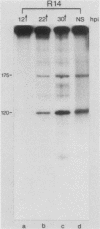
Abstract
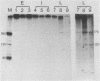
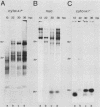
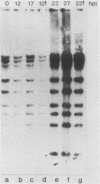
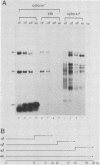
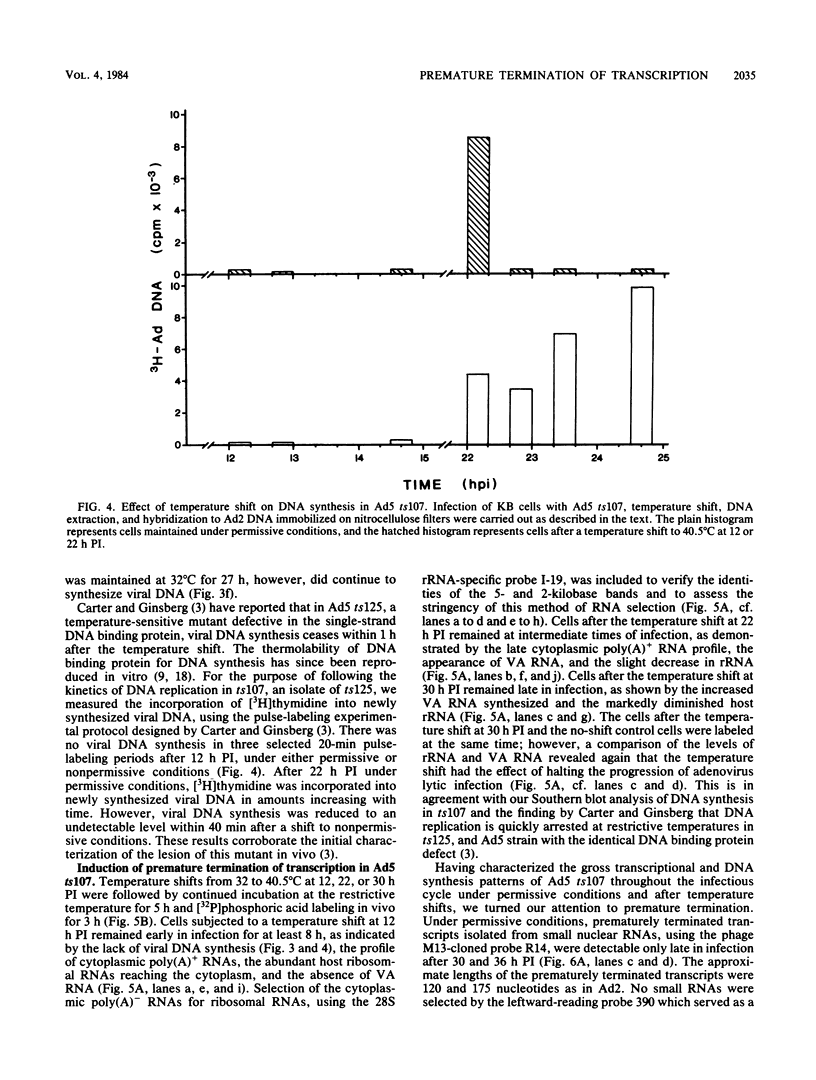
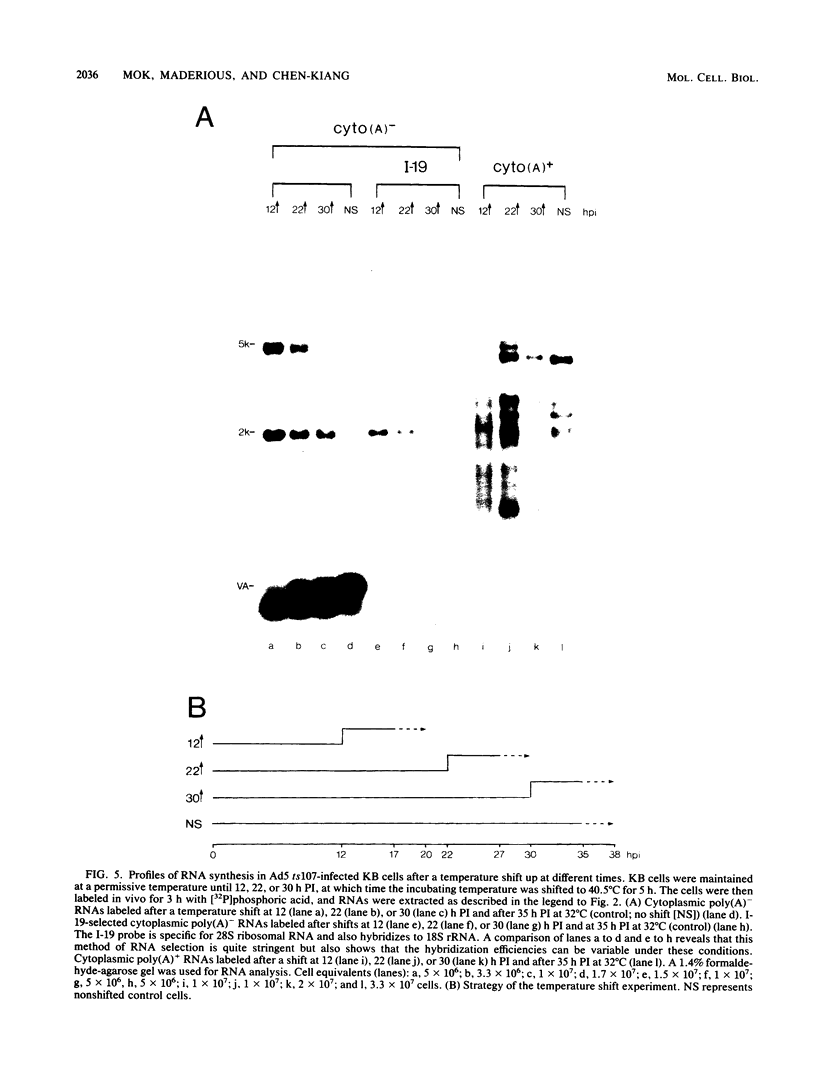
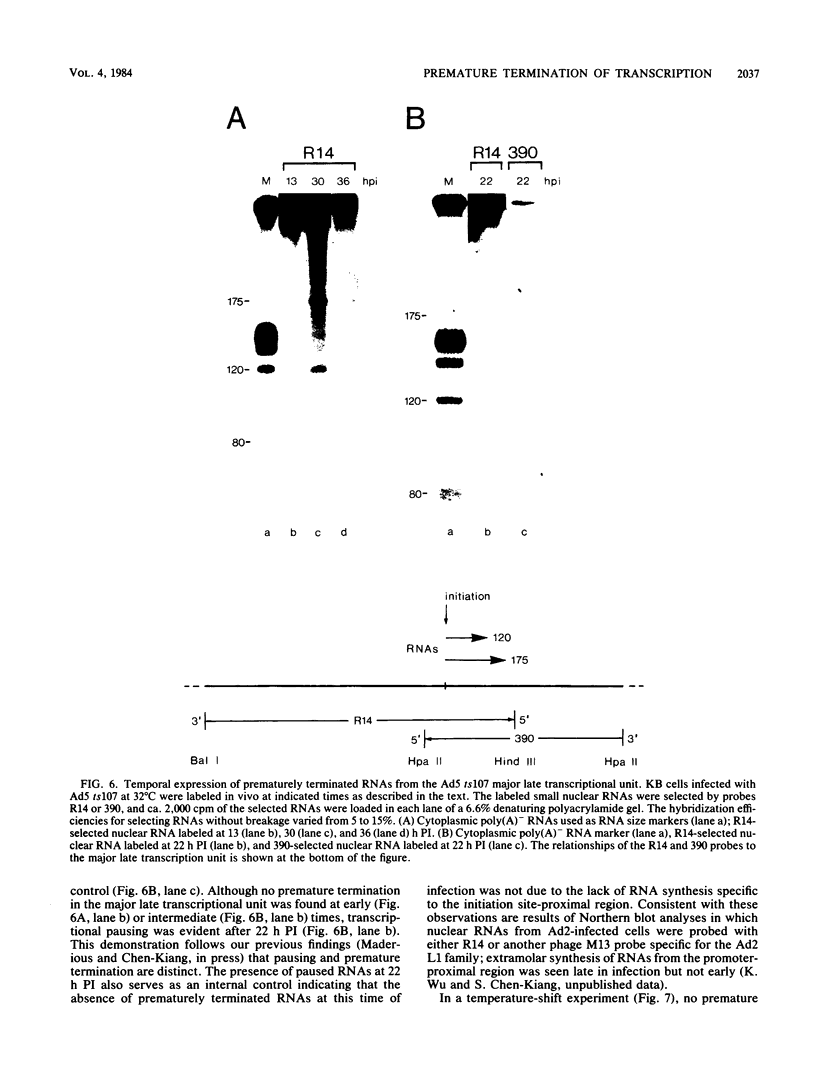
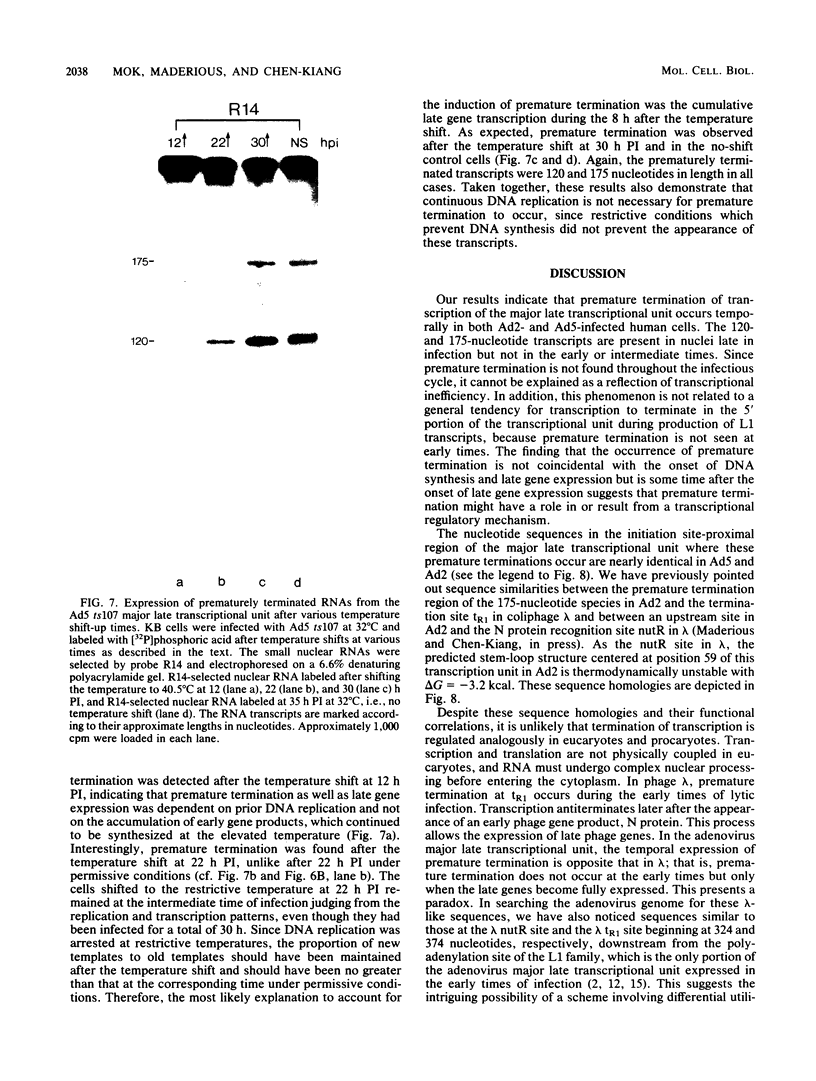
We have recently demonstrated pausing and premature termination of transcription by eucaryotic RNA polymerase II at specific sites in the major late transcriptional unit of adenovirus type 2 in vivo and in vitro. In further developing this as a system for studying eucaryotic termination control, we found that prematurely terminated transcripts of 175 and 120 nucleotides also occur in adenovirus type 5-infected cells. In both cases, premature termination occurs temporally, being found only during late times of infection, not at early times before DNA replication or immediately after the onset of DNA replication when late gene expression has begun (intermediate times). To examine the phenomenon of premature termination further, a temperature-sensitive mutant virus, adenovirus type 5 ts107, was used to uncouple DNA replication and transcription. DNA replication is defective in this mutant at restrictive temperatures. We found that premature termination is inducible at intermediate times by shifting from a permissive temperature to a restrictive temperature, allowing continuous transcription in the absence of continuous DNA replication. No premature termination occurs when the temperature is shifted up at early times before DNA replication. Our data suggest that premature termination of transcription is dependent on both prior synthesis of new templates and cumulative late gene transcription but does not require continuous DNA replication.
Full text
PDF

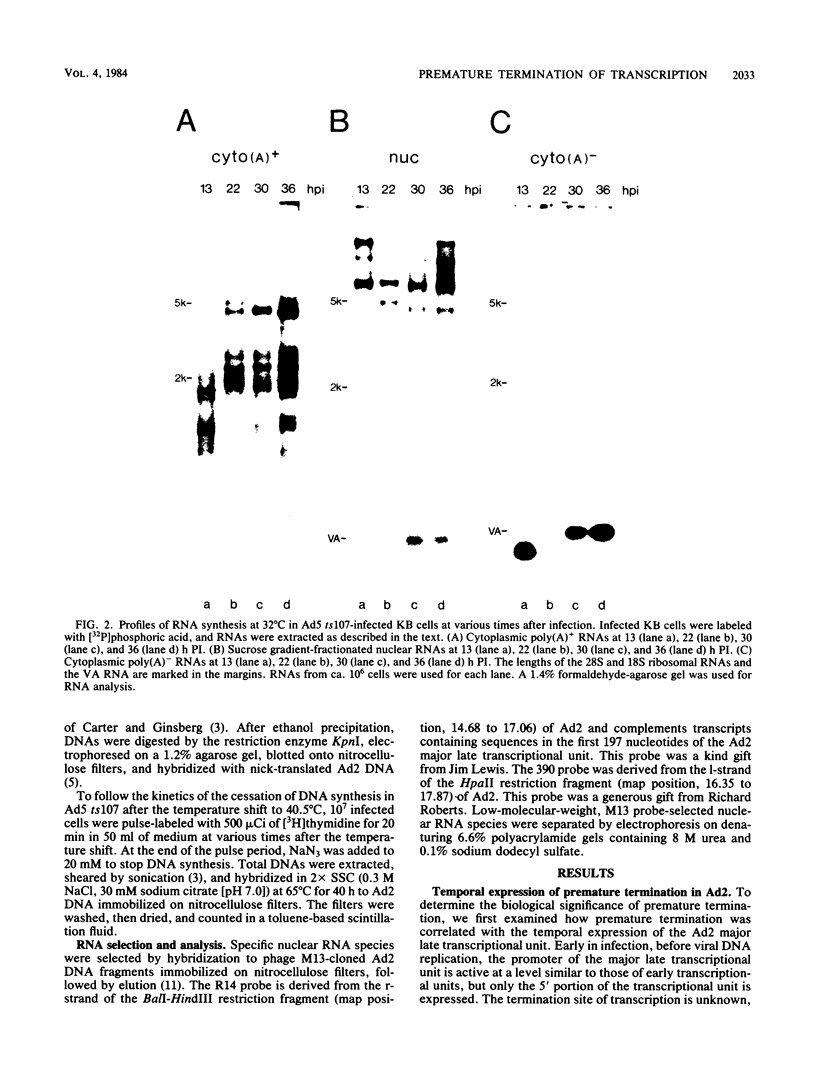
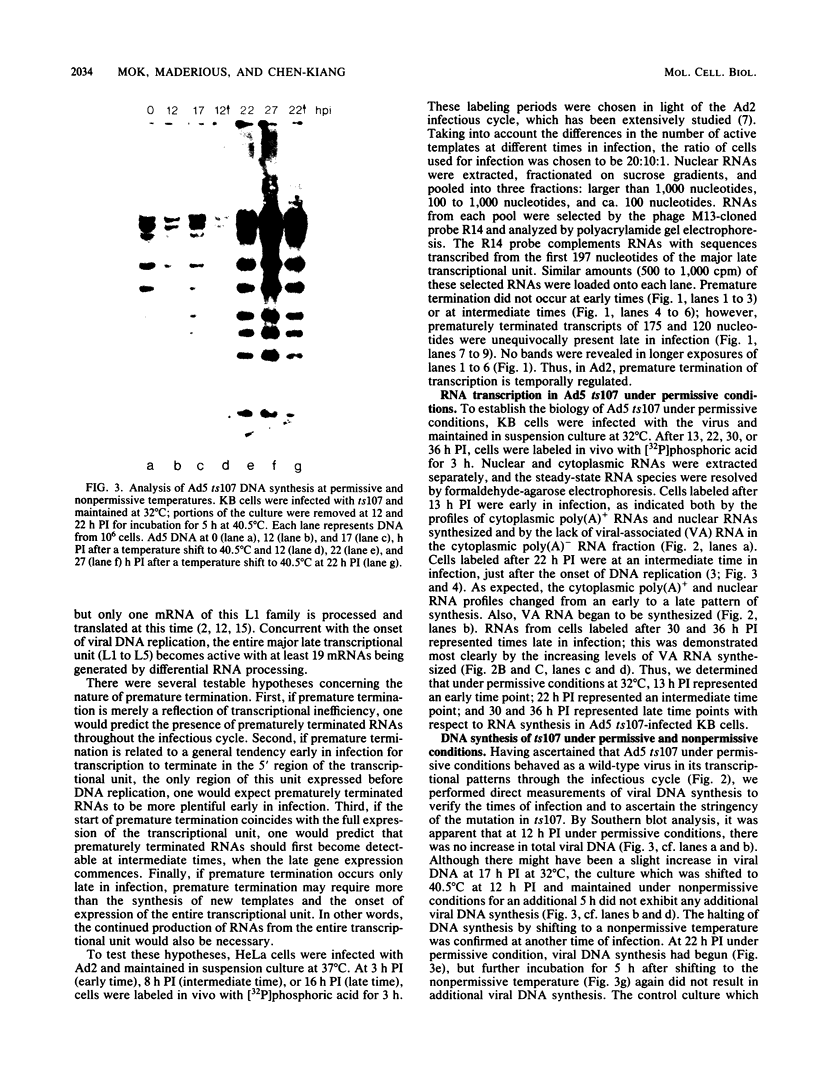

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Carter T. H., Ginsberg H. S. Viral transcription in KB cells infected by temperature-sensitive "early" mutants of adenovirus type 5. J Virol. 1976 Apr;18(1):156–166. doi: 10.1128/jvi.18.1.156-166.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S., Nevins J. R., Darnell J. E., Jr N-6-methyl-adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol. 1979 Dec 15;135(3):733–752. doi: 10.1016/0022-2836(79)90174-8. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Wolgemuth D. J., Hsu M. T., Darnell J. E., Jr Transcription and accurate polyadenylation in vitro of RNA from the major late adenovirus 2 transcription unit. Cell. 1982 Mar;28(3):575–584. doi: 10.1016/0092-8674(82)90212-4. [DOI] [PubMed] [Google Scholar]
- Crossland L. D., Raskas H. J. Identification of adenovirus genes that require template replication for expression. J Virol. 1983 Jun;46(3):737–748. doi: 10.1128/jvi.46.3.737-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Kelly J. D., Cohen E. H. Transcription terminates in yeast distal to a control sequence. Cell. 1983 Jun;33(2):607–614. doi: 10.1016/0092-8674(83)90441-5. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Nicolas J. C., van Schaik F. M., Sussenbach J. S. Structure and function of DNA binding proteins from revertants of adenovirus type 5 mutants with a temperature-sensitive DNA replication. Virology. 1983 Jan 30;124(2):425–433. doi: 10.1016/0042-6822(83)90358-6. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S. G., Jelinek W., Darnell J. E. 3'-Terminal addition to HeLa cell nuclear and cytoplasmic poly (A). J Mol Biol. 1977 Jun 15;113(1):219–235. doi: 10.1016/0022-2836(77)90051-1. [DOI] [PubMed] [Google Scholar]
- Shaw A. R., Ziff E. B. Transcripts from the adenovirus-2 major late promoter yield a single early family of 3' coterminal mRNAs and five late families. Cell. 1980 Dec;22(3):905–916. doi: 10.1016/0092-8674(80)90568-1. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- van Bergen B. G., van der Vliet P. C. Temperature-sensitive initiation and elongation of adenovirus DNA replication in vitro with nuclear extracts from H5ts36-, H5ts149-, and H5ts125-infected HeLa cells. J Virol. 1983 May;46(2):642–648. doi: 10.1128/jvi.46.2.642-648.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]