Abstract
Pheochromocytoma is a rare but potentially lethal chromaffin cell tumor with currently no effective treatment. Peptide hormone receptors are frequently overexpressed on endocrine tumor cells and can be specifically targeted by various anti-tumor peptide analogs. The present study carried out on mouse pheochromocytoma cells (MPC) and a more aggressive mouse tumor tissue-derived (MTT) cell line revealed that these cells are characterized by pronounced expression of the somatostatin receptor 2 (sst2), growth hormone-releasing hormone (GHRH) receptor and the luteinizing hormone-releasing hormone (LHRH) receptor. We further demonstrated significant anti-tumor effects mediated by cytotoxic somatostatin analogs, AN-162 and AN-238, by LHRH antagonist, Cetrorelix, by the cytotoxic LHRH analog, AN-152, and by recently developed GHRH antagonist, MIA-602, on MPC and for AN-152 and MIA-602 on MTT cells. Studies of novel anti-tumor compounds on these mouse cell lines serve as an important basis for mouse models of metastatic pheochromocytoma, which we are currently establishing.
Keywords: pheochromocytoma, peptide analogs, targeted tumor therapy
1. Introduction
Pheochromocytoma are potentially lethal tumors of adrenal and extra-adrenal (also called paragangliomas) chromaffin cells (Harding et al., 2005). Currently there are no reliable markers to distinguish benign from malignant tumors and there is no curative therapy (Scholz et al., 2007).
To date, combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine (CVD) and radiotherapy with 131I-metaiodobenzylguanidine (MIBG) represent the two primary modes of treatment for malignant pheochromocytoma (Chrisoulidou et al., 2007). Combinations of etoposide and cisplatin, cisplatin and 5-fluorouracil, cytokine arabinoside, anthracine, and more recently, temozolomide and thalidomide also have benefit in some patients (Eisenhofer et al., 2008). However, complete remissions are rare and these therapies can only be regarded as palliative (Fassnacht et al., 2009). Thus, there is a need for identification of new targets for treatment of metastatic pheochromocytoma along with development of appropriate therapeutic agents for those targets.
Targeted therapy based on peptide analogs binding specifically to neuropeptide hormone receptors on cancer cells provides unique treatment strategy with potentially more tissue specificity and less systemic toxicity than conventional chemotherapeutic approaches (Reubi, 2003). Some support for this approach is provided by case reports (Valkema et al., 2002) and a recent publication including 28 patients treated with radio-labeled DOTATOC (Forrer et al., 2008). Since altered neuropeptide hormone receptor expression leading to hypersecretion of hormones or enhanced tumor growth is a frequent feature of adrenal tumors (Willenberg et al., 1998; Lacroix et al., 2001), targeting these aberrant receptors with novel chemotherapeutic peptide analogs may provide a logical therapeutic alternative to other approaches for pheochromocytomas and other tumors (Ziegler et al., 2009). Such targeted therapies should enable higher tumoral concentrations of antineoplastic agents compared to current systemic chemotherapeutic therapies and thereby lead to improved antitumor treatment efficacy with reduced systemic toxicity (Buchholz et al., 2006).
Over the last few decades several targeted cytotoxic hormone analogs have been synthesized by Schally et al. to yield new classes of antineoplastic agents (Schally et al., 2001). These compounds include the cytotoxic analogs of bombesin (AN-215), of somatostatin AN-238 and AN-162, of luteinizing hormone-releasing hormone (LHRH) AN-152, and others, synthesized by coupling doxorubicin or 2 pyrrolinodoxorubicin (2-pyrrolino-DOX) (AN-201) to the respective hormone analogs (Nagy et al., 1996). Recently, a new class of GHRH antagonists (MIA-313, MIA-602, MIA-604, and MIA-610) was generated by Schally and co-workers and shown to be effective anti-neoplastic agents, as for example demonstrated on ovarian cancer (Klukovits et al., 2012). Both MIA-602 and the LHRH antagonist Cetrorelix (Buchholz et al., 2009) seem to be highly promising anti-cancer compounds on endocrine tumors (Schally, 2008) and both compounds were also part of our investigation on adrenomedullary tumors.
