Abstract
Background and Aims
The large monophyletic genus Mimosa comprises approx. 500 species, most of which are native to the New World, with Central Brazil being the main centre of radiation. All Brazilian Mimosa spp. so far examined are nodulated by rhizobia in the betaproteobacterial genus Burkholderia. Approximately 10 Mya, transoceanic dispersal resulted in the Indian subcontinent hosting up to six endemic Mimosa spp. The nodulation ability and rhizobial symbionts of two of these, M. hamata and M. himalayana, both from north-west India, are here examined, and compared with those of M. pudica, an invasive species.
Methods
Nodules were collected from several locations, and examined by light and electron microscopy. Rhizobia isolated from them were characterized in terms of their abilities to nodulate the three Mimosa hosts. The molecular phylogenetic relationships of the rhizobia were determined by analysis of 16S rRNA, nifH and nodA gene sequences.
Key Results
Both native Indian Mimosa spp. nodulated effectively in their respective rhizosphere soils. Based on 16S rRNA, nifH and nodA sequences, their symbionts were identified as belonging to the alphaproteobacterial genus Ensifer, and were closest to the ‘Old World’ Ensifer saheli, E. kostiensis and E. arboris. In contrast, the invasive M. pudica was predominantly nodulated by Betaproteobacteria in the genera Cupriavidus and Burkholderia. All rhizobial strains tested effectively nodulated their original hosts, but the symbionts of the native species could not nodulate M. pudica.
Conclusions
The native Mimosa spp. in India are not nodulated by the Burkholderia symbionts of their South American relatives, but by a unique group of alpha-rhizobial microsymbionts that are closely related to the ‘local’ Old World Ensifer symbionts of other mimosoid legumes in north-west India. They appear not to share symbionts with the invasive M. pudica, symbionts of which are mostly beta-rhizobial.
Keywords: Mimosa hamata, Mimosa himalayana, Mimosa pudica, Thar Desert, nodulation, Cupriavidus, Burkholderia, Ensifer, bacterial symbionts, rhizobia, Betaproteobacteria, nitrogen fixation, arid regions
INTRODUCTION
The large monophyletic genus Mimosa (Mimosoideae; Fabaceae) consists of >500 species, mainly native to the New World (Barneby, 1991; Simon et al., 2011). Species vary in habit from tall trees and shrubs to vines and herbs and they are found in a wide variety of habitats from wet to dry, growing on many different soils, including those that are low in nutrients and organic matter, low in pH and iron rich. Mimosa was considered by Barneby (1991) to have ‘differentiated profusely in tropical and warm temperate savanna habitats’, and it is particularly abundant and diverse in the cerrado and caatinga biomes of Brazil, where there are many endemics (Barneby, 1991; Simon and Proença, 2000; Simon et al., 2011). Despite this high endemism, a few species have become pan-tropical invasive weeds, the most notorious of these being M. diplotricha (synonym M. invisa), M. pigra and M. pudica (Barneby, 1991; Chen et al., 2005a; Parker et al., 2007; Simon et al., 2011). Nodulation by N2-fixing bacteria (rhizobia) has been observed in almost all of approx. 100 Mimosa spp. that have been examined (dos Reis Junior et al., 2010). Indeed, it is likely that their ability to nodulate profusely in alien environments has greatly assisted the spread of the invasive Mimosa spp. outside their predominantly native Americas (Chen et al., 2005a; Parker et al., 2007; Andrus et al., 2012).
It is partly because of the seriousness of invasive Mimosa spp. as aggressive weeds that their bacterial symbionts have attracted a lot of interest in recent years, particularly as initial studies of invasive M. diplotricha, M. pudica and M. pigra in Taiwan showed that they were almost exclusively nodulated by strains of Betaproteobacteria (Chen et al., 2001, 2003a, b, 2005a). Legume nodulation by Betaproteobacteria (‘beta-rhizobia’) is a relatively recently described phenomenon; ‘rhizobia’ were formerly considered to consist exclusively of a limited number of genera in the order Rhizobiales in the Alphaproteobacteria (Graham, 2008; Sprent, 2009). Since their initial discovery, a considerable body of evidence has accumulated to show that legumes, particularly Mimosa spp. (Chen et al., 2001, 2003a, b, 2005a, b, 2006; Barrett and Parker, 2005, 2006; Elliott et al., 2007a; Andam et al., 2007; Bontemps et al., 2010; Mishra et al., 2012), but also other mimosoids and some papilionoids, such as Cyclopia (Elliott et al., 2007b), Rhynchosia (Garau et al., 2009), common bean (Phaseolus vulgaris) (Talbi et al., 2010) and Lebeckia spp. (Howieson et al., 2013), may form effective nodules with bacteria in the genera Burkholderia and Cupriavidus (Ralstonia) (see review by Gyaneshwar et al., 2011).
The consistent isolation of beta-rhizobia from Mimosa nodules worldwide suggested a special relationship between them and this legume genus, and this was investigated by a large study of symbionts of Mimosa spp. native to the cerrado and caatinga biomes of Central Brazil. These biomes are home to >250 Mimosa spp., most of them endemics to either the biomes as a whole or to specific (mainly highland) regions within them (Simon and Proença, 2000; Simon et al., 2011). The surveys by Bontemps et al. (2010) and dos Reis Junior et al. (2010) showed that >95 % of the nodules from approx. 70 Mimosa spp. from the cerrado/caatinga contained Burkholderia spp. as their symbionts. These studies thus demonstrated that Burkholderia spp. are the predominant symbionts of Mimosa in its largest centre of radiation, i.e. Brazil. In addition, Bontemps et al. (2010) showed that there was high congruence between the core ‘housekeeping’ (16S rRNA, recA) and symbiosis-related (nifH, nodC) genes in the microsymbionts, and suggested that the symbiosis between Mimosa and Burkholderia spp. was ‘ancient’ (approx. 50 Myr old) and, therefore, unlikely to have been the result of recent transfer(s) of symbiosis-related genes from alpha-rhizobia.
In contrast to Brazil, in the second major centre of Mimosa radiation in the central highlands of Mexico, which has approx. 100 species (Barneby, 1991), it would appear that most of the endemic Mimosa spp. are nodulated not by Betaproteobacteria but by Rhizobium or Ensifer (synonym Sinorhizobium). This was first suggested by a study on just a single native Mexican species, M. affinis (Wang et al., 1999), and then confirmed by a wider study on approx. 30 central Mexican species by C. Bontemps, Université de Lorraine, France and M. A. Rogel, Centro de Ciencias Genómicas, Mexico (unpubl. res.). This difference between Brazil and Mexico suggests that geographical separation/location (and possibly soil type) and host phylogenetic relationships (Simon et al., 2011) have played a part in determining symbiont selection by Mimosa in the New World. In addition to the two major centres of Mimosa radiation in Brazil and Mexico there are two smaller ones in the Old World: Madagascar, with approx. 30 endemic species, and the Indian subcontinent with six (M. angustisiliqua, M. barberi, M. hamata, M. himalayana, M. prainiana and M. rubicaulis) (Gamble, 1920; Barneby, 1991; Simon et al., 2011). These Old World species are phylogenetically nested in South American Mimosa, and it has been hypothesized that they arrived in Asia approx. 6 – 10 Mya via trans-Atlantic dispersal (Simon et al., 2011).
Little is known about the symbionts of the Old World Mimosa spp., but given that they are closely related to South American species it might be expected that they would have retained their ability to nodulate with similar bacterial symbionts, i.e. with Burkholderia strains (Bontemps et al., 2010). This appears to be the case with at least one species, M. himalayana, as it could nodulate effectively with the promiscuous Mimosa symbiont B. phymatum STM815T, and ineffectively with C. taiwanensis LMG 19424T (Elliott et al., 2007a). The same symbiotic phenotype was evidenced by several South American species tested with these strains (Elliott et al., 2007a; dos Reis Junior et al., 2010). However, a recent study of legumes native to the Thar Desert in Rajasthan in western India showed that the symbionts of M. hamata, a species closely related to M. himalayana, include strains of Ensifer that are related to E. saheli (Gehlot et al., 2012). The only other published study on Mimosa symbionts from India is that of Verma et al. (2004), who described two strains of C. taiwanensis, BHU1 and MS1, isolated from nodules on the non-native species, M. pudica, collected in the north (Uttar Pradesh) and south (Tamil Nadu) of India, respectively.
India thus represents a unique situation regarding Mimosa symbionts as, unlike other parts of sub-tropical and tropical Asia and Australasia, such as southern China (Liu et al., 2011, 2012), Taiwan (Chen et al., 2001, 2003b, 2005a), Australia (Parker et al., 2007), New Guinea (Elliott et al., 2009), the Philippines (Andrus et al., 2012) and New Caledonia (Klonowska et al., 2012) that harbour only invasive species (particularly M. pudica, which is common to them all), India also has native Mimosa spp. This raises the possibility of interaction(s) between the symbionts of the native/invasive species and their respective hosts. The present study, therefore, was aimed at: (1) examining in more detail the symbionts of native and invasive Mimosa spp. to determine their diversity and potential origins; and (2) determining if the native species share their environments and/or rhizobial symbionts with the invasive M. pudica.
MATERIALS AND METHODS
Collection of plant materials and soils for rhizobial ‘trap’ experiments and isolation of nodule symbionts
The sites in Rajasthan (RJ) from which the native Indian Mimosa species were sampled are characterized as semi-arid, whereas all the M. pudica sites are characterized as humid sub-tropical, with the exception of Bangalore (KA) which has a tropical wet/dry climate. Details are given in Table 1, where abbreviations for the locations can also be found in the footnote.
Table 1.
