41. [DOI] [PubMed] [Google Scholar]
- 259.Hughes-Fulford M. Li C.F. The role of FGF-2 and BMP-2 in regulation of gene induction, cell proliferation and mineralization. J Orthop Surg Res. 2011;6:8. doi: 10.1186/1749-799X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Higashino K. Viggeswarapu M. Bargouti M. Liu H. Titus L. Boden S.D. Stromal cell-derived factor-1 potentiates bone morphogenetic protein-2 induced bone formation. Tissue Eng Part A. 2011;17:523. doi: 10.1089/ten.tea.2010.0168. [DOI] [PubMed] [Google Scholar]
- 261.Vinatier C. Mrugala D. Jorgensen C. Guicheux J. Noël D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27:307. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 262.Bos P.K. van Osch G.J. Frenz D.A. Verhaar J.A. Verwoerd-Verhoef H.L. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthritis Cartilage. 2001;9:382. doi: 10.1053/joca.2000.0399. [DOI] [PubMed] [Google Scholar]
- 263.Freyria A.M. Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury. 2012;43:259. doi: 10.1016/j.injury.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 264.Brochhausen C. Lehmann M. Halstenberg S. Meurer A. Klaus G. Kirkpatrick C.J. Signalling molecules and growth factors for tissue engineering of cartilage-what can we learn from the growth plate? J Tissue Eng Regen Med. 2009;3:416. doi: 10.1002/term.192. [DOI] [PubMed] [Google Scholar]
- 265.Redini F. Galera P. Mauviel A. Loyau G. Pujol J.P. Transforming growth factor β stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett. 1988;234:172. doi: 10.1016/0014-5793(88)81327-9. [DOI] [PubMed] [Google Scholar]
- 266.Tsukazaki T. Usa T. Matsumoto T. Enomoto H. Ohtsuru A. Namba H., et al. Effect of transforming growth factor-beta on the insulin-like growth factor-I autocrine/paracrine axis in cultured rat articular chondrocytes. Exp Cell Res. 1994;215:9. doi: 10.1006/excr.1994.1307. [DOI] [PubMed] [Google Scholar]
- 267.van Beuningen H.M. Glansbeek H.L. van der Kraan P.M. van den Berg W.B. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage. 2000;8:25. doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- 268.Edwards S.H. Intra-articular drug delivery: the challenge to extend drug residence time within the joint. Vet J. 2011;190:15. doi: 10.1016/j.tvjl.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 269.Pesesse L. Sanchez C. Henrotin Y. Osteochondral plate angiogenesis: a new treatment target in osteoarthritis. Joint Bone Spine. 2011;78:144. doi: 10.1016/j.jbspin.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 270.Wang Q.Y. Dai J. Kuang B. Zhang J. Yu S.B. Duan Y.Z., et al. Osteochondral angiogenesis in rat mandibular condyles with osteoarthritis-like changes. Arch Oral Biol. 2012;57:620. doi: 10.1016/j.archoralbio.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 271.Panseri S. Russo A. Cunha C. Bondi A. Di Martino A. Patella S., et al. Osteochondral tissue engineering approaches for articular cartilage and subchondral bone regeneration. Knee Surg Sports Traumatol Arthrosc. 2012;20:1182. doi: 10.1007/s00167-011-1655-1. [DOI] [PubMed] [Google Scholar]
- 272.Salvay D.M. Shea L.D. Inductive tissue engineering with protein and DNA-releasing scaffolds. Mol Biosyst. 2006;2:36. doi: 10.1039/b514174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ripamonti U. Ferretti C. Heliotis M. Soluble and insoluble signals and the induction of bone formation: molecular therapeutics recapitulating development. J Anat. 2006;209:447. doi: 10.1111/j.1469-7580.2006.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Luvizuto E.R. Tangl S. Zanoni G. Okamoto T. Sonoda C.K. Gruber R., et al. The effect of BMP-2 on the osteoconductive properties of beta-tricalcium phosphate in rat calvaria defects. Biomaterials. 2011;32:3855. doi: 10.1016/j.biomaterials.2011.01.076. [DOI] [PubMed] [Google Scholar]
- 275.Cinar G. Ceylan H. Urel M. Erkal T.S. Deniz Tekin E. Tekinay A.B., et al. Amyloid inspired self-assembled Peptide nanofibers. Biomacromolecules. 2012;13:3377. doi: 10.1021/bm301141h. [DOI] [PubMed] [Google Scholar]
- 276.Shalumon K.T. Chennazhi K.P. Tamura H. Kawahara K. Nair S.V. Jayakumar R. Fabrication of three-dimensional nano, micro and micro/nano scaffolds of porous poly(lactic acid) by electrospinning and comparison of cell infiltration by Z-stacking/three-dimensional projection technique. IET Nanobiotechnol. 2012;6:16. doi: 10.1049/iet-nbt.2011.0028. [DOI] [PubMed] [Google Scholar]