Recently two mouse pheochromocytoma cell (MPC) lines, one more malignant (MTT) than the other have been established as originally generated from heterozygous neurofibromin-1 knock-out mice (Powers et al., 2000). Based on these cell lines various mouse models were derived, which are useful for studying metastatic pheochromocytoma and testing novel therapies (Martiniova et al., 2009; Martiniova et al., 2011; Korpershoek et al., 2012; Nolting and Grossman, 2012). Furthermore, these models are also physiologically relevant to human pheochromocytoma because of the tumoral production of norepinephrine in athymic nude mice after tail vein injection of the MPC cells (Ohta et al., 2006). Additionally, both MPC and MTT cells were recently stably transfected with luciferase and/or GFP for further tracing tumor spread in vivo by bioluminescence (Giubellino et al., 2012).
Aberrantly expressed neuropeptide hormone receptors are frequently found in a subgroup of adrenocortical tumors, especially in ACTH-independent macronodular adrenal hyperplasia. Based on this we explored a large pheochromocytoma microarray data base performed by our collaborating partners (Brouwers et al., 2006) and demonstrated significant differential expression of neuropeptide hormone receptors among human pheochromocytoma specimens (Ziegler et al., 2009). Based on these microarray data we performed a comprehensive analysis of mRNA expression of various relevant receptors in human adrenal tumors and cell lines.
The present analysis extends our previous study of neuropeptide hormone receptor expression to the MPC and MTT cell lines. We also studied possible anti-tumor effects of the most promising peptide analogs targeting neuropeptide hormone receptors identified to be expressed in these cell lines.
2. Materials and methods
2.1. Cell culture
MPC and MTT cells were cultured on collagen coated flasks in RPMI 1640 including HEPES (GIBCO) with 10% horse serum (GIBCO), 5% FBS superior (BIOCHROM) and 0,1% Gentamicin (GIBCO) in a humidified 5% CO2/95% O2 atmosphere at 37°C. The culture medium was changed every second day. Neuropeptide antagonists and agonists were used at concentrations of 10−5–10−8 for 24 to 72 h.
2.2. Immunohistochemical analysis of tumor cells
Immunohistochemical stainings were preformed on deparaffinized slides of tumor cells (n=3) using an automated immunostainer (Benchmark Ventana) according to the manufacturer’s protocols. The primary antibody for Somatostatin Receptor Type 2 (SSTR2) was Rabbit anti mouse/anti-human polyclonal antibody (Novus Biologicals). For GHRHR we employed Rabbit anti-human (Lifespan Bio Sciences) and Rabbit anti-mouse (ABCAM) antibodies and for detecting LHRH/GnRHR we used Rabbit anti-mouse (Novus Biologicals) and Rabbit anti-human (Santa Cruz) antibodies, respectively. We used the VENTANA amplification kit as well as avidin-biotin labeling and 3, 3’- diaminobenzidine to amplify and visualize the signal. Slides were counterstained with H&E. Staining with isotype control antibodies was performed to confirm the staining specificity.
2.3. Cell viability and apoptosis assays
For evaluating cell viability we used the CellTiter-Glo Luminescent Cell Viability Assay (Promega). This assay determines the number of viable cells in culture based on quantification of the ATP present, an indicator of metabolically active cells. For analysing programmed cell death we employed the Caspase-Glo 3/7 Assay (Promega), which provides a homogeneous luminescent assay that measures caspase-3/7 activities. All assays were performed according to the manufacturer’s protocols and guidelines.
2.4. Electron microscopy
For evaluating the ultrastructure of our tumor cells, they were fixed in 2.5 M glutaraldehyde in 0.1 M cacodylate buffer and refixed in 1% osmiumtetroxid solution. After dehydration in an ascending ethanol series, specimens were embedded in EPON. Ultrathin slices (60 nm) were afterwards stained with lead acetate und uranyl acetate to obtain a suitable contrast, and analyzed using an electron microscope.
2.5. Neuropeptides employed
In our current investigation we employed somatostatin octapeptide analog RC-160, targeted cytotoxic somatostatin analogs AN-162 and AN-238, LHRH antagonist Cetrorelix, cytotoxic LHRH analog AN-152 as well as GHRH antagonist MIA-602. Doxorubicin (DOX) and Dox hydrochloride were obtained from Chemex Export-Import. All analogs were synthesized in the laboratories of one of us (A.V.S.) (Zarandi et al., 1994).
2.6. Statistical analyses
In all experiments, statistical differences between experimental groups relative to appropriate controls were determined by ANOVA. Data are presented as means ± SEM. Significance of differences was tested by analysis of variance with Bonferroni’s post hoc test. Differences were considered significant at values of P < 0.05. Cells from at least 2 different passages were used for each experimental series; n represents the number of cells or tissue culture dishes investigated.