Sites from which Mimosa seeds and nodules were collected, their climatic types, soil characteristics (pH, %N) and nodulation of Mimosa spp. in rhizosphere soil used for ‘trapping’ of rhizobia
| Site (State)* | Coordinates | Altitude (m) | Site from which nodules and/or soil was sampled. Climate. | Soil pH | Soil %N | Mimosa spp. native to the soil | Mimosa spp. used to trap rhizobia† |
|---|---|---|---|---|---|---|---|
| Jodhpur (RJ) | 26°14′49·85″N/73°1′18·65″E | 230·61 | Field near Bhagat ki Kothi (New Campus, JNVU) in the native range of M. hamata. Semi-arid (rainfall <300 mm p.a.). | 8·2 | 0·0091 | M. hamata‡ | M. hamata (E), M. himalayana (E), M. pudica (E) |
| Deh (Nagaur) (RJ) | 27°18′30·40″N/73°54′53·51″E | 303·38 | Soil from rhizosphere of M. hamata in the Thar Desert. Semi-arid. | 8·3 | 0·0102 | M. hamata | M. hamata (E), M. himalayana (E) |
| Fatehpur (Sikar) (RJ) | 27°58′0·43″N/74°58′21·02″E | 328·61 | Soil from rhizosphere of M. hamata bordering the Thar Desert. Semi-arid. | 8·5 | 0·0085 | M. hamata | M. hamata (E) |
| Chhapar (Churu) (RJ) | 27°45′43·57″N/74°27′12·25″E | 329·8 | Soil from rhizosphere of M. hamata bordering the Thar Desert. Semi-arid. | 8·7 | 0·0097 | M. hamata | M. hamata (E), M. himalayana (E) |
| Bikaner (RJ) | 28°1′49·04″N/73°15′30·63″E | 238·3 | Soil from rhizosphere of M. hamata in the Thar Desert. Semi-arid. | 8·4 | 0·0078 | M. hamata | M. hamata (E) |
| Barmer (RJ) | 25°39′54·66″N/72°0′54·03″E | 227·1 | Soil from rhizosphere of M. hamata bordering the Thar Desert. Semi-arid. | 8·6 | 0·0071 | M. hamata | M. hamata (E), M. himalayana (E) |
| Bijoliya (Bhilwara) (RJ) | 25°7′25·78″N/75°16′24·28″E | 508·79 | Soil from rhizosphere of M. himalayana collected from field within its native range. Semi-arid with higher rainfall than the Thar Desert (rainfall = 600 mm p.a.) | 7·8 | 0·0216 | M. himalayana | M. hamata (–), M. himalayana (E), M. pudica (–) |
| Agra (UP) | 27°16′60·00″N/77°58′0·00″E | 324·85 | Nursery seedlings collected from the field. Humid sub-tropical. | 7·2 | 0·0352 | M. pudica‡ | ND |
| Bokaro (JH) | 23°45′27·10″N/85°53′36·52″E | 232·42 | Konar, riverside near BTPS, Kothara. Humid sub-tropical. | 6·9 | 0·0432 | M. pudica‡ | M. hamata (I), M. himalayana (E), M. pudica (E) |
| Bangalore (KA) | 13°0′39·54″N/77°34′13·70″E | 895·79 | Nursery seedlings in the campus of Indian Wood Science Technology (IWST). Wet and dry tropical. | 6·8 | 0·0352 | M. pudica‡ | ND |
| Haridwar (UT) | 30°5′14·65″N/78°15′55·47″E | 327·45 | Plants on roadside near Rishikesh. Humid sub-tropical. | 7·5 | 0·0322 | M. pudica‡ | ND |
| Jorhat (AS) | 26°46′57·25″N/94°17′35·92″E | 91 | Field-grown plant in the grounds of the Rain Forest Research Institute (RFRI). Humid sub-tropical. | 5·2 | 0·065 | M. pudica‡ | M. hamata (–), M. himalayana (–), M. pudica (E) |
| Shillong (ME) | 25°39′18·83″N/91°53′52·85″E | 3216 | Plants on roadside in Barapani area near Shillong. Humid sub-tropical. | 4·9 | 0·280 | M. pudica‡ | M. hamata (–), M. himalayana (–), M. pudica (E) |
* Standard abbreviations used: AS, Assam; JH, Jharkhand; KA, Karnataka; ME, Meghalaya; RJ, Rajasthan; UP, Uttar Pradesh; UT, Uttarakhand.
† E, effective; I, ineffective; –, no nodules; ND, not determined.
‡ Nodules sampled directly from plants in the field.
Nodules were collected from some M. hamata plants growing naturally, e.g. near Jodhpur, Rajasthan (Gehlot et al., 2012), but most M. hamata nodules were sampled from the roots of plants grown in pots using soil taken from the rhizosphere of M. hamata growing in its native range in various locations in the Thar Desert of Rajasthan (Table 1, Supplementary Data Fig. S1). Soil for ‘trapping’ M. himalayana rhizobia was sampled from the rhizosphere of this species growing in its native range in eastern Rajasthan (Bijoliya), which is characterized by a higher altitude and precipitation than that in the native range of M. hamata (Table 1). The M. pudica nodules/rhizospheric soils were sampled from plants growing in several parts of India, encompassing sites in the north-west (Haridwar, UT), centre (Agra, UP), west (Jodhpur, RJ), south (Bangalore, KA), east (Bokaro, JH) and north-east (Jorhat, AS; Shillong, ME) of the country (Table 1, Supplementary Data Fig. S1).
To trap symbionts of M. hamata, M. himalayana and M. pudica growing in the various rhizosphere soils, seeds of each species were germinated as previously described (Elliott et al., 2007a), and the seedlings were then sown into soil in pots (8 kg soil per pot) and grown in a greenhouse for up to 12 weeks, at which time the plants were harvested and nodules were sampled from the roots. Bacteria were axenically isolated from single nodules, purified from single colonies and cultivated on yeast-mannitol (YM) medium (Vincent, 1970) essentially as described by Bontemps et al. (2010). Some of the nodules were also cut in half to determine if they were potentially effective, as judged by the appearance of a pink colouration due to the presence of leghaemoglobin (Lb). Pink nodules were then placed in vials containing 2·5 % glutaraldehyde in 50 mm phosphate buffer (pH 7·5) for microscopical analysis.
In addition to rhizobial trapping experiments in Indian soils, M. hamata and M. himalayana were also sown in soil taken from the rhizosphere of Brazilian Mimosa spp. at Embrapa-CENARGEN, Brasília, Brazil.
Microscopy and immunolabelling of Mimosa nodules
Nodules were embedded in resin and sectioned for light and transmission electron microscopy (TEM) coupled with in situ immunogold labelling with antibodies raised against Burkholderia phymatum STM815T and Cupriavidus taiwanensis LMG 19424T according to Elliott et al. (2007a). These antibodies have been shown previously to be specific, respectively, to the genus Burkholderia and to the species C. taiwanensis (Elliott et al., 2007a; dos Reis Junior et al., 2010). To confirm their symbiotic effectiveness, the nodule sections were also labelled with an antibody that was raised against the NifH protein of the nitrogenase enzyme (dos Reis Junior et al., 2010). Non-immune serum was used as a negative control in all immunogold assays.
Genetic characterization of Mimosa-nodulating rhizobia
Potential rhizobial symbionts were isolated from nodules collected from the sites and/or trap plants described above (Table 1, Supplementary Data Fig. S1). Three nodules were sampled from each plant; in general, one symbiotic isolate per nodule was then obtained. All bacteria were grown in YM broth or on YM agar plates. The isolates were grouped based on their place of origin, and then further selected based upon their colony morphology on YM plates compared with known rhizobial type strains, and finally on their ability to nodulate their host species of Mimosa. Confirmed nodulating strains from each group from each location were then further characterized by PCR amplification and sequencing of their 16S rRNA genes, and representative strains from each 16S rRNA cluster were selected for sequencing of their nifH and nodA genes (Table 2). PCR amplifications were performed with genomic DNA that was extracted as described in Moulin et al. (2004). For all strains, the nearly full-length 16S rRNA gene was amplified and sequenced with primers AGAGTTTGATCCTGGCTCAG and AAGGAGGTGATCCAGCC (Weisburg et al., 1991). Partial nifH fragments from the isolates were amplified with primers CGTTTTACGGCAAGGGCGGTATCGGCA and TCCTCCAGCTCCTCCATGGTGATCGG (Perret and Broughton, 1998) for Alphaproteobacteria or with primers CGCIWTYTACGGIAARGGIGG and GGIKCRTAYTSGATIACIGTCAT for Betaproteobacteria (Chen et al., 2003b). Partial nodA fragments were amplified with primers TGCRGTGGAARNTRNNCTGGGAAA and GGNCCGTCRTCRAAWGTCARGTA (Haukka et al., 1998) for Alphaproteobacteria, with primers NodAF, AGTTGGGCCGGMGCNAGGCCTGA, and NodAR1, CAACGAACTGTTAATTGGCA, for Burkholderia strains, and with primers nodA F, 5′TGCRGTGGARDCTRYGCTGGGAAA 3′, and nodA R, 5′ TCACARCTCKGGCCCGTTCCG-3′, for Cupriavidus strains (Mishra et al., 2012). The PCR conditions for amplification were essentially as described earlier (Bontemps et al., 2010; Gehlot et al., 2012). The amplified gene products were purified using the QIAquickTM PCR purification kit. Sequencing was performed at Xcelris Genomics, Ahmedabad, India, using a ABI SOLiD V4·0 System, at the University of Wisconsin Madison DNA Sequencing Facility, and at the National Kaohsiung Marine University using an Applied Biosystems ABI Prism 3730 sequencer.
Table 2.
