Abstract
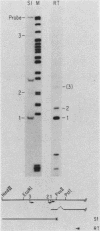
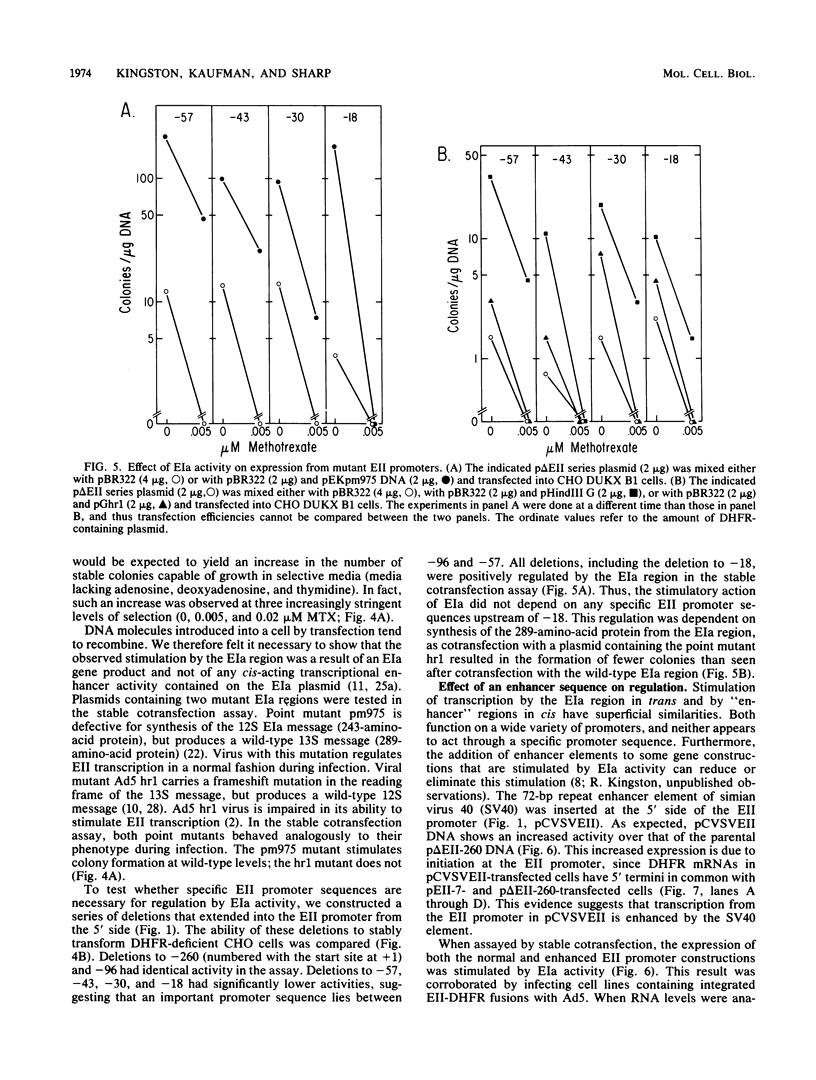
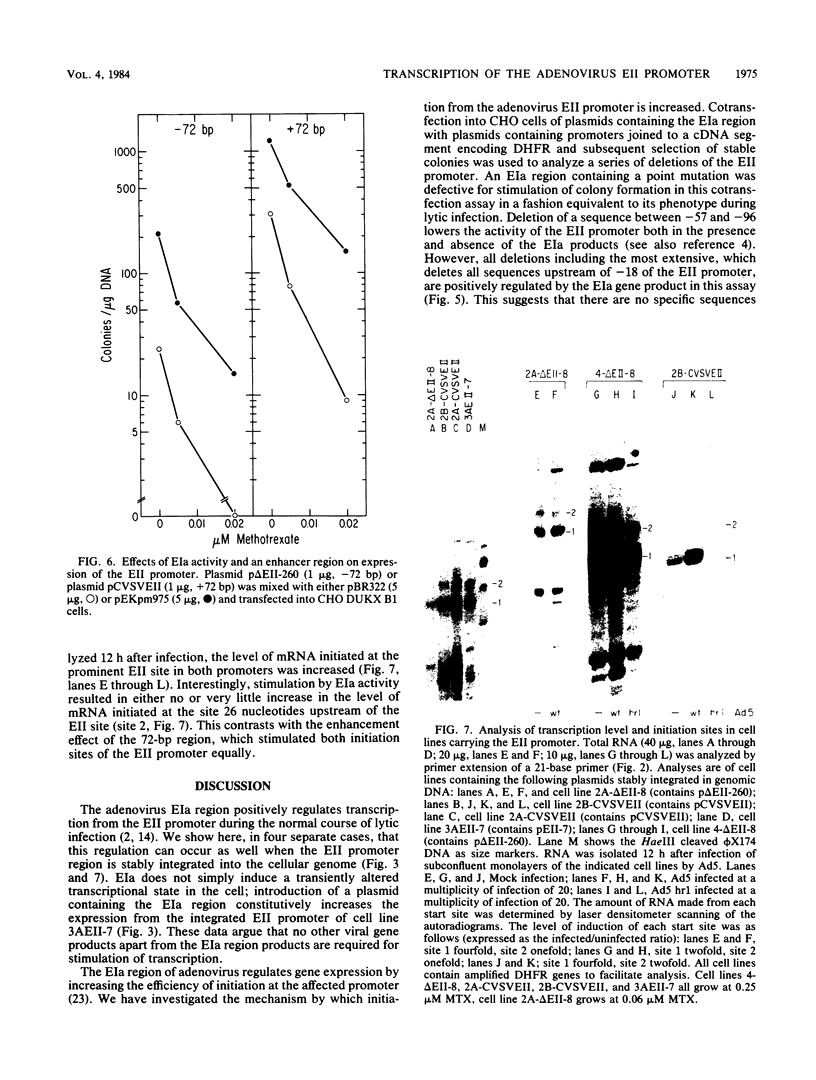
During adenovirus infection, the EII promoter is positively regulated by products of the EIa region. We have studied this regulation by fusing a DNA segment containing the adenovirus EII promoter to a dihydrofolate reductase cDNA segment. Expression of this hybrid gene is stimulated in trans when cell lines containing an integrated copy are either transfected with plasmids carrying the EIa region or infected with adenovirus. This suggests that EIa activity regulates transcription of the EII promoter in the absence of other viral proteins and that this stimulation can occur when the EII promoter is organized in cellular chromatin. Transcription from the EII promoter is initiated at two sites in cell lines lacking EIa activity. Introduction of the EIa region preferentially stimulated transcription from one of these two sites. A sensitive, stable cotransfection assay was used to test for specific EII sequences required for stimulation. EIa activity stimulates all mutant promoters; the most extensive deletion retained only 18 base pairs of sequences upstream of the initiation site. We suggest that regulation of a promoter by the EIa region does not depend on the presence of a set of specific sequences, but instead reflects a characteristic of promoters that have been exogenously introduced into cells. Insertion of the 72-base-pair repeat of simian-virus 40 in cis enhances transcription from the EII promoter. The stimulatory effects of EIa activity and of the simian virus 40 sequence are additive and appear to differ mechanistically.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Ziff E. B. Promoters and heterogeneous 5' termini of the messenger RNAs of adenovirus serotype 2. J Mol Biol. 1981 Jun 25;149(2):189–221. doi: 10.1016/0022-2836(81)90298-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Bos J. L., ten Wolde-Kraamwinkel H. C. The E1b promoter of Ad12 in mouse L tk- cells is activated by adenovirus region E1a. EMBO J. 1983;2(1):73–76. doi: 10.1002/j.1460-2075.1983.tb01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkaim R., Goding C., Kédinger C. The adenovirus-2 EIIa early gene promoter: sequences required for efficient in vitro and in vivo transcription. Nucleic Acids Res. 1983 Oct 25;11(20):7105–7117. doi: 10.1093/nar/11.20.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Galibert F., Hérissé J., Courtois G. Nucleotide sequence of the EcoRI-F fragment of adenovirus 2 genome. Gene. 1979 May;6(1):1–22. doi: 10.1016/0378-1119(79)90081-7. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Hillman D., Berk A. J. Adenovirus early region 1A protein activates transcription of a nonviral gene introduced into mammalian cells by infection or transfection. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1193–1197. doi: 10.1073/pnas.81.