Results
Immunohistochemical analyses
We established abundant receptor protein expression for somatostatin subtype 2 (sst2), for growth hormone releasing-hormone (GHRH) and for luteinizing hormone-releasing hormone (LHRH) on MPC and MTT cells (Figure 1).
Figure 1.
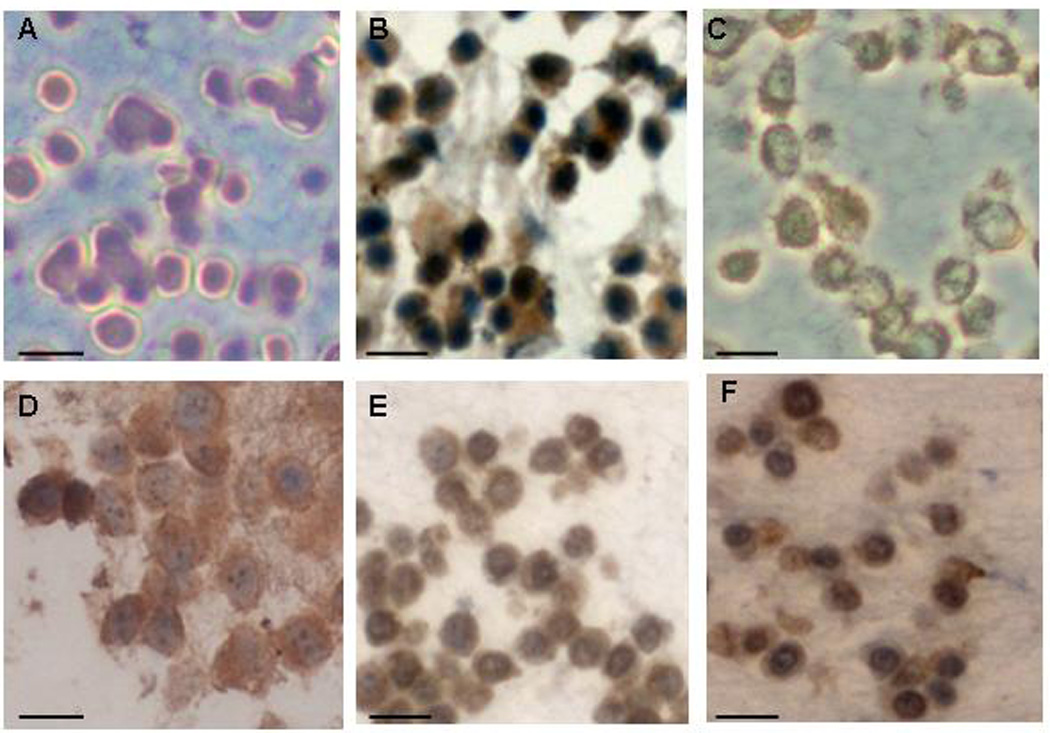
Immuohistochemical analyses: protein expression of the neuropeptide hormone receptors on mouse pheochromocytoma cells (A-F). Negative control (A). MPC express sst2 (B), GHRHR (C) and LHRHR (D) and on MTT cells we found a pronounced expression of GHRHR (E) and LHRHR (F) (Scale bars: 20 µm).
Effect of peptide analogs on MPC
AN-162 and AN-238 targeting SSTR
Employing cytotoxic analogs of somatostatin, AN-162 and AN-238 (10−6 mol/l), we found strong and significant reductions in cell viability over three days of incubation of MPC with these anti-cancer compounds, while the non-targeted and more systemically acting anti-cancer drug doxorubicin was at the same concentration (10−6 mol/l) and study time (24–72h) not affecting MPC viability (Fig. 2A). When we applied lower concentrations of the somatostatin analogs (10−7–10−8 mol/l) we could not document significant anti-tumor effects. In our study we could also demonstrate AN-162 (24–48h) and AN-238 (24h) to significantly increase caspase 3/7 activity with a most effective concentration of 10−6 mol/l (Fig. 2B). Lower concentrations of the cytotoxic somatostatin analogs (10−7–10−8) were not effective or not as effective as 10−6 mol/l, as was the case for doxorubicin.
Figure 2.