Rhizobial strains isolated from native and invasive Mimosa spp. in India and Brazil and their putative identification via matching of their 16S rRNA gene sequences with those in the databases; data are also shown for nodulation tests of selected strains with M. hamata (Mha), M. himalayana (Mhi) and M. pudica (Mp)
| Strain no. | Plant host (no. of isolates obtained) | Geographical origin (State) | 16S rRNA GenBank accession no. | Closest 16S rRNA BLASTN match (% similarity) | nifH GenBank accession no. | nodA GenBank accession no. | Mha | Mhi | Mp |
|---|---|---|---|---|---|---|---|---|---|
| MH1b | M. hamata (2) | Nagaur (Rajasthan) | GQ355314 | E. saheli LMG 7837T (99 %) | JQ951757 | JQ951758 | E | ND | |
| MH3 | M. hamata (2) | Sikar (Rajasthan) | GQ355315 | E. saheli LMG 7837T (99 %) | JQ951759 | JQ951760 | E | – | – |
| MH3a* | M. hamata | Sikar (Rajasthan) | JN867012 | E. saheli LMG 7837T (99 %) | JQ951761 | E | – | – | |
| MH8* | M. hamata (7) | Jodhpur (Rajasthan) | JN867013 | E. saheli LMG 7837T (99 %) | KC478282 | JQ951762 | E | – | – |
| MH9 | M. hamata | Jodhpur (Rajasthan) | GQ355316 | E. saheli LMG 7837T (99 %) | JQ951763 | E | ND | – | |
| MH32 | M. hamata (5) | Chhapar (Rajasthan) | JX843749 | E. saheli LMG 7837T (99 %) | JX843757 | JX843746 | E | ND | – |
| MH37 | M. hamata (3) | Bikaner (Rajasthan) | JX843750 | E. saheli LMG 7837T (99 %) | JX843758 | JX843747 | E | E | – |
| MH40 | M. hamata (3) | Barmer (Rajasthan) | JX843751 | E. saheli LMG 7837T (99 %) | JX843759 | JX843748 | E | E | – |
| MHM1 | M. himalayana (7) | Bijoliya (Rajasthan) | JQ951764 | E. saheli LMG 7837T (99 %) | JX843760 | JX843744 | – | E | – |
| MHM2 | M. himalayana | Bijoliya (Rajasthan) | JQ951766 | E. saheli LMG 7837T (99 %) | – | E | – | ||
| MHM3 | M. himalayana | Bijoliya (Rajasthan) | JQ951768 | E. saheli LMG 7837T (99 %) | – | E | – | ||
| MHM4 | M. himalayana | Bijoliya (Rajasthan) | JQ951770 | E. saheli LMG 7837T (99 %) | – | E | – | ||
| MHM12 | M. himalayana | Bijoliya (Rajasthan) | JQ951772 | E. saheli LMG 7837T (99 %) | JQ951773 | JQ951774 | – | E | – |
| MHM22 | M. himalayana (4) | Jodhpur (Rajasthan) | JQ951776 | E. saheli LMG 7837T (99 %) | JX112774 | JQ951777 | – | E | – |
| MHM24 | M. himalayana (3) | Nagaur (Rajasthan) | JX843752 | E. saheli LMG 7837T (99 %) | JX843761 | JX112778 | – | E | – |
| MHM32 | M. himalayana (3) | Chhapar (Rajasthan) | JQ951778 | E. saheli LMG 7837T (99 %) | JX112775 | JQ951779 | – | E | – |
| MHM40 | M. himalayana (3) | Barmer (Rajasthan) | JX843753 | E. saheli LMG 7837T (99 %) | JX843762 | JX843745 | – | E | – |
| MP3 | M. pudica (2) | Bangalore (Karnataka) | JQ951791 | C. oxalaticus DSM 1105T (97 %) | JX843754 | JQ951780 | I | I | E |
| MP6 | M. pudica (2) | Haridwar (Uttarakhand) | GQ355321 | C. taiwanensis LMG 19424T (99 %) | JX843755 | JX843742 | ND | ND | E |
| MP7 | M. pudica (4) | Jodhpur (Rajasthan) | GQ355322 | C. taiwanensis LMG 19424T (99 %) | JQ951781 | JQ951782 | ND | ND | E |
| MP10 | M. pudica (3) | Agra (Uttar Pradesh) | GQ355325 | R. vallis CCBAU 65647T (100 %) | JQ951784 | JQ951783 | I | ND | E |
| MP15 | M. pudica (3) | Agra (Uttar Pradesh) | GQ355324 | C. taiwanensis LMG 19424T (99 %) | JX843756 | JX843743 | – | – | E |
| MP20 | M. pudica (5) | Bokaro (Jharkhand) | GQ355318 | B. phymatum STM815T (99 %) | JQ951785 | JQ951786 | I | E | E |
| MPB1 | M. pudica (10) | Barapani (Meghalaya) | KC287136 | B. mimosarum PAS44T (99 %) | KC440177 | KC478283 | ND | ND | E |
| MPB6 | M. pudica | Barapani (Meghalaya) | KC287137 | B. mimosarum PAS44T (99 %) | KC440178 | ND | ND | E | |
| MPB8 | M. pudica | Barapani (Meghalaya) | KC287138 | B. mimosarum PAS44T (99 %) | KC440179 | KC478284 | ND | ND | E |
| MPB11 | M. pudica | Barapani (Meghalaya) | KC287139 | B. mimosarum PAS44T (99 %) | KC440180 | ND | ND | E | |
| MPJ1 | M. pudica (4) | Jorhat (Assam) | JQ951792 | B. phymatum STM815T (99 %) | JQ951788 | JX843740 | ND | ND | E |
| MPJ4 | M. pudica (4) | Jorhat (Assam) | JQ951793 | B. mimosarum PAS44T (99 %) | JQ951789 | JX843739 | ND | ND | E |
| MPJ11 | M. pudica (4) | Jorhat (Assam) | JQ951794 | C. taiwanensis LMG 19424T (98 %) | JQ951790 | JX843741 | ND | ND | E |
| STM815T | M. pudica† | French Guiana | NR_027555 | B. phymatum STM815T (100 %) | AJ505319 | AJ505318 | I | E† | E† |
| LMG19424T | M. pudica | Taiwan | NR_028800 | C. taiwanensis LMG 19424T (100 %) | NC_010529 | AJ505311 | I | I† | E† |
| Mim-1 | M. affinis | Mexico | DQ648573 | R. etli bv. mimosae Mim-1 (100 %) | – | E | I‡ | ||
| MHM (B) 2 III | M. himalayana (7) | Brazil | KC791149 | E. mexicanum ITTG-R7T (99 %) | ND | ND | ND | ||
| MHM (B) 5 | M. himalayana | Brazil | KC791150 | E. mexicanum ITTG-R7T (99 %) | ND | ND | ND | ||
| MHM (B) 8 | M. himalayana | Brazil | KC791151 | E. mexicanum ITTG-R7T (99 %) | ND | ND | ND |
* Previously reported by Gehlot et al. (2012).
† See Elliott et al. (2007a) for details.
‡ See Elliott et al. (2009) for details.
Phylogenetic and taxonomic analysis
For molecular phylogenetic analyses, sequences of type strains and/or NCBI reference (NR) sequences were downloaded from NCBI. The GenBank accession numbers are listed in parentheses for the 16S rRNA, nifH and nodA genes used in this analysis. All the sequences were aligned using CLUSTAL W (Thompson et al., 1997) and the alignment was exported to molecular evolutionary genetics analysis (MEGA) format in MEGA5 software (Tamura et al., 2011). The evolutionary history was inferred using the neighbour-joining method (Saitou and Nei, 1987). Evolutionary distances were computed using the Kimura two-parameter method in units of the number of base substitutions per site (Kimura, 1980). To obtain confidence values, the original data set was resampled 1000 times using the bootstrap analysis method (Felsenstein, 1985). The MEGA5 software (Tamura et al., 2011) was used for construction of phylogenetic trees, inferring distances and percentage similarity.
Nodulation tests with wild-type and GUS-marked strains
Representative strains from all three species were tested for nodulation of their original hosts (M. hamata, M. himalayana, M. pudica), and some strains were also selected for cross-inoculation tests on the same three hosts and on M. affinis, a Mexican species that is known to prefer to nodulate with alpha-rhizobia (Wang et al., 1999; Elliott et al., 2009). More detailed nodulation tests combined with microscopy were performed with selected strains that were marked with a pCAM121 transposon containing constitutively expressed glucuronidase (GUS) (Wilson et al., 1995). Briefly, Escherichia coli strain β2155 (Dehio and Meyer, 1997), which requires diaminopimelic acid, was transformed with the plasmid, pCAM121, and the transposon was then mobilized into the M. pudica isolates Cupriavidus sp. MP3 and B. phymatum MP20 by conjugation. The transconjugants were selected on YM agar containing 100 µg ml−1 spectinomycin and screened for GUS activity on YM agar containing 20 µg ml−1 X-gluc. One colony showing GUS activity and no apparent growth defect was selected for nodulation studies. Seeds of M. hamata, M. himalayana, M. pudica and M. affinis were scarified with concentrated sulphuric acid for 5 min, washed with sterile distilled water five times and germinated on water agar (1 %) plates. Seven-day-old seedlings were transferred to 150-mL glass tubes containing sterile vermiculite and inoculated with 109–1010 cells of various bacterial strains grown on YM medium. The inoculated seedlings were then incubated in a growth chamber at 25 °C either under a 16/8-h light/dark cycle or under a natural day/night cycle. Un-inoculated seedlings served as controls. The number of nodules, their appearance (e.g. if they were expressing Lb) and the health of the host plants was determined at 30 d after inoculation (dai) for M. pudica and M. affinis and at 40 dai for M. hamata and M. himalayana. Representative nodules from all species/strain combinations were also prepared for light microscopy and TEM as described above.