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Spector D. J., Raskas H. J. In vitro translation products specified by the transforming region of adenovirus type 2. J Virol. 1979 Sep;31(3):621–629. doi: 10.1128/jvi.31.3.621-629.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison T., Graham F., Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977 Mar;77(1):319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Galos R., Williams J. Isolation of type 5 adenovirus mutants with a cold-sensitive host range phenotype: genetic evidence of an adenovirus transformation maintenance function. Virology. 1982 Oct 15;122(1):109–124. doi: 10.1016/0042-6822(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Richter J. D., Weeks D. L., Smith L. D. Regulation of adenovirus transcription by an E1a gene in microinjected Xenopus laevis oocytes. Mol Cell Biol. 1983 Dec;3(12):2131–2142. doi: 10.1128/mcb.3.12.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J Mol Biol. 1982 Aug 25;159(4):601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Elkaim R., Kédinger C., Sassone-Corsi P., Chambon P. Specific in vitro initiation of transcription on the adenovirus type 2 early and late EII transcription units. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7383–7387. doi: 10.1073/pnas.78.12.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Ginsberg H. S., Blanchard J. M., Wilson M. C., Darnell J. E., Jr Regulation of the primary expression of the early adenovirus transcription units. J Virol. 1979 Dec;32(3):727–733. doi: 10.1128/jvi.32.3.727-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Osborne T. F., Arvidson D. N., Tyau E. S., Dunsworth-Browne M., Berk A. J. Transcription control region within the protein-coding portion of adenovirus E1A genes. Mol Cell Biol. 1984 Jul;4(7):1293–1305. doi: 10.1128/mcb.4.7.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The protein products of the myc and myb oncogenes and adenovirus E1a are structurally related. Nature. 1983 Dec 22;306(5945):803–806. doi: 10.1038/306803a0. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Bird S., Rowe W. P., Weinberg R. A. Identification of DNA fragments carrying ecotropic proviruses of AKR mice. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4554–4558. doi: 10.1073/pnas.76.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Green M. R., Maniatis T. cis and trans activation of globin gene transcription in transient assays. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7428–7432. doi: 10.1073/pnas.80.24.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Käs E., Carothers A. M., Chasin L. A. Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell. 1983 Jun;33(2):405–412. doi: 10.1016/0092-8674(83)90422-1. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. L., Jones N. C. E1A control of gene expression is mediated by sequences 5' to the transcriptional starts of the early viral genes. Mol Cell Biol. 1983 Jul;3(7):1222–1234. doi: 10.1128/mcb.3.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]