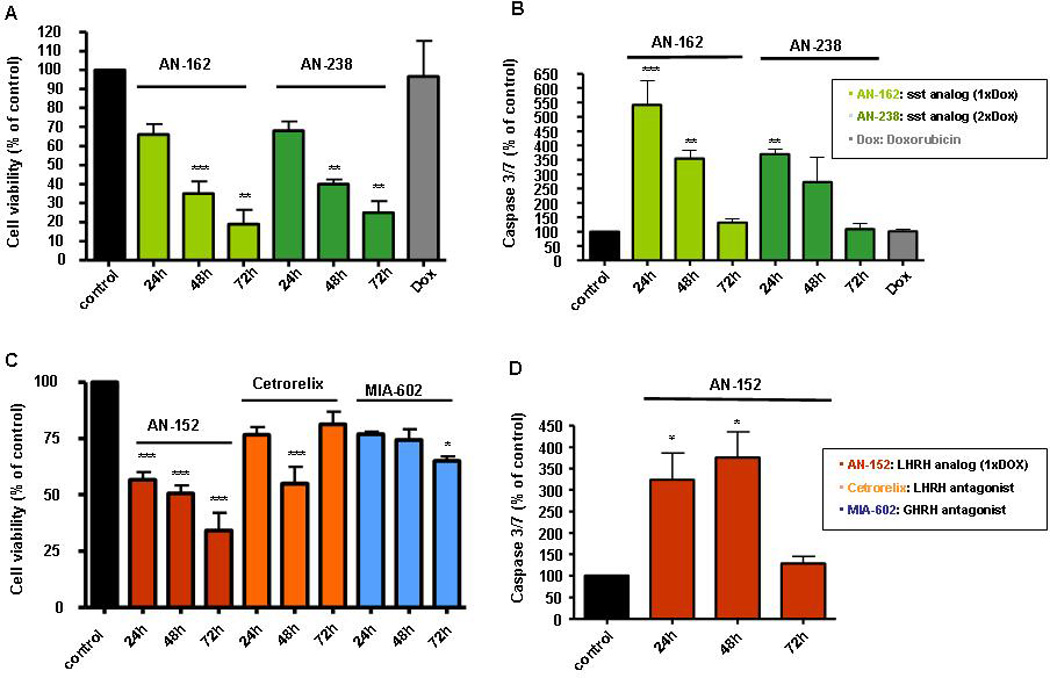
Anti-tumor effects of peptide analogs targeting ss2, LHRHR and GHRHR in MPC. AN-162 and AN-238 significantly influenced MPC cell viability over 24–72h (A) and highly significant increased caspase 3/7 activity (B) over 24–72h (10−6 mol/l). Similarly, GHRH antagonist MIA-602, LHRH antagonist Cetrorelix and LHRH analog AN-152 reduced MPC cell viability (C). AN-152 also significantly increased programmed MPC cell death (D) (n= 3–6, *P < 0.05, **P < 0.01, ***P < 0.001 as compared to control for all assays).
Effects of MIA-602 as well as of Cetrorelix and AN-152 targeting GHRHR and LHRHR
Since also receptors for LHRH and GHRH were strongly expressed on our analyzed adrenal tumor cell lines and tumor tissues, we were interested to evaluate possible anti-tumor effects mediated by the cytotoxic LHRH analog AN-152, by the LHRH antagonist Cetrorelix as well as by the GHRH antagonist MIA-602 on MPC. While AN-152 (10−6 mol/l) highly significant reduced cell viability over three days, Cetrorelix (10−6 mol/l) showed highly significant anti-tumor effects only after 48h. Lower concentrations (10−7–10−8) of both compounds were again not effective on MPC (Fig. 2C). Studying the effects of MIA-602, we found this GHRH antagonist to slightly but significantly reduce MPC survival after 72h, at a concentration of 10−6 mol/l. Furthermore, mediated by activation of caspases 3 and 7, AN-152 (10−7–10−8 mol/l) directed MPC into programmed cell death (24h-72h) (Fig. 2D).
Effect of peptide analogs on MTT cells
AN-152 and MIA-602 targeting LHRHR and GHRHR
Similar to MPC cells, the more aggressive MTT cells demonstrated pronounced expression of LHRH and GHRH receptors. AN-152 (10−6 mol/l) was again found to strongly and highly significant reduce MTT cell viability after 24h. Interestingly, after 2–3 days both 10−6 mol/l and 10−7 mol/l had a pronounced and significant effect on MTT cell survival, while there was no effect of AN-152 at 10−8 mol/l (Fig. 3A). Furthermore, at both 10−6 and 10−7 mol/l, AN-152 strongly induced programmed cell death with a most prominent effect after 24h (10−6 mol/l) (Fig. 3B). Finally, since also the GHRH receptor was abundantly expressed on MTT cells we were interested to studied possible anti-tumor effects mediated by the GHRH antagonist MIA-602 and could show that MIA-602 decreased MTT cell survival only at a rather high concentration of 10−5 mol/l after 2–3 days (Fig. 3C). Studies on the possible effects of the targeted cytotoxic somatostatin analogs AN-162 and AN-238 on MTT cells are still ongoing.