RESULTS
Nodulation of native and invasive Mimosa spp. in India
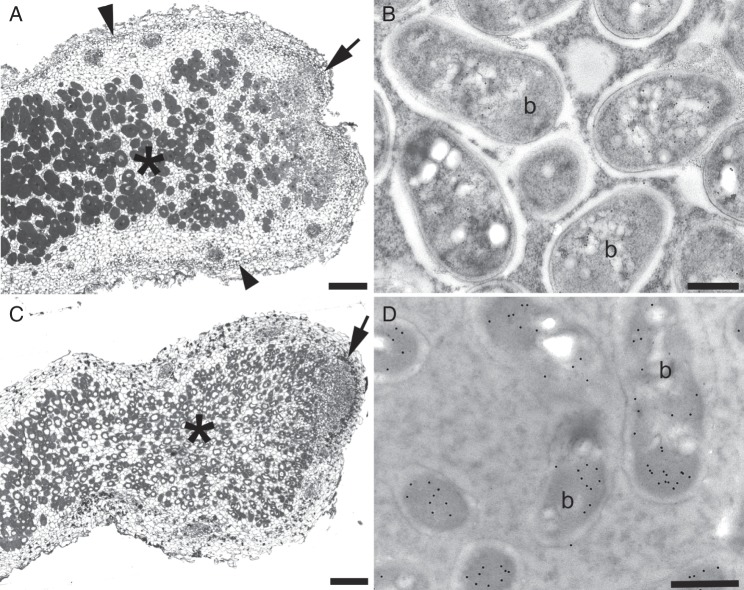
Mimosa hamata (Fig. 1A, B) is native to the Thar Desert and to surrounding semi-arid regions of Rajasthan and north-west India (Gehlot et al., 2012). The other native Indian species in this study, M. himalayana (Fig. 1C, D), is much more widespread (Ali, 1973; Shetty and Singh, 1987; Bora and Kumar, 2003), and generally prefers higher altitude (non-desert) regions in Rajasthan and in other parts of northern India that have significantly higher rainfall than the Thar Desert. In this study, the two native species were not found to inhabit the same environments. Nodulation of M. hamata growing near Jodhpur has previously been reported by Gehlot et al. (2012), and the ability of this species to nodulate in this semi-arid environment was confirmed in the present study via trap experiments using soil from several other locations in the Thar Desert (Table 1, Fig. 1E, Supplementary Data Fig. S1). In the case of M. himalayana, soil was obtained from the rhizosphere of natural stands of plants growing near Bijoliya in the east of Rajasthan (Fig. S1). This soil, which was more fertile than the M. hamata rhizospheric soils from the Thar Desert (Table 1), was used to trap rhizobia with M. himalayana seedlings that had been sown into it. Mature nodules had formed on M. himalayana by 2 months after seeds had been sown into the soil, similar to the time taken for M. hamata nodules to form when grown in pots of soil under the conditions used in the present study. Nodules on both species were branched and appeared to be indeterminate (Fig. 1E, F). This was confirmed by light microscopy of longitudinal sections, which demonstrated that M. hamata nodules were similar to those on other Mimosa spp. from semi-arid environments (dos Reis Junior et al., 2010), i.e. indeterminate with a pronounced meristem and invasion zone, and with an outer cortex with a ‘corky’ hypodermis layer (Fig. 2A, C), with cells containing phenolic compounds and/or tannins. The structure of nodules on M. himalayana was similar to that of M. hamata nodules, and has been described previously by Elliott et al. (2007a). TEM coupled with immunogold labelling with an antibody against the NifH protein of nitrogenase confirmed that bacteroids in field-grown or trap soil-grown nodules expressed this enzyme (Fig. 2B, D), strongly suggesting that both species form symbiotic N2-fixing nodules in the field and/or in their native soils.
Fig. 1.
Native Indian Mimosa spp. in the wild. (A) Mimosa hamata is a shrub that grows in the Thar Desert of Rajasthan. It grows to approx. 3 m maximum height, and the plant in this photograph is approx. 2 m. (B) Detail of the foliage and flowers of M. hamata; the spiny stems and the spherical pink inflorescences are very typical of the genus Mimosa. (C) Mimosa himalayana has a similar growth habit to M. hamata and it grows to a similar size, but it prefers wetter environments, in which it grows among other lush vegetation. (D) Detail of the foliage and flowers of M. himalayana; note that the stems, foliage and flowers are very similar to the closely related M. hamata. (E) Large branched nodules (arrow) on an M. hamata plant grown in soil taken from the rhizosphere of a plant growing in the Thar Desert of Rajasthan. (F) Nodules (*) on an M. himalayana plant grown in soil taken from the rhizosphere of a plant growing in the Bijoliya region of Rajasthan. Scale bars: (E) = 1 cm; (F) = 500 µm.
Fig. 2.
Light microscopy (A, C) and transmission electron microscopy (TEM) combined with immunogold labelling with an antibody against the NifH (Fe-)protein of nitrogenase (B, D) of M. hamata (A, B) and M. himalayana (C, D) nodules. Longitudinal sections of the nodules (A, C) show them to be broadly similar to those on other Mimosa spp., i.e. typically indeterminate with a persistent meristem (arrows) and an infected zone of N2-fixing cells (*), but in the case of M. hamata they also have a pronounced hypodermis (arrowheads in A). The bacteroids (b) in nodules from both species strongly express the NifH protein (B, D). Scale bars: (A, C) = 200 µm; (B, D) = 500 nm.
Nodules from the invasive M. pudica that were sampled from several parts of India were also examined by microscopy, and the structure of these was as reported previously (Chen et al., 2003a). Sections of nodules of all three species were also probed with antibodies specific to the common beta-rhizobial Mimosa symbionts, B. phymatum and C. taiwanensis (Elliott et al., 2007a; dos Reis Junior et al., 2010). None of the nodules examined from either of the native species was recognized by these antibodies (a section of an M. hamata nodule that was probed with the C. taiwanensis antibody is shown in Supplementary Data Fig. S2A), but nodules of M. himalayana that had been nodulated by B. phymatum STM815T from the study of Elliott et al. (2007a) reacted strongly with the B. phymatum antibody (Fig. S2B). Mimosa pudica nodules obtained from trap plants grown in soil from the rhizosphere of M. hamata near Jodhpur (RJ) (Table 1) were strongly labelled with the C. taiwanensis antibody (Fig. S2C), but not the B. phymatum antibody (Fig. S2D), and this was also the case with M. pudica nodules sampled directly from plants at three other locations at Agra (UP) (Fig. S2E), Bangalore (KA) and Haridwar (UT) (data not shown). On the other hand, nodule samples from another location, Bokaro (JH), in eastern India, were strongly labelled with the B. phymatum antibody (Fig. S2F).
The native and invasive Mimosa spp. were also tested for nodulation in some of the rhizospheric soils (Table 1). Mimosa himalayana nodulated in several of the M. hamata rhizospheric soils from the Thar Desert (Table 2), but M. hamata failed to nodulate in the more fertile M. himalayana rhizosphere soil from Bijoliya (RJ). Neither of the native species was able to nodulate in any of the M. pudica rhizospheric soils, with the exception of the Bokaro (JH) soil, in which M. himalayana (but not M. hamata) nodulated. Mimosa pudica was able to nodulate readily in the M. hamata rhizospheric soil from Jodhpur (Table 2), but not in the M. himalayana rhizosphere soil from Bijoliya (data not shown).
Mimosa hamata grew poorly and only formed the occasional ineffective nodule in Brazilian cerrado soil (Fig. 3A, B), whereas M. himalayana grew well and nodulated profusely and effectively (Fig. 3C, D). Sections of the nodules from neither species reacted with the B. phymatum and C. taiwanensis antibodies (data not shown), which strongly suggests that neither of these beta-rhizobial types is present in the nodules (dos Reis Junior et al., 2010).
Fig. 3.
Mimosa hamata (A, B) and M. himalayana (C, D) grown in Brazilian cerrado soil for 3 months. Note that there are no (or few) nodules on M. hamata (A) and that the plant is small and unhealthy. This is reflected in the structure of the single nodule taken from an M. hamata plant (B); it is clearly ineffective and contains areas of degraded tissue (d). In contrast, M. himalayana is green and healthy and well nodulated (arrows in C), and the nodules are effective in appearance (D). An arrow indicates the nodule meristem in (B) and (D), and the infected, N2-fixing zone is indicated by an asterisk (*) in each case. Scale bars: (A) = 1 cm; (B) = 2 cm; (C, D) = 200 µm.
