Abstract
PC12 cells, derived from a rat pheochromocytoma, were mutagenized and selected in media containing agents known to elevate intracellular concentrations of cyclic AMP (cAMP). More than 40 clones were isolated by selection with cholera toxin or 2-chloroadenosine or both. The variants that were deficient in accumulating cAMP were obtained by using a protocol in which 1 microM 8-bromo-cAMP was included in addition to the agonist. Certain of these variants were partially characterized with respect to the site of altered cAMP metabolism. The profiles of adenylate cyclase activity responsiveness of certain variants to guanosine-5'-(beta, gamma-imido) triphosphate and to forskolin resembled those of UNC and cyc phenotypes of S49 lymphoma cells, which are functionally deficient in the GTP-sensitive coupling protein, Ns. Other variants were characterized by increased cyclic nucleotide phosphodiesterase activity at low substrate concentration. Diverse morphological traits were observed among the variants, but it was not possible to assign them to a particular cAMP phenotype. Two revertants of a PC12 mutant were isolated and observed to have regained a cellular cAMP response to 2-chloroadenosine and to forskolin. It is hoped that these PC12 mutants will have utility for defining cAMP-mediated functions, including any links to the action of nerve growth factor, in cells derived from the neural crest.
Full text
PDF

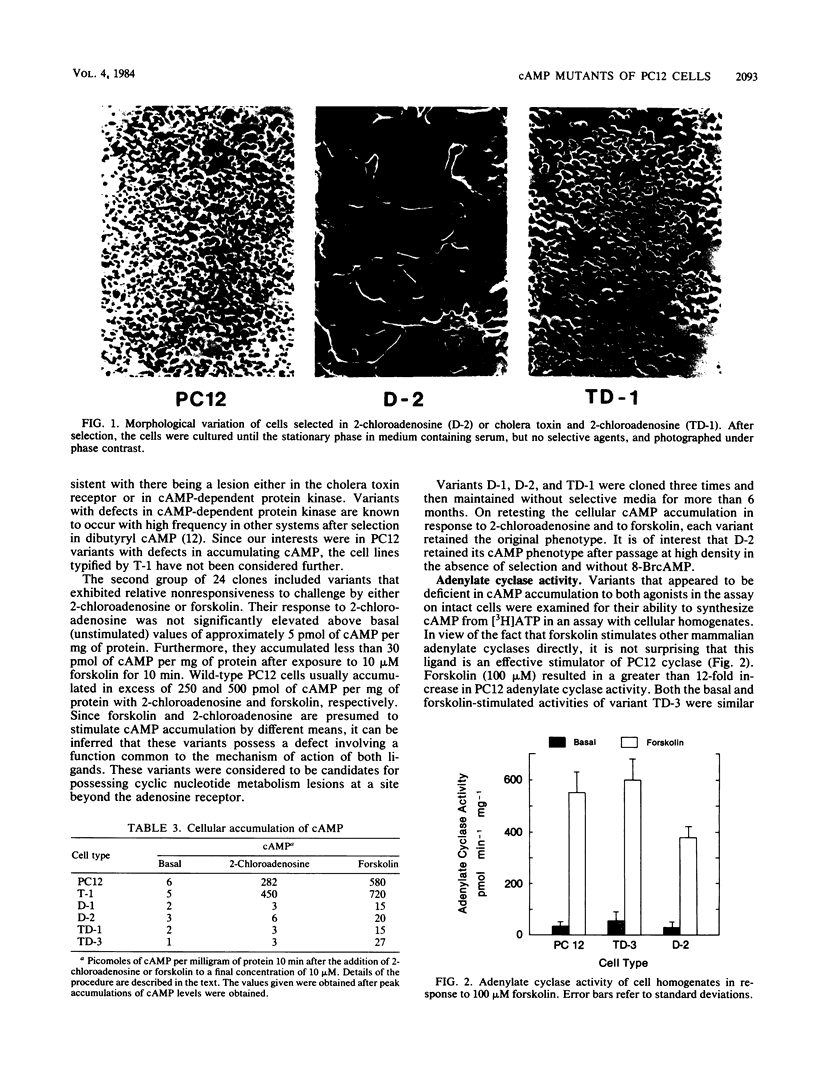
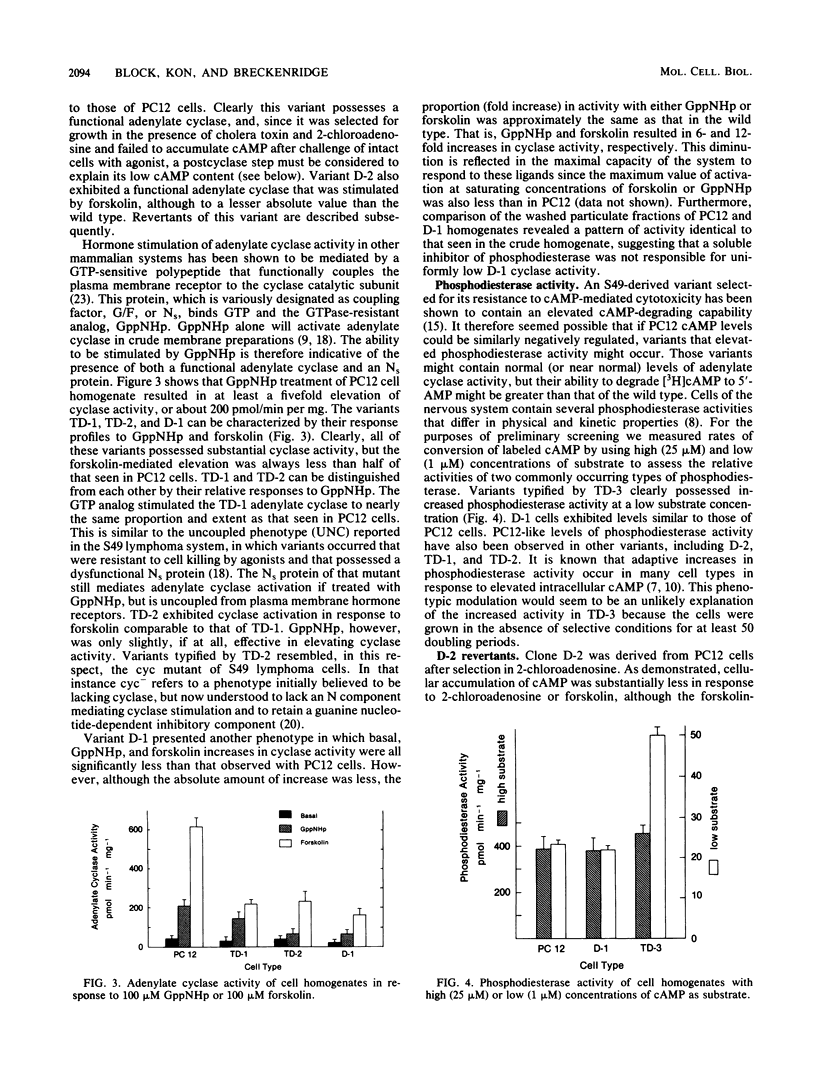
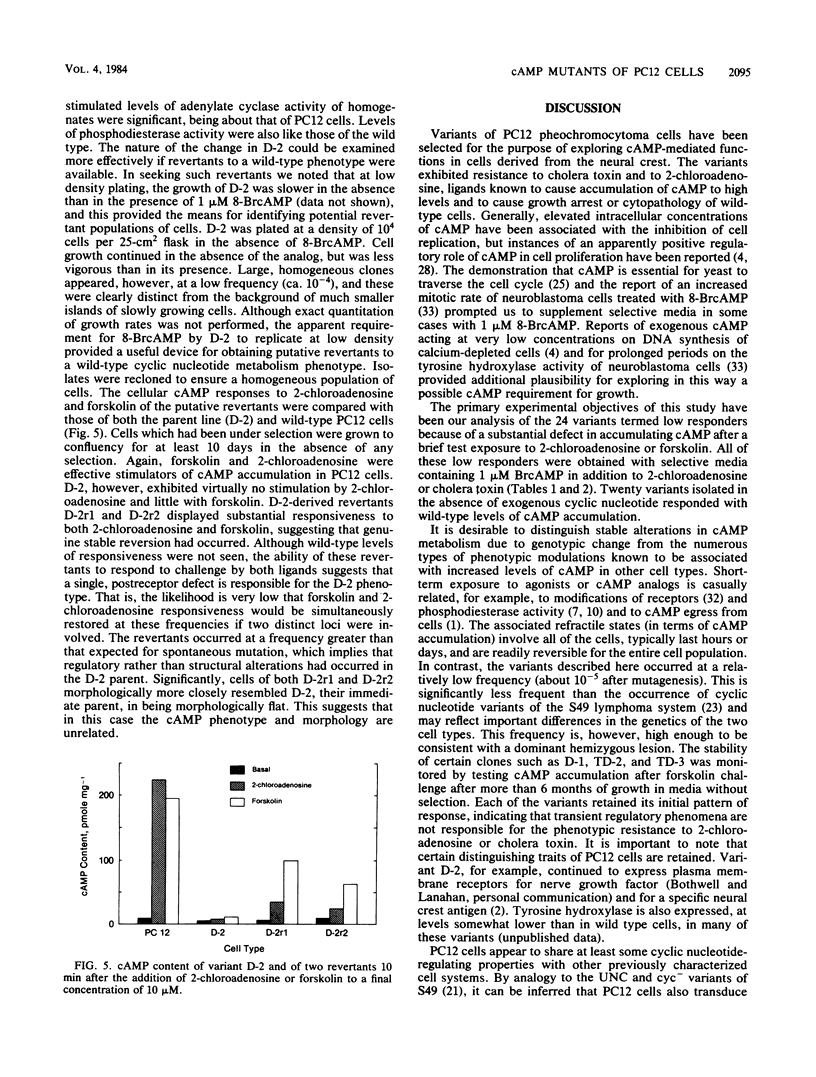


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block T., Bothwell M. The nerve growth factor receptor on PC12 cells: interconversion between two forms with different binding properties. J Neurochem. 1983 Jun;40(6):1654–1663. doi: 10.1111/j.1471-4159.1983.tb08139.x. [DOI] [PubMed] [Google Scholar]
- Bothwell M. A., Schechter A. L., Vaughn K. M. Clonal variants of PC12 pheochromocytoma cells with altered response to nerve growth factor. Cell. 1980 Oct;21(3):857–866. doi: 10.1016/0092-8674(80)90449-3. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A., Wolff D. J. Calcium-dependent adenylate cyclase from rat cerebral cortex. Reversible activation by sodium fluoride. J Biol Chem. 1977 Aug 25;252(16):5677–5685. [PubMed] [Google Scholar]