Figure 3.
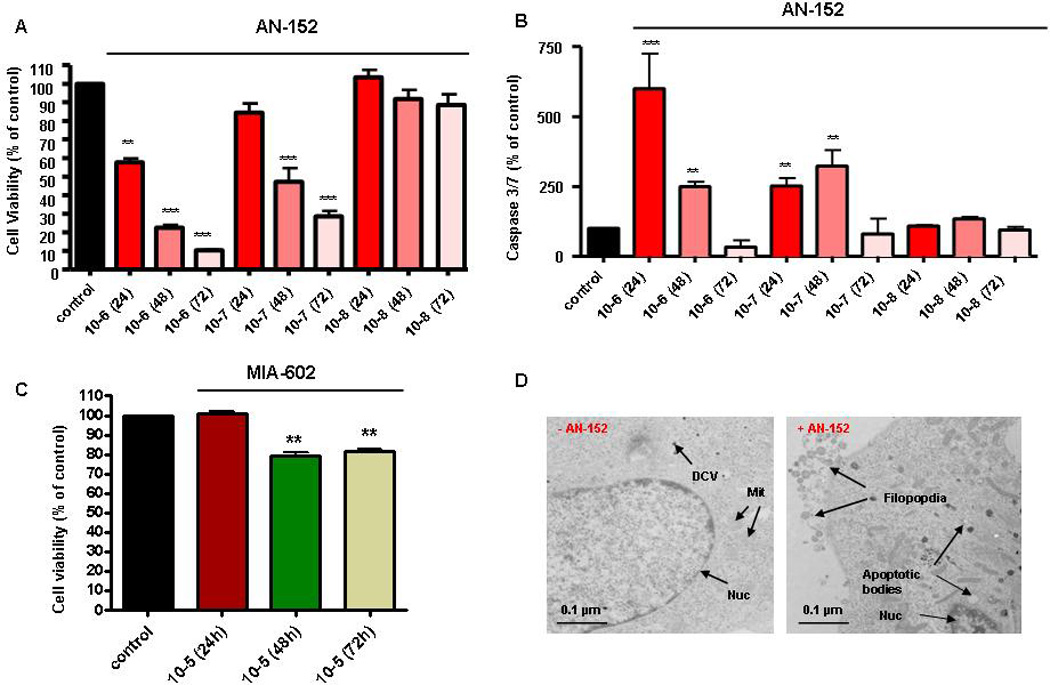
Anti-tumor effects of peptide analogs targeting LHRHR and GHRHR in MTT cells. AN-152 (10−6–10−7 mol/l) highly significant reduced MTT cell viability (A) and induced cell apoptosis (B) over 24–72h. (C) MIA-602 (10−5 mol/l) significantly reduced cell viability of more malignant MTT cells (48–72h). (D) Ultrastructural analyses. Confirmation of pro-apoptotic mode of cell death induced by AN-152, including shrinking of the cytoplasm away from the plasma membrane, apoptotic bodies, internucleosomal DNA fragmentation, or condensation of the cytoplasm while retaining mitochondria and endomembrane structure (Scale bar, 0.2 µm); DCV, dense-core vesicles; MIT, mitochondria; Nuc, nucleus.
Ultrastructural analyses
We could further confirm this pro-apoptotic mode of cell death induced by AN-152 on an ultrastructural level. Application of AN-152 (10−6 mol/l) revealed characteristic apoptotic changes, including apoptotic bodies, filopodia and internucleosomal DNA fragmentation, while MTT cells without AN-152 showed characteristics of normal chromaffin cells with a relatively low number of dense core vesicles (Fig. 3D).
Discussion
This study, which extends previous work in other tumor cells (Ziegler et al., 2009) to MPC and MTT cell lines relevant to neuroendocrine tumors, provides further evidence that targeted hormone receptor therapy might provide a novel approach for metastatic pheochromocytoma. Our study revealed an abundant expression of various neuropeptide hormone receptors on MPC and MTT cells as well as significant anti-tumor effects when applying highly potent and long-lasting peptide hormone analogs, specifically targeting these overexpressed receptors. Although until now, the functional role and importance of mutated and upregulated peptide receptors occasionally detected in various tumors including pheochromocytoma are not known, the present results suggest that targeting these overexpressed receptors might provide a promising therapeutic approach for patients with these tumors (Schally et al., 2011).