Molecular characterization of symbionts of native and invasive Mimosa spp. in India
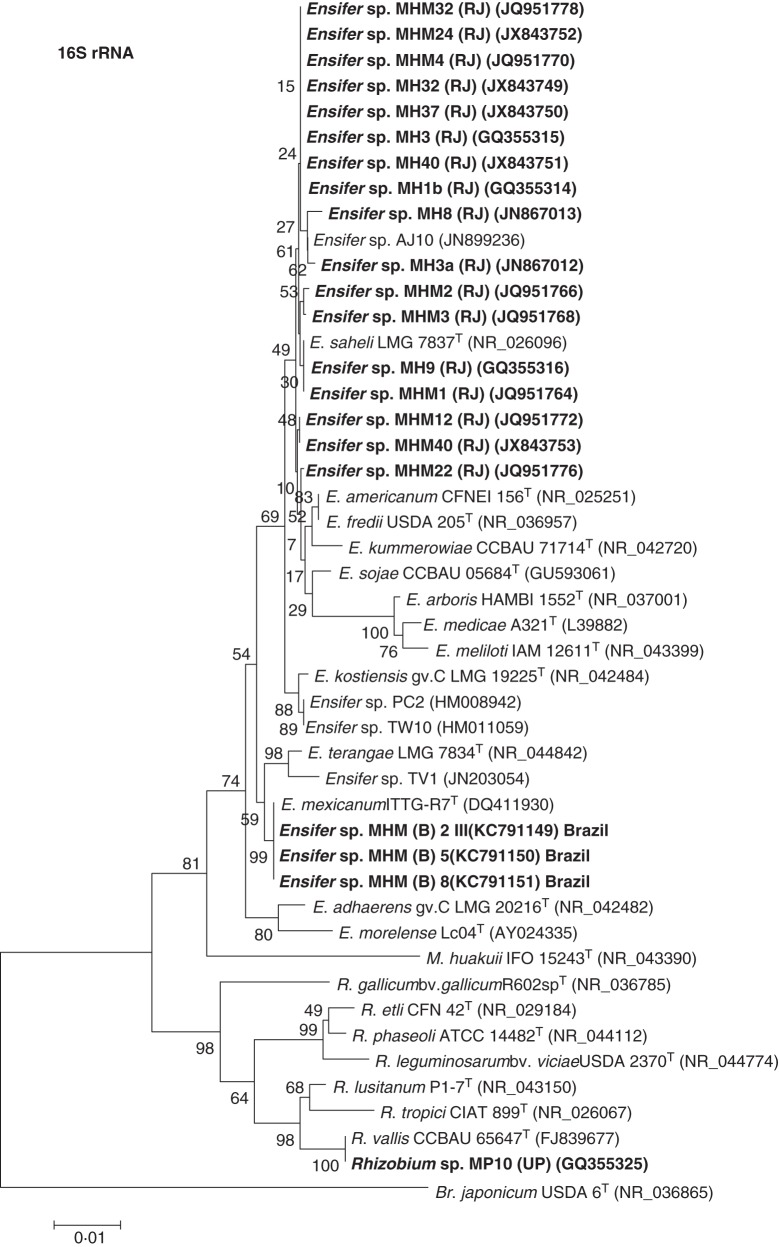
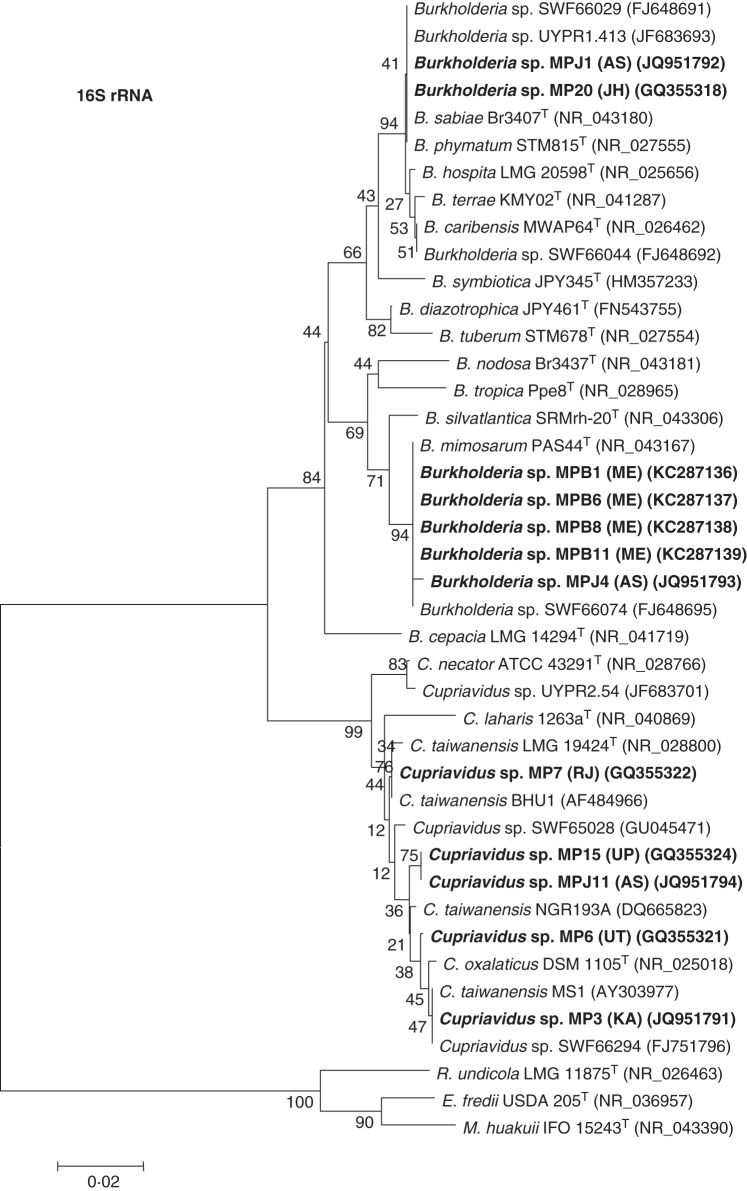
Rhizobia were isolated from nodules of native and invasive Mimosa spp. under axenic conditions and their phylogenetic relationships were determined by analysis of 16S rRNA gene sequences (Figs 4 and 5). In addition to strains MH3a and MH8 that were directly isolated, respectively, from M. hamata nodules sampled near Sikar and Jodhpur (RJ) by Gehlot et al. (2012), six further defined strains were isolated from M. hamata nodules obtained from soil trapping experiments using soil from four more sites in the Thar Desert of Rajasthan (Tables 1 and 2). Five defined rhizobial strains were isolated from M. himalayana nodules obtained from trapping experiments using soil from the rhizosphere of M. himalayana sampled from Bijoliya in eastern Rajasthan, and four additional strains were isolated from M. himalayana nodules on seedlings grown in M. hamata rhizospheric soil from four sites in the Thar Desert (Table 1). As shown in Fig. 4, all the strains from M. hamata and M. himalayana grouped together and showed highest 16S rRNA gene sequence similarity to sequences from Ensifer saheli in the Alphaproteobacteria. The 16S rRNA sequences of the rhizobia isolated from M. himalayana plants grown and nodulated in Brazilian cerrado soil also placed these in Ensifer, but in this case they were more closely related to E. mexicanum (Fig. 4). The identities of the strains nodulating the native species contrast with those isolated from the invasive species M. pudica as, with the exception of MP10 from Agra (UP) which was related to Rhizobium vallis (Table 2, Fig. 4), all of the symbiotically effective M. pudica isolates belonged to genera/species in the Betaproteobacteria (Fig. 5).
Fig. 4.
Neighbour-joining phylogenetic tree for 16S rRNA gene sequences of Ensifer and Rhizobium strains isolated from native Indian Mimosa species with type/reference strains and close relatives. Bootstrap values calculated for 1000 replications are indicated at the internodes. The scale bar indicates 1 % substitutions per site. GenBank accession numbers are given in parentheses. Abbreviations: Br., Bradyrhizobium; E., Ensifer; M., Mesorhizobium; R., Rhizobium; T, type strain; (NR), NCBI reference sequence. Strains with the prefixes MH and MHM were isolated from M. hamata and M. himalayana, respectively. Strains isolated in the present study are marked in bold.
Fig. 5.
Neighbour-joining phylogenetic tree for 16S rRNA gene sequences of Burkholderia and Cupriavidus strains isolated from the invasive species Mimosa pudica with type/reference strains and close relatives. Bootstrap values calculated for 1000 replications are indicated at the internodes. The scale bar indicates 2 % substitutions per site. GenBank accession numbers are given in parentheses. Abbreviations: B., Burkholderia; C., Cupriavidus; E., Ensifer; M., Mesorhizobium; R., Rhizobium; T, type strain; (NR), NCBI reference sequence. Strains isolated in the present study are marked in bold.
All the betaproteobacterial isolates from M. pudica nodules sampled in Bangalore (KA), Agra (UP) and Haridwar (UT) and one isolate from Jorhat (AS) were related to C. taiwanensis, as were the isolates ‘trapped’ by M. pudica seedlings that were grown in M. hamata rhizosphere soil from Jodhpur (Fig. 5). These strains all clustered with the C. taiwanensis type strain, LMG 19424T, but strain MP3 from Bangalore (KA) was closer to the South Indian Cupriavidus sp. strain from Tamil Nadu (MS1) than to the north Indian one from Uttar Pradesh (BHU1), both of which had been isolated from M. pudica nodules by Verma et al. (2004). In contrast to C. taiwanensis being the apparently predominant symbiont of M. pudica in north-western, central and southern India, mostly bacteria showing maximum 16S rRNA sequence similarity to the common M. pudica-nodulating Burkholderia spp., B. mimosarum and B. phymatum, were isolated from M. pudica growing in eastern (Bokaro, JH) and north-eastern (Jorhat, AS; Shillong, ME) parts of India (Fig. 5).
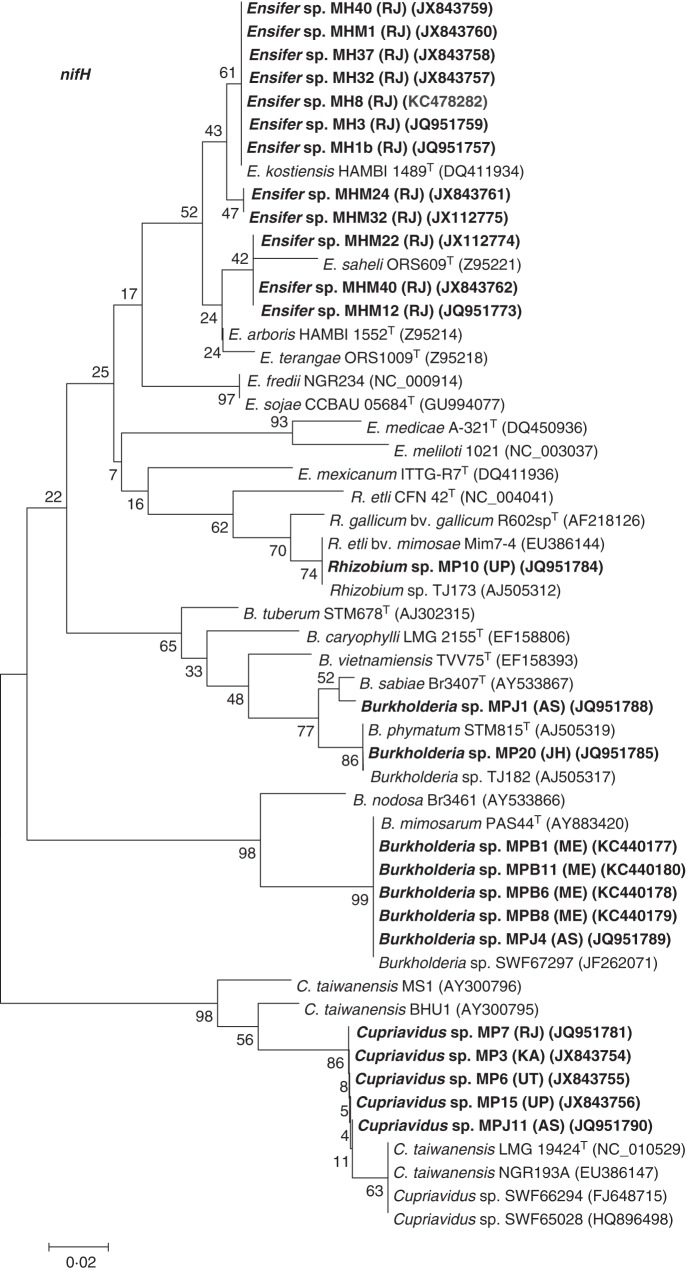
To determine the relatedness of the rhizobia of the invasive and native Mimosa spp. further, the DNA sequences of genes that are essential for N2 fixation (nifH) and symbiosis (nodA) were analysed (Figs 6 and 7). The nifH gene encodes the iron (Fe-) protein component of the nitrogenase enzyme complex and is essential for mutualistic N2-fixing symbioses, although it is not specific to rhizobia and is present in all free-living diazotrophs (Young, 2005). A phylogenetic analysis of the nifH sequences of the strains that nodulated the native Indian M. hamata and M. himalayana showed them to be clustered together and that they were closest to the E. kostiensis type strain HAMBI 1489T, which was isolated from Acacia senegal in Sudan (Nick et al., 1999), with the next closest sequence being that of the E. saheli type strain, ORS609T, from Sesbania cannabina (de Lajudie et al., 1994). In the case of the M. pudica isolates, the nifH sequence from Rhizobium sp. MP10 clustered with that of R. etli bv. mimosae Mim7-4, a symbiont of M. affinis from Mexico (Wang et al., 1999) and with R. etli TJ173 from M. pudica nodules in Taiwan (Elliott et al., 2009), whereas the M. pudica-nodulating C. taiwanensis and B. phymatum strains showed maximum similarity to the nifH sequences of their respective type strains, C. taiwanensis LMG 19424T and B. phymatum STM815T, but the Cupriavidus nifH sequences were different from those of the previously isolated Indian Cupriavidus strains BHU1 and MS1 (Verma et al., 2004). Finally, the nifH sequences of B. mimosarum MPJ4 and MPB1, MPB6, MPB8 and MPB11 showed highest similarity to that of the B. mimosarum type strain, PAS44T (Fig. 6).