- Brostrom C. O., Wolff D. J. Calcium-dependent cyclic nucleotide phosphodiesterase from brain: comparison of adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate as substrates. Arch Biochem Biophys. 1976 Jan;172(1):301–311. doi: 10.1016/0003-9861(76)90079-5. [DOI] [PubMed] [Google Scholar]
- Browning E. T., Brostrom C. O., Groppi V. E., Jr Altered adenosine cyclic 3',5'-monophosphate synthesis and degradation by C-6 astrocytoma cells following prolonged exposure to norepinephrine. Mol Pharmacol. 1976 Jan;12(1):32–40. [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Regulation of adenosine 3',5'-cyclic monophosphate phosphodiesterase activity in fibroblasts by intracellular concentrations of cyclic adenosine monophosphate (3T3-dibutyryl cyclic AMP-SV40-transformed cells-michaelis constants-L cells-prostaglandin E 1 ). Proc Natl Acad Sci U S A. 1972 Feb;69(2):459–462. doi: 10.1073/pnas.69.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darfler F. J., Mahan L. C., Koachman A. M., Insel P. A. Stimulation of forskolin of intact S49 lymphoma cells involves the nucleotide regulatory protein of adenylate cyclase. J Biol Chem. 1982 Oct 25;257(20):11901–11907. [PubMed] [Google Scholar]
- Downs R. W., Jr, Aurbach G. D. The effects of forskolin on adenylate cyclase in S49 wild type and cyc- cells. J Cyclic Nucleotide Res. 1982;8(4):235–242. [PubMed] [Google Scholar]
- Friedrich U., Coffino P. Mutagenesis in S49 mouse lymphoma cells: induction of resistance to ouabain, 6-thioguanine, and dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1977 Feb;74(2):679–683. doi: 10.1073/pnas.74.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M. Genetic approaches to cyclic AMP effects in cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):329–330. doi: 10.1016/0092-8674(80)90342-6. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Liem R. K., Shelanski M. L. Regulation of a high molecular weight microtubule-associated protein in PC12 cells by nerve growth factor. J Cell Biol. 1983 Jan;96(1):76–83. doi: 10.1083/jcb.96.