After receptor-ligand interaction at the cell membrane, the ligand (usually agonist) is internalized, as e.g. demonstrated for the targeted cytotoxic LHRH analog AN-152 (Emons et al., 2010). In this way, radiotracers, important for tumor imaging could easily accumulate in the cancer cell or the internalized ligand may also be able to selectively destroy the target cell, or inhibit hormone secretion as shown for somatostatin and its analogs (Schally et al., 2004). Also our present study adds further evidence for significant anti-tumor effects mediated by the cytotoxic somatostatin analogs AN-162 and AN-238 on pheochromocytoma cells in vitro.
Another class of potent anti-tumor compounds are analogs of LHRH as demonstrated already 30 years ago on prostate cancer (Tolis et al., 1982). Currently the LHRH analog AN-152 (AEZS-108) is provided to patients enrolled in phase II clinical trials suffering form prostate, ovarian or endometrial cancer (Engel et al., 2012). Additionally, in experimental models, analogs of LHRH have been shown to exert direct effects on human breast and bladder cancers through specific LHRH receptors, while non targeted doxorubicin was not as effective and more toxic (Schally et al., 2011; Szepeshazi et al., 2012). Also the side effects of targeted cytotoxic LHRH analogs are expected to be only minor (Emons et al., 2009). Besides AN-152 also the LHRH antagonist Cetrorelix exerted a strong anti-tumor effect on our chromaffin cell tumor model. Interestingly, Cetrorelix has recently been shown to reduce prostate size and prostatic hyperplasia via influencing inflammatory cytokines (Rick et al., 2011).
A third class of promising and highly effective anticancer compounds are GHRH antagonists. These agents show widespread anti-tumor effects and were already successfully employed over many years by Schally and co-workers in numerous experimental tumor models (Schally et al., 2008). In our study, we especially focused on MIA-602 and found significant reductions in cell viability on both MPC and MTT cells. Furthermore, just recently, MIA-602 has been shown to significantly decrease cell viability and well known parameters of tumor spreading on three different human cancers (Bellyei et al., 2010).
In summary, our present study on mouse pheochromocytoma cells demonstrated significant anti-tumor effects of peptide analogs targeting sst2, GHRHR and LHRHR in adrenomedullary tumors. Together with our previous investigation (Ziegler et al., 2009) we provide further evidence for a successful application of targeted peptide hormone receptor therapy on adrenal tumor cells. Future in vivo studies on two different mouse metastatic pheochromocytoma models, based on subcutaneous and intravenous application of MPC and MTT cells, will hopefully shed more light on the possible therapeutic efficiency of various peptide analogs in experimental and pre-clinically models of metastatic pheochromocytoma.
Acknowledgments
This work was supported by The Deutsche Forschungsgemeinschaft (Grants ZI-1362/2-1(C.G.Z. & G.E.), and BE-2607/1-1 (R.B. & J.P)). We are very thankful to Prof. Arthur Tischer and Dr. James Powers for providing us both the MPC as well as the MTT cell lines.