Fig. 6.
Neighbour-joining tree of nifH gene sequences showing the phylogenetic relationships of the root nodule bacteria isolated from Mimosa spp. Bootstrap values calculated for 1000 replications are indicated at the internodes. GenBank accession numbers are given in parentheses. The scale bar represents 2 % nucleotide substitutions per site. Abbreviations: B., Burkholderia; C., Cupriavidus; E., Ensifer; R., Rhizobium; T, type strain. Strains with the prefixes MH, MHM and MP were isolated from M. hamata, M. himalayana and M. pudica, respectively. Strains isolated in the present study are marked in bold.
Fig. 7.
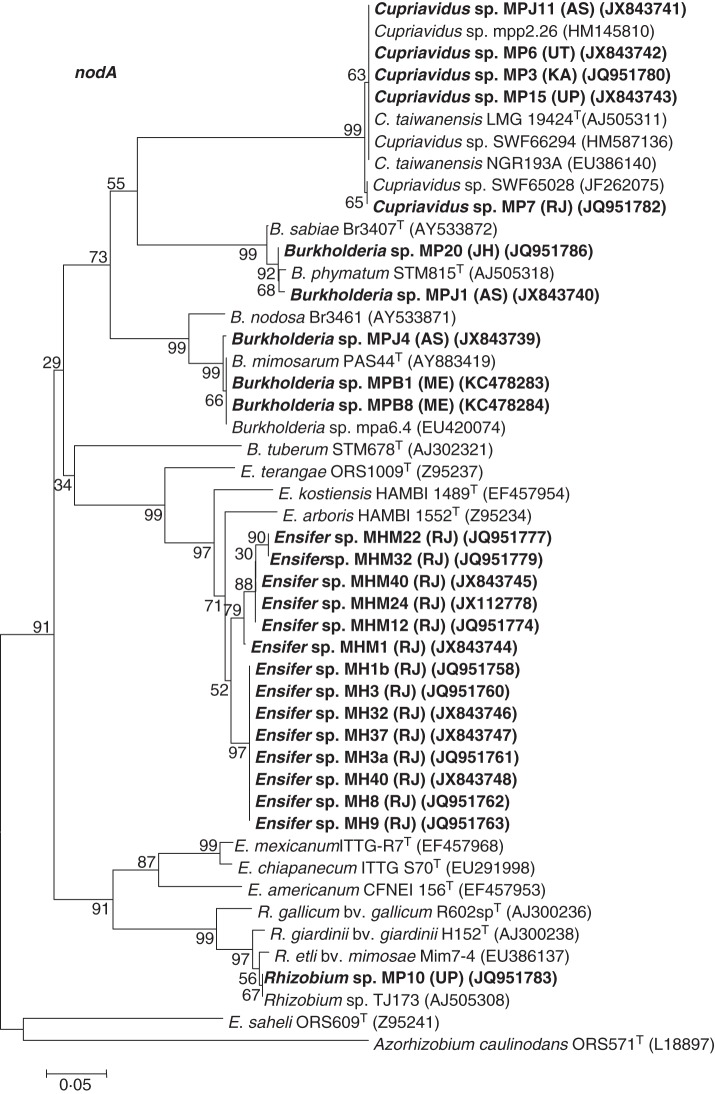
Neighbour-joining phylogenetic tree for nodA gene sequences of nodulating strains isolated from Mimosa spp. with close relatives and type strains. Bootstrap values calculated for 1000 replications are indicated at the internodes. The scale bar indicates 5 % nucleotide substitutions per site. GenBank accession numbers are given in parentheses. Abbreviations: B., Burkholderia; C., Cupriavidus; E., Ensifer; R., Rhizobium; T, type strain. Strains with the prefixes MH, MHM and MP were isolated from M. hamata, M. himalayana and M. pudica, respectively. Strains isolated in the present study are marked in bold.
In contrast to nifH genes, the nod genes are present only in legume-nodulating rhizobia, in which they are involved in the synthesis of Nod factors. In most legume–rhizobial symbioses studied to date, these are essential components of the signal exchange between the soil-dwelling rhizobia and the roots of their potential legume host, an exchange which will ultimately lead to the formation of functional N2-fixing nodules (Sprent, 2009). Slight alterations (‘decorations’) on the chemical structure of the lipo-chito-oligosaccharide backbone of the Nod factors can greatly affect the host range of a particular rhizobial strain (Pueppke and Broughton, 1999; Kobayashi and Broughton, 2008). Analysis of the sequences of the nodA genes of the various native and invasive Mimosa isolates (Fig. 7) showed that the Ensifer strains from M. hamata and M. himalayana clustered together, but they were not closely related to any type strains, with their sequences being closest to the African strains E. arboris HAMBI 1552T from Prosopis chilensis, E. kostiensis HAMBI 1489T from Acacia senegal and E. terangae ORS1009T from A. laeta (de Lajudie et al., 1994; Haukka et al., 1998; Nick et al., 1999). For the M. pudica rhizobial strain, as with its nifH sequence (Fig. 6), the nodA sequence from Rhizobium sp. MP10 showed highest similarity to those of the Mimosa-nodulating strains R. etli biovar mimosae Mim7-4 and R. etli TJ173 (Fig. 7). The nodA sequences of the Cupriavidus strains were all similar to the C. taiwanensis type strain, LMG 19424T from Taiwan, whereas those of the Burkholderia strains MP20 and MPJ1 were closest to B. phymatum STM815T, and those of Burkholderia strains MPJ4, MPB1 and MPB8 were closest to B. mimosarum PAS44T (Fig. 7).
Cross inoculation studies using wild-type and GUS-marked strains
All of the strains isolated from the Indian native and invasive Mimosa spp. featured in Figs 4–7 were tested positive for nodulation on their original hosts (Table 2). Some of these strains were also tested for nodulation of the other species in this study. None of the M. hamata and M. himalayana strains was capable of nodulating M. pudica (Table 2), but M. hamata strains, such as Ensifer sp. MH37 and MH40, could effectively nodulate both M. hamata and M. himalayana (Supplementary Data Fig. S3A, B), whereas the opposite was not true, i.e. no M. himalayana strains could nodulate M. hamata (Fig. S3A, Table 2). A Mexican Mimosa strain, R. etli bv. mimosae Mim-1, which was isolated from M. affinis by Wang et al. (1999) and which can effectively nodulate this species (Elliott et al., 2009), was also capable of nodulating M. himalayana (Fig. S3C) but not M. hamata (data not shown). Mimosa affinis could be nodulated by the M. hamata Ensifer sp. strains MH37 and MH40, but the nodules were small, white and ineffective (Fig. S3D). Mimosa affinis could not be nodulated by the M. himalayana Ensifer sp. strain MHM12 (data not shown).
It was previously shown that M. himalayana can be effectively nodulated by the M. pudica-nodulating strain B. phymatum STM815T and ineffectively nodulated by C. taiwanensis LMG 19424T (Elliott et al., 2007a). This has been confirmed in the present study (data not shown), and we have also found that M. hamata is ineffectively nodulated by both these strains (Table 2). However, neither of these beta-rhizobial strains was isolated in India, and so Cupriavidus sp. strain MP3 (from Karnataka), B. phymatum strain MP20 (from Jharkhand) and Rhizobium sp. MP10, all of which were capable of nodulating M. pudica effectively (Table 2), were tested on M. hamata and M. himalayana. As with the type strain of C. taiwanensis, LMG 19424T, MP3 nodulated both the native species ineffectively, whereas MP20 followed the same pattern as the B. phymatum type strain, STM815T, and nodulated M. himalayana effectively but M. hamata ineffectively (Table 2). Rhizobium sp. MP10, like many Rhizobium strains isolated from Mimosa nodules (Barrett and Parker, 2006; Elliott et al., 2009; Mishra et al., 2012), is not completely effective on its original host, M. pudica, producing plants with prematurely senescing nodules and yellow–green leaves, and it was also ineffective at nodulating M. hamata (Table 2). Additional studies were performed using variants of strains MP3 and MP20 that were marked with a transposon-based constitutively expressed gusA gene (Wilson et al., 1995), and these confirmed the nodulation phenotypes of the wild-type strains (e.g. on M. hamata; Supplementary Data Fig. S3E, F).
DISCUSSION
The native Indian species Mimosa hamata and M. himalayana are nodulated by Ensifer (Sinorhizobium) spp.