- 277.Matson J.B. Stupp S.I. Self-assembling peptide scaffolds for regenerative medicine. Chem Commun (Camb) 2012;48:26. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chen Z. Li Z. Lin Y. Yin M. Ren J. Qu X. Biomineralization inspired surface engineering of nanocarriers for pH-responsive, targeted drug delivery. Biomaterials. 2012;34:1364. doi: 10.1016/j.biomaterials.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 279.Bae S.E. Choi J. Joung Y.K. Park K. Han D.K. Controlled release of bone morphogenetic protein (BMP)-2 from nanocomplex incorporated on hydroxyapatite-formed titanium surface. J Control Release. 2012;160:676. doi: 10.1016/j.jconrel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 280.Lee S.C. Kwon I.K. Park K. Hydrogels for delivery of bioactive agents: a historical perspective. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.07.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lienemann P.S. Lutolf M.P. Ehrbar M. Biomimetic hydrogels for controlled biomolecule delivery to augment bone regeneration. Adv Drug Deliv Rev. 2012;64:1078. doi: 10.1016/j.addr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 282.Fan H. Vitharana S.N. Chen T. O'Keefe D. Middaugh C.R. Effects of pH and polyanions on the thermal stability of fibroblast growth factor 20. Mol Pharm. 2007;4:232. doi: 10.1021/mp060097h. [DOI] [PubMed] [Google Scholar]
- 283.Sands R.W. Mooney D.J. Polymers to direct cell fate by controlling the microenvironment. Curr Opin Biotechnol. 2007;18:448. doi: 10.1016/j.copbio.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Biondi M. Ungaro F. Quaglia F. Netti P.A. Controlled drug delivery in tissue engineering. Adv Drug Deliv Rev. 2008;60:229. doi: 10.1016/j.addr.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 285.Lim S.H. Mao H.Q. Electrospun scaffolds for stem cell engineering. Adv Drug Deliv Rev. 2009;61:1084. doi: 10.1016/j.addr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 286.Chen R.R. Silva E.A. Yuen W.W. Mooney D.J. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 287.Pepper M.S. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 288.Wang H. Leeuwenburgh SCG. Li Y. Jansen J.A. The Use of Micro- and nanospheres as functional components for bone tissue regeneration. Tissue Eng Part B Rev. 2011;18:24. doi: 10.1089/ten.teb.2011.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Niu X. Feng Q. Wang M. Guo X. Zheng Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release. 2009;134:111. doi: 10.1016/j.jconrel.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 290.Perets A. Baruch Y. Weisbuch F. Shoshany G. Neufeld G. Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res A. 2003;65:489. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 291.Shi X. Su K. Varshney R.R. Wang Y. Wang D.A. Sintered microsphere scaffolds for controlled release and tissue engineering. Pharm Res. 2011;28:1224. doi: 10.1007/s11095-010-0359-4. [DOI] [PubMed] [Google Scholar]
- 292.Wang X. Wenk E. Zhang X. Meinel L. Vunjak-Novakovic G. Kaplan D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release. 2009;134:81. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Silva A.K. Richard C. Bessodes M. Scherman D. Merten O.W. Growth factor delivery approaches in hydrogels. Biomacromolecules. 2009;10:9. doi: 10.1021/bm801103c. [DOI] [PubMed] [Google Scholar]
- 294.Guo X. Park H. Young S. Kretlow J.D. van den Beucken J.J. Baggett L.S., et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ravindran S. Roam J.L. Nguyen P.K. Hering T.M. Elbert D.L. McAlinden A. Changes of chondrocyte expression profiles in human MSC aggregates in the presence of PEG microspheres and TGF-beta3. Biomaterials. 2011;32:8436. doi: 10.1016/j.biomaterials.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Duan B. Wang M. Zhou W.Y. Cheung W.L. Li Z.Y. Lu W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomaterialia. 2010;6:4495. doi: 10.1016/j.actbio.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 297.Zhou W. Lee S. Wang M. Cheung W. Ip W. Selective laser sintering of porous tissue engineering scaffolds from poly(L-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J Mater Sci. 2008;19:2535. doi: 10.1007/s10856-007-3089-3. [DOI] [PubMed] [Google Scholar]
- 298.Miranda E.S. Silva T.H. Reis R.L. Mano J.F. Nanostructured natural-based polyelectrolyte multilayers to agglomerate chitosan particles into scaffolds for tissue engineering. Tissue Eng Part A. 2011;17:2663. doi: 10.1089/ten.tea.2010.0635. [DOI] [PubMed] [Google Scholar]
- 299.Wang H. Hansen M.B. Löwik DWPM. van Hest JCM. Li Y. Jansen J.A., et al. Oppositely charged gelatin nanospheres as building blocks for injectable and biodegradable gels. Adv Mater. 2011;23:H119. doi: 10.1002/adma.201003908. [DOI] [PubMed] [Google Scholar]
- 300.Van Tomme S.R. van Steenbergen M.J. De Smedt S.C. van Nostrum C.F. Hennink W.E. Self-gelling hydrogels based on oppositely charged dextran microspheres. Biomaterials. 2005;26:2129. doi: 10.1016/j.biomaterials.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 301.Wang Q. Wang J. Lu Q. Detamore M.S. Berkland C. Injectable PLGA based colloidal gels for zero-order dexamethasone release in cranial defects. Biomaterials. 2010;31:4980. doi: 10.1016/j.biomaterials.2010.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]