Literature
- Bellyei S, Schally AV, Zarandi M, Varga JL, Vidaurre I, Pozsgai E. GHRH antagonists reduce the invasive and metastatic potential of human cancer cell lines in vitro. Cancer Lett. 2010;293:31–40. doi: 10.1016/j.canlet.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Brouwers FM, Elkahloun AG, Munson PJ, Eisenhofer G, Barb J, Linehan WM, Lenders JW, De Krijger R, Mannelli M, Udelsman R, Ocal IT, Shulkin BL, Bornstein SR, Breza J, Ksinantova L, Pacak K. Gene expression profiling of benign and malignant pheochromocytoma. Ann. N. Y. Acad. Sci. 2006;1073:541–556. doi: 10.1196/annals.1353.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz S, Keller G, Schally AV, Halmos G, Hohla F, Heinrich E, Koester F, Baker B, Engel JB. Therapy of ovarian cancers with targeted cytotoxic analogs of bombesin, somatostatin, and luteinizing hormone-releasing hormone and their combinations. Proc. Natl. Acad. Sci. U S A. 2006;103:10403–10407. doi: 10.1073/pnas.0602971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz S, Seitz S, Schally AV, Engel JB, Rick FG, Szalontay L, Hohla F, Krishan A, Papadia A, Gaiser T, Brockhoff G, Ortmann O, Diedrich K, Köster F. Triple-negative breast cancers express receptors for luteinizing hormone-releasing hormone (LHRH) and respond to LHRH antagonist cetrorelix with growth inhibition. Int. J. Oncol. 2009;35:789–796. doi: 10.3892/ijo_00000391. [DOI] [PubMed] [Google Scholar]
- Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr. Relat. Cancer. 2007;14:569–585. doi: 10.1677/ERC-07-0074. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Siegert G, Kotzerke J, Bornstein SR, Pacak K. Current progress and future challenges in the biochemical diagnosis and treatment of pheochromocytomas and paragangliomas. Horm. Metab. Res. 2008;40:329–337. doi: 10.1055/s-2008-1073156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emons G, Kaufmann M, Gorchev G, Tsekova V, Gründker C, Günthert AR, Hanker LC, Velikova M, Sindermann H, Engel J, Schally AV. Dose escalation and pharmacokinetic study of AEZS-108 (AN-152), an LHRH agonist linked to doxorubicin, in women with LHRH receptor-positive tumors. Gynecol. Oncol. 2010;119:457–461. doi: 10.1016/j.ygyno.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Engel J, Emons G, Pinski J, Schally AV. AEZS-108: a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert. Opin. Investig. Drugs. 2012;21:891–899. doi: 10.1517/13543784.2012.685128. [DOI] [PubMed] [Google Scholar]
- Fassnacht M, Kreissl MC, Weismann D, Allolio B. New targets and therapeutic approaches for endocrine malignancies. Pharmacol. Ther. 2009;123:117–141. doi: 10.1016/j.pharmthera.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Forrer F, Riedweg I, Maecke HR, Mueller-Brand J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q. J. Nucl. Med. Mol. Imaging. 2008;52:334–340. [PubMed] [Google Scholar]
- Giubellino A, Woldemichael GM, Sourbier C, Lizak MJ, Powers JF, Tischler AS, Pacak K. Characterization of two mouse models of metastatic pheochromocytoma using bioluminescence imaging. Cancer Lett. 2012;316:46–52. doi: 10.1016/j.canlet.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JL, Yeh MW, Robinson BG, Delbridge LW, Sidhu SB. Potential pitfalls in the diagnosis of phaeochromocytoma. Med. J. Aust. 2005;182:637–640. [PubMed] [Google Scholar]
- Klukovits A, Schally AV, Szalontay L, Vidaurre I, Papadia A, Zarandi M, Varga JL, Block NL, Halmos G. Novel antagonists of growth hormone-releasing hormone inhibit growth and vascularization of human experimental ovarian cancers. Cancer. 2012;118:670–680. doi: 10.1002/cncr.26291. [DOI] [PubMed] [Google Scholar]
- Korpershoek E, Pacak K, Martiniova L. Murine models and cell lines for the investigation of pheochromocytoma: applications for future therapies? Endocr. Pathol. 2012;23:43–54. doi: 10.1007/s12022-012-9194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix A, Ndiaye N, Tremblay J, Hamet P. Ectopic and abnormal hormone receptors in adrenal Cushing's syndrome. Endocr. Rev. 2001;22:75–110. doi: 10.1210/edrv.22.1.0420. [DOI] [PubMed] [Google Scholar]
- Martiniova L, Lai EW, Elkahloun AG, Abu-Asab M, Wickremasinghe A, Solis DC, Perera SM, Huynh TT, Lubensky IA, Tischler AS, Kvetnansky R, Alesci S, Morris JC, Pacak K. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin. Exp. Metastasis. 2009;26:239–250. doi: 10.1007/s10585-009-9236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiniova L, Lu J, Chiang J, Bernardo M, Lonser R, Zhuang Z, Pacak K. Pharmacologic modulation of serine/threonine phosphorylation highly sensitizes PHEO in a MPC cell and mouse model to conventional chemotherapy. PLoS One. 2011;6:e14678. doi: 10.1371/journal.pone.0014678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Schally AV, Armatis P, Szepeshazi K, Halmos G, Kovacs M, Zarandi M, Groot K, Miyazaki M, Jungwirth A, Horvath J. Cytotoxic analogs of luteinizing hormonereleasing hormone containing doxorubicin or 2-pyrrolinodoxorubicin, a derivative 500–1000 times more potent. Proc. Natl. Acad. Sci. U S A. 1996;93:7269–7273. doi: 10.1073/pnas.93.14.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolting S, Grossman AB. Signaling pathways in pheochromocytomas and paragangliomas: prospects for future therapies. Endocr. Pathol. 2012;23:21–33. doi: 10.1007/s12022-012-9199-6. [DOI] [PubMed] [Google Scholar]
- Ohta S, Lai EW, Taniguchi S, Tischler AS, Alesci S, Pacak K. Animal models of pheochromocytoma including NIH initial experience. Ann. N. Y. Acad. Sci. 2006;1073:300–305. doi: 10.1196/annals.1353.034. [DOI] [PubMed] [Google Scholar]
- Powers JF, Evinger MJ, Tsokas P, Bedri S, Alroy J, Shahsavari M, Tischler AS. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000;302:309–320. doi: 10.1007/s004410000290. [DOI] [PubMed] [Google Scholar]
- Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- Rick FG, Schally AV, Block NL, Halmos G, Perez R, Fernandez JB, Vidaurre I, Szalontay L. LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate. 2011;71:736–747. doi: 10.1002/pros.21289. [DOI] [PubMed] [Google Scholar]
- Schally AV. New approaches to the therapy of various tumors based on peptide analogs. Horm. Metab. Res. 2008;40:315–322. doi: 10.1055/s-2008-1073142. [DOI] [PubMed] [Google Scholar]
- Schally AV, Comaru-Schally AM, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga JL, Halmos G. Hypothalamic hormones and cancer. Front. Neuroendocrinol. 2001;22:248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- Schally AV, Engel JB, Emons G, Block NL, Pinski J. Use of analogs of peptide hormones conjugated to cytotoxic radicals for chemotherapy targeted to receptors on tumors. Curr. Drug Deliv. 2011;8:11–25. doi: 10.2174/156720111793663598. [DOI] [PubMed] [Google Scholar]
- Schally AV, Szepeshazi K, Nagy A, Comaru-Schally AM, Halmos G. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell Mol. Life Sci. 2004;61:1042–1068. doi: 10.1007/s00018-004-3434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- Scholz T, Eisenhofer G, Pacak K, Dralle H, Lehnert H. Clinical review: Current treatment of malignant pheochromocytoma. J. Clin. Endocrinol. Metab. 2007;92:1217–1225. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- Szepeshazi K, Schally AV, Keller G, Block NL, Benten D, Halmos G, Szalontay L, Vidaurre I, Jaszberenyi M, Rick FG. Receptor-targeted therapy of human experimental urinary bladder cancers with cytotoxic LH-RH analog AN-152 [AEZS- 108] Oncotarget. 2012;22 doi: 10.18632/oncotarget.546. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, Comaru-Schally AM, Schally AV. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc. Natl. Acad. Sci. U S A. 1982;79:1658–1662. doi: 10.1073/pnas.79.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, De Jong FH, Christiansen A, Kam BL, De Herder WW, Stridsberg M, Lindemans J, Ensing G, Krenning EP. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin. Nucl. Med. 2002;32:110–122. doi: 10.1053/snuc/2002.31025. [DOI] [PubMed] [Google Scholar]
- Willenberg HS, Stratakis CA, Marx C, Ehrhart-Bornstein M, Chrousos GP, Bornstein SR. Aberrant interleukin-1 receptors in a cortisol-secreting adrenal adenoma causing Cushing's syndrome. N. Engl. J. Med. 1998;339:27–31. doi: 10.1056/NEJM199807023390105. [DOI] [PubMed] [Google Scholar]
- Zarandi M, Horvath JE, Halmos G, Pinski J, Nagy A, Groot K, Rekasi Z, Schally AV. Synthesis and biological activities of highly potent antagonists of growth hormone-releasing hormone. Proc. Natl. Acad. Sci. U S A. 1994;91:12298–12302. doi: 10.1073/pnas.91.25.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler CG, Brown JW, Schally AV, Erler A, Gebauer L, Treszl A, Young L, Fishman LM, Engel JB, Willenberg HS, Petersenn S, Eisenhofer G, Ehrhart-Bornstein M, Bornstein SR. Expression of neuropeptide hormone receptors in human adrenal tumors and cell lines: antiproliferative effects of peptide analogs. Proc. Natl. Acad. Sci. U S A. 2009;106:15879–15884. doi: 10.1073/pnas.0907843106. [DOI] [PMC free article] [PubMed] [Google Scholar]