Mimosa hamata, a species which is native to the Thar Desert of Rajasthan and to semi-arid parts of neighbouring Pakistan (Barneby, 1991; Kumar and Sane, 2003), was shown in a study by Gehlot et al. (2012) to be nodulated near the city of Jodhpur and in Sikar district by rhizobial strains in Ensifer. This was confirmed in the present study by the isolation and characterization of strains from trap experiments using soils from the rhizosphere of M. hamata from several other locations in the Thar Desert. These strains were shown to form effective nodules on their host, and as no other symbiotic bacterial types were isolated from M. hamata, it is reasonable to state that it is preferentially nodulated by Ensifer spp. in its native range. This is reinforced by the demonstration that M. hamata cannot be nodulated effectively (or at all) by other Mimosa-nodulating strains; this includes alpha- and betaproteobacterial strains known to be promiscuous nodulators of several Mimosa spp. (Elliott et al., 2007a, 2009; dos Reis Junior et al., 2010; Gyaneshwar et al., 2011) and strains isolated from other Mimosa spp. in India (this study).
Mimosa himalayana is another native Indian species, but it prefers wetter and more fertile environments than its close relative M. hamata. It is also more widespread than M. hamata, and is native to several states in northern India, as well as neighbouring countries, such as Afghanistan and Nepal, where, as its name suggests, it is often found growing in highland regions bordering the Himalayas (Ali, 1973; Shetty and Singh, 1987; Barneby, 1991; Bora and Kumar, 2003). As with M. hamata, M. himalayana was also nodulated in the present study by Ensifer strains when it was sown into soil sampled from the rhizosphere of mature plants in its native range in eastern Rajasthan. The strains that were isolated from both the native Indian Mimosa spp. were closely related to each other on the basis of their 16S rRNA sequences, and were also somewhat related to E. saheli, a species known to nodulate Acacia spp. (de Lajudie et al., 1994) and is the most commonly isolated symbiont from several other native legumes in the Thar Desert, including all the mimosoids examined (Gehlot et al., 2012). Indeed, Ensifer spp. are often the preferred symbionts of mimosoid legumes, such as those in the genera Acacia (sensu lato), Acaciella, Calliandra, Leucaena and Prosopis, that are native and/or introduced to tropical and sub-tropical ecosystems in both the Old World (de Lajudie et al., 1994; McInroy et al., 1999; Nick et al., 1999; Räsänen et al., 2001; Bala and Giller, 2001; Bala et al., 2003; Wolde-Meskel et al., 2005; Ben Romdhane et al., 2006; Benata et al., 2008; Xu et al., 2013) and the New World (Moreira et al., 1998; Toledo et al., 2003; Lloret et al., 2007; Rincón-Rosales et al., 2009). Generally speaking, the Ensifer strains nodulating Old World mimosoids are in (or related to) the species E. arboris, E. kostiensis, E. saheli and E. terangae, whereas those from the New World belong to a group represented by E. americanum, E. chiapenecum and E. mexicanum (Rincón-Rosales et al., 2009).
Although their association with mimosoid legumes is well established by these examples, Ensifer spp. have not previously been reported as symbionts of Mimosa spp. in their native ranges (Barrett and Parker, 2005, 2006; Andam et al., 2007; Bontemps et al., 2010; Mishra et al., 2012). Moreover, although an Ensifer strain (TJ170) was isolated from nodules on invasive M. pudica in Taiwan by Chen et al. (2003b), this strain was not capable of nodulation, and so the present study is the first published demonstration that Ensifer strains can effectively nodulate Mimosa spp.
When considering relationships between legumes and their symbionts, core ‘housekeeping’ genes, such as rrs (16S rRNA) and recA, can only tell part of the story, as the ability of rhizobia to nodulate and fix N2 with particular legume hosts depends on their symbiosis-related genes (nod and nif), which in many rhizobial genera, including Ensifer, are borne on mobile Sym plasmids (Martinez-Romero, 2009; Sprent, 2009; Rogel et al., 2011). In spite of their potential to be transferred between bacterial types via horizontal gene transfer (HGT), the phylogenetic trees for symbiosis-related genes, such as nifH and nodA, are often similar to each other and to the core genomes of both alpha- and betaproteobacterial legume symbionts (Rincón-Rosales et al., 2009; Bontemps et al., 2010; Mishra et al., 2012). Ensifer strains that nodulate mimosoids are a case in point, as the phylogenetic trees for their nifH and nodA genes, for example, generally follow those for their housekeeping genes (such as 16S rRNA), and accordingly they also show a clear separation between the Ensifer strains isolated from the Old and New Worlds (Haukka et al., 1998; Rincón-Rosales et al., 2009). However, when the nifH and nodA genes from the native Indian Mimosa symbionts were examined in the present study, they were shown to have different phylogenetic relationships. Although the nifH genes of strains from both the native Indian species clustered together in two clades that were relatively close to each other and to other Old World Ensifer mimosoid symbionts, particularly E. kostiensis (Haukka et al., 1998), their nodA genes were different from any described rhizobial strains, being closely grouped together in a distinct clade that was distant from the nearest described rhizobial species, E. kostiensis and E. arboris. This was particularly true of the nodA sequences of strains from M. hamata, which were in a sub-clade that was distinct from those of the M. himalayana strains.
Given that the nod genes, including nodA, are those that confer host selectivity upon rhizobia (Kobayashi and Broughton, 2008; Martinez-Romero, 2009; Cummings et al., 2009; Rogel et al., 2011), the different phylogenetic patterns of their nodA genes suggested that the host ranges of the M. hamata and M. himalayana symbionts were different, and so the ability of representative strains from the M. hamata and M. himalayana symbionts to nodulate various Mimosa hosts was examined. These experiments showed that M. hamata strains nodulated M. himalayana, but that the reverse was not true, i.e. M. himalayana strains could not nodulate M. hamata.
Taken together, these data demonstrate that the symbiosis-related genes of native Indian Mimosa spp. are more closely related to Old World Ensifer mimosoid symbionts than to New World ones, but that the nodA genes are in separate groups from each other and from other mimosoid Ensifer strains. In the case of M. hamata, this has resulted in the species being highly dependent on being nodulated by symbionts with very specific nodA sequences, and so it might be appropriate to consider that the M. hamata Ensifer symbionts described in the present study belong to a new ‘symbiovar’ (Rogel et al., 2011). Mimosa himalayana is a slightly different case, as although it appears to nodulate preferentially in its native soil with Ensifer strains that are closely related to M. hamata symbionts (with which it can also nodulate), it differs from M. hamata in that it is more promiscuous and can nodulate with other rhizobial types, including Burkholderia (Elliott et al., 2007a).
The invasive Mimosa species in India M. pudica is mainly nodulated by Cupriavidus and Burkholderia
Mimosa pudica is a widespread invasive plant in India, and is present in most (if not all) states, where it is found as a weed growing on roadsides, wasteground and pastures. It generally prefers wetter and more fertile environments, and so has not been recorded in (for example) arid and/or semi-arid regions, such as the Thar Desert (Shetty and Singh, 1987; Kumar and Sane, 2003). As with many sub-tropical and tropical South East Asian countries in which it has been introduced (Chen et al., 2003b; Elliott et al., 2009; Liu et al., 2011, 2012; Klonowska et al., 2012; Andrus et al., 2012), M. pudica in India is mainly nodulated by Betaproteobacteria in the genera Burkholderia and Cupriavidus (Verma et al., 2004; this study). The degree to which M. pudica is nodulated by each beta-rhizobial genus, Burkholderia or Cupriavidus, appears to depend upon the location; in Taiwan and New Caledonia it is almost exclusively nodulated by Cupriavidus (Chen et al., 2003b; Klonowska et al., 2012), whereas in southern China and the Philippines it is nodulated by a relatively equal proportion of both genera. In India, it was previously shown by Verma et al. (2004) that M. pudica was nodulated by Cupriavidus in two locations, one in the north (Uttar Pradesh) and the other in the south (Tamil Nadu). The present study has confirmed that Cupriavidus strains similar to C. taiwanensis are common symbionts of M. pudica in several other locations in India, but has gone further and shown for the first time that strains in the species B. mimosarum and B. phymatum are also common M. pudica symbionts, and even that some Rhizobium strains (e.g. MP10, which is similar to R. vallis; Wang et al., 2011) can be symbiotic with this species in India. Our study of M. pudica symbionts in India has some parallels with that of Liu et al. (2012) from southern China, in which the same three species, C. taiwanensis, B. mimosarum and B. phymatum, were always found to nodulate M. pudica in varying proportions depending upon location, but the present study differs from Liu et al. (2012) in that some sites in India were dominated by one symbiont type, e.g. Haridwar (UT), Agra (UP) and Bangalore (KA) by C. taiwanensis, Bokaro (JH) by B. phymatum, and Shillong (ME) by B. mimosarum, whereas others, such as Jorhat (AS), had M. pudica plants that were nodulated with all three symbiont types.
Are soil characteristics and/or plant taxonomy and geographical isolation responsible for the selection of symbionts by native and invasive Indian Mimosa spp.?
The results from this study have shown clearly that the rhizobial symbionts of native and invasive Mimosa spp. in India are distinct and host-specific, and are most likely not shared between the two types. In the case of the native species, M. hamata and M. himalayana are both nodulated by Ensifer, but the fact that these symbionts are more closely related to those of other Mimosoideae in the same region suggests that their geographical isolation of approx. 10 Myr from the main centres of Mimosa diversity in the New World (Simon et al., 2011) has resulted in these Mimosa spp. evolving a relationship with variants of the ‘local’ mimosoid symbionts rather than with the Burkholderia symbionts (Bontemps et al., 2010) of their closest New World relatives in Brazil (Simon et al., 2011). Indeed, in the case of M. hamata, it has become so adapted to its particular environment in the Thar Desert that it appears no longer to be capable of nodulating effectively with other Mimosa-nodulating rhizobia of any type, and this could be related to the high pH and low fertility of the soils in this region (Sprent and Gehlot, 2010; Gehlot et al., 2012). Mimosa himalayana, by contrast, which is a more widespread species than M. hamata, has retained the ability of its South American ancestors to nodulate with Burkholderia (Elliott et al., 2007a), and thus it also appears to be adaptable to several soil types. It can nodulate in low-fertility Thar Desert soils and in more fertile soils in its native range and in Bokaro (JH). Of potentially even more significance is the fact that, unlike the closely related M. hamata, M. himalayana can nodulate so effectively in Brazilian cerrado Mimosa rhizospheric soils. However, given its ability to nodulate with Burkholderia and the preponderance of native and endemic Mimosa spp. nodulated by Burkholderia in cerrado soils (Bontemps et al., 2010; dos Reis Junior et al., 2010), it is surprising that the symbionts isolated from the M. himalayana trap plants were all closely related to E. mexicanum, a species originally isolated from nodules on Acaciella spp. in Mexico (Toledo et al., 2003; Rincón-Rosales et al., 2009). Further studies are currently being undertaken to determine the origin (i.e. the original hosts) of these symbionts in Brazil, and to characterize them in terms of their symbiosis-related genes.