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppi V. E., Steinberg F., Kaslow H. R., Walker N., Bourne H. R. Identification by direct photoaffinity labeling of an altered phosphodiesterase in a mutant S49 lymphoma cell. J Biol Chem. 1983 Aug 25;258(16):9717–9723. [PubMed] [Google Scholar]
- Gunning P. W., Landreth G. E., Bothwell M. A., Shooter E. M. Differential and synergistic actions of nerve growth factor and cyclic AMP in PC12 cells. J Cell Biol. 1981 May;89(2):240–245. doi: 10.1083/jcb.89.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroff G., Dickens G., End D., Londos C. The action of adenosine analogs on PC12 cells. J Neurochem. 1981 Dec;37(6):1431–1439. doi: 10.1111/j.1471-4159.1981.tb06312.x. [DOI] [PubMed] [Google Scholar]
- Haga T., Ross E. M., Anderson H. J., Gilman A. G. Adenylate cyclase permanently uncoupled from hormone receptors in a novel variant of S49 mouse lymphoma cells. Proc Natl Acad Sci U S A. 1977 May;74(5):2016–2020. doi: 10.1073/pnas.74.5.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Heumann R., Kachel V., Thoenen H. Relationship between NGF-mediated volume increase and "priming effect" in fast and slow reacting clones of PC12 pheochromocytoma cells. Role of cAMP. Exp Cell Res. 1983 Apr 15;145(1):179–190. doi: 10.1016/s0014-4827(83)80019-6. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. D., Hanoune J., Birnbaumer L. Guanine nucleotide inhibition of cyc- S49 mouse lymphoma cell membrane adenylyl cyclase. J Biol Chem. 1982 Dec 25;257(24):14723–14725. [PubMed] [Google Scholar]
- Hollenbeck R. A., Chuang D. M., Costa E. Translocation of cytosol protein kinase into nuclei and the induction of tyrosine hydroxylase in NBD-2 neuroblastoma cells. Brain Res. 1979 Aug 10;171(3):481–487. doi: 10.1016/0006-8993(79)91052-7. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Farfel Z., Bourne H. R. Genetic analysis of hormone-sensitive adenylate cyclase. Adv Cyclic Nucleotide Res. 1980;13:1–37. [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Oshima Y., Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg M., Wilson S., Higashida H., Rotter A., Krueger K., Busis N., Ray R., Kenimer J. G., Adler M. Modulation of synapse formation by cyclic adenosine monophosphate. Science. 1983 Nov 18;222(4625):794–799. doi: 10.1126/science.6314503. [DOI] [PubMed] [Google Scholar]
- Rabe C. S., Schneider J., McGee R., Jr Enhancement of depolarization-dependent neurosecretion from PC12 cells by forskolin-induced elevation of cyclic AMP. J Cyclic Nucleotide Res. 1982;8(6):371–384. [PubMed] [Google Scholar]
- Rozengurt E. Cyclic AMP: a growth-promoting signal for mouse 3T3 cells. Adv Cyclic Nucleotide Res. 1981;14:429–442. [PubMed] [Google Scholar]
- Schimmer B. P., Tsao J. Isolation of forskolin-resistant adrenal cells defective in the adenylate cyclase system. J Biol Chem. 1984 May 10;259(9):5376–5379. [PubMed] [Google Scholar]
- Schulze I., Perez-Polo J. R. Nerve growth factor and cyclic AMP: opposite effects on neuroblastoma-substrate adhesion. J Neurosci Res. 1982;8(2-3):393–411. doi: 10.1002/jnr.490080227. [DOI] [PubMed] [Google Scholar]
- Treisman G. J., Bagley S., Gnegy M. E. Calmodulin-sensitive and calmodulin-insensitive components of adenylate cyclase activity in rat striatum have differential responsiveness to guanyl nucleotides. J Neurochem. 1983 Nov;41(5):1398–1406. doi: 10.1111/j.1471-4159.1983.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Waldo G. L., Northup J. K., Perkins J. P., Harden T. K. Characterization of an altered membrane form of the beta-adrenergic receptor produced during agonist-induced desensitization. J Biol Chem. 1983 Nov 25;258(22):13900–13908. [PubMed] [Google Scholar]
- Waymire J. C., Gilmer-Waymire K., Haycock J. W. Cyclic-AMP-induced differentiation in neuroblastoma is independent of cell division rate. Nature. 1978 Nov 9;276(5684):194–195. doi: 10.1038/276194a0. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Shooter E. M. The biology and mechanism of action of nerve growth factor. Annu Rev Biochem. 1982;51:845–868. doi: 10.1146/annurev.bi.51.070182.004213. [DOI] [PubMed] [Google Scholar]