In contrast to the native species, no M. pudica plants from any of the sites/soils were nodulated with Ensifer spp., even when they were nodulated after being sown into M. hamata rhizospheric soils from Jodhpur. It thus appears that M. pudica in India is nodulated by the same types of symbionts as in other Asian locations, including neighbouring China (Liu et al., 2011, 2012), and that as with other invasive legumes (e.g. Acacia saligna; Crisóstomo et al., 2013) it most likely brings its symbionts with it as it invades new territories, including those that are already occupied by native Mimosa spp. These symbionts are (mainly) a combination of beta-rhizobial types, and the type (or combination of types) depends on the location, but what is it about each location that might be involved in their selection? Soil characteristics are considered to be important for the selection of symbionts by invasive Mimosa spp., especially soil pH (Bontemps et al., 2010; dos Reis Junior et al., 2010; Mishra et al., 2012; Liu et al., 2012) and fertility (Elliott et al., 2009). Low fertility (i.e. low soil N-concentration) generally favours the selection of Burkholderia as symbionts by Mimosa spp., almost to the complete exclusion of other rhizobial types, but this dominance is broken in favour of C. taiwanensis as soil N-concentrations increase (Elliott et al., 2009). In the case of pH, it has been noted from studies on M. pudica symbionts in French Guiana (Mishra et al., 2012) and southern China (Liu et al., 2012) that soils with values below pH 7·0 harbour plants that are generally nodulated by Burkholderia spp., whereas plants growing in soils with higher pH values are likely to have C. taiwanensis as their symbionts. With the exception of the low-fertility alkaline soils in Jodhpur (RJ) (pH 8·2) that resulted in M. pudica trap plants selecting C. taiwanensis, and the acidic soils in Shillong (ME) (pH. 4·9) that resulted in M. pudica selecting B. mimosarum, there are no clear reasons as to why the soils in many of the locations in the present study produced the particular M. pudica symbionts that they did based upon pH and fertility alone. However, sampling from M. pudica was very low for each site, as the study was designed only to get a wider picture of the variety of symbionts nodulating this invasive species in India, and more in-depth sampling will almost certainly show that the diversity of M. pudica symbionts at each site is much more complex than has been demonstrated here.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Prof. N. S. Shekhawat, Dr H. R. Dagla, Dr J. C. Tarafdar and Dr Neelam Poonar for their valuable suggestions, soil information and sample collections during field trips, Valter Baura and Emanuel de Souza at UFPR for sequencing (funded by INCT-FBN/CNPq), and Esperanza Martinez-Romero for R. etli bv. mimosae Mim-1 and seeds of M. affinis. This work was supported in part by a grant from the College of Letters and Sciences, University of Wisconsin Milwaukee, to P.G. and Department of Biotechnology, Government of India funded research project (BT/PR11461/AGR/21/270/2008) to Gehlot Hukam. N.T., A.T., I.S.S. and N.P. would like to thank the Council of Scientific and Industrial Research (CSIR) and the University Grant Commission (UGC), New Delhi, for financial assistance in the form of senior research fellowships.
LITERATURE CITED
- Ali SI. Mimosaceae In. In: Nasir E, Ali SI, editors. Flora of western Pakistan. Vol. 36. Karachi, Pakistan: Stewart Herbarium; 1973. pp. 1–41. [Google Scholar]
- Andam CP, Mondo SJ, Parker MA. Monophyly of nodA and nifH genes across Texan and Costa Rican populations of Cupriavidus nodule symbionts. Applied and Environmental Microbiology. 2007;73:4686–4690. doi: 10.1128/AEM.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrus AD, Andam C.P, Parker MA. American origin of Cupriavidus bacteria associated with invasive Mimosa legumes in the Philippines. FEMS Microbiology Ecology. 2012;80:747–750. doi: 10.1111/j.1574-6941.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- Bala A, Giller KE. Symbiotic specificity of tropical tree rhizobia for host legumes. New Phytology. 2001;149:495–507. doi: 10.1046/j.1469-8137.2001.00059.x. [DOI] [PubMed] [Google Scholar]
- Bala A, Murphy P, Giller KE. Distribution and diversity of rhizobia nodulating agroforestry legumes in soils from three continents in the tropics. Molecular Ecology. 2003;12:917–929. doi: 10.1046/j.1365-294x.2003.01754.x. [DOI] [PubMed] [Google Scholar]
- Barneby RC. Sensitivae Censitae: a description of the genus Mimosa Linnaeus (Mimosaceae) in the New World. Memoirs of the New York Botanical Garden. 1991;65:1–835. [Google Scholar]
- Barrett CF, Parker MA. Prevalence of Burkholderia sp. nodule symbionts on four mimosoid legumes from Barro Colorado Island, Panama. Systematic and Applied Microbiolgy. 2005;28:57–65. doi: 10.1016/j.syapm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Barrett CF, Parker MA. Coexistence of Burkholderia, Cupriavidus, and Rhizobium sp. nodule bacteria on two Mimosa spp. in Costa Rica. Applied and Environmental Microbiology. 2006;72:1198–1206. doi: 10.1128/AEM.72.2.1198-1206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Romdhane S, Nasr H, Samba-Mbaye R, Neyra M, Ghorbal MH, de Lajudie P. Genetic diversity of Acacia tortilis ssp. raddiana rhizobia in Tunisia assessed by 16S and 16S-23S rDNA genes analysis. Journal of Applied Microbiology. 2006;100:436–445. doi: 10.1111/j.1365-2672.2005.02765.x. [DOI] [PubMed] [Google Scholar]
- Benata H, Mohammed O, Noureddine B, et al. Diversity of bacteria that nodulate Prosopis juliflora in the eastern area of Morocco. Systematic and Applied Microbiology. 2008;31:378–386. doi: 10.1016/j.syapm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Bontemps C, Elliott GN, Simon MF, et al. Burkholderia species are ancient symbionts of legumes. Molecular Ecology. 2010;19:44–52. doi: 10.1111/j.1365-294X.2009.04458.x. [DOI] [PubMed] [Google Scholar]
- Bora PJ, Kumar Y. Floristic diversity of Assam: Study of Pabitora Wildlife Sanctuary. Delhi: Daya Publishing House; 2003. [Google Scholar]
- Chen W-M, Laevens S, Lee TM, Coenye T, de Vos P, Mergeay M, Vandamme P. Ralstonia taiwanensis sp. nov., isolated from root nodules of Mimosa species and sputum of a cystic fibrosis patient. International Journal Systematic and Evolutionary Microbiology. 2001;51:1729–1735. doi: 10.1099/00207713-51-5-1729. [DOI] [PubMed] [Google Scholar]
- Chen W-M, James EK, Prescott AR, Kierans M, Sprent JI. Nodulation of Mimosa spp. by the β-proteobacterium Ralstonia taiwanensis. Molecular Plant–Microbe Interactions. 2003a;16:151–1061. doi: 10.1094/MPMI.2003.16.12.1051. [DOI] [PubMed] [Google Scholar]
- Chen W-M, Moulin L, Bontemps C, Vandamme P, Béna G, Boivin-Masson C. Legume symbiotic nitrogen fixation by β-proteobacteria is widespread in nature. Journal of Bacteriology. 2003b;185:7266–7272. doi: 10.1128/JB.185.24.7266-7272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-M, de Faria S.M, Straliotto R, et al. Proof that Burkholderia forms effective symbioses with legumes: a study of novel Mimosa-nodulating strains from South America. Applied and Environmental Microbiology. 2005a;71:7461–7471. doi: 10.1128/AEM.71.11.7461-7471.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-M, James EK, Chou J-H, Sheu S-Y, Yang SZ, Sprent JI. Beta-rhizobia from Mimosa pigra, a newly-discovered invasive plant in Taiwan. New Phytologist. 2005b;168:661–675. doi: 10.1111/j.1469-8137.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- Chen W-M, James EK, Coenye T, et al. Burkholderia mimosarum sp. nov., isolated from root nodules of Mimosa spp. from Taiwan and South America. Intenational Journal of Systematic and Evolutionary Microbiology. 2006;56:1847–1851. doi: 10.1099/ijs.0.64325-0. [DOI] [PubMed] [Google Scholar]
- Crisóstomo JA, Rodríguez-Echeverría S, Freitas H. Co-introduction of exotic rhizobia to the rhizosphere of the invasive legume Acacia saligna, an intercontinental study. Applied Soil Ecology. 2013;64:118–126. [Google Scholar]
- Cummings SP, Gyaneshwar P, Vinuesa P, et al. Nodulation of Sesbania species by Rhizobium (Agrobacterium) strain IRBG74 and other rhizobia. Environmental Microbiology. 2009;11:2510–2525. doi: 10.1111/j.1462-2920.2009.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehio C, Meyer M. Maintenance of broad-host range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal transfer from Escherichia coli. Journal of Bacteriology. 1997;179:538–540. doi: 10.1128/jb.179.2.538-540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lajudie P, Willems A, Pot B, et al. Polyphasic taxonomy of rhizobia. Emendation of the genus Sinorhizobium and description of Sinorhizobium meliloti comb. nov., Sinorhizobium saheli sp. nov. & Sinorhizobium teranga sp. nov. International Journal of Systematic Bacteriology. 1994;44:715–733. [Google Scholar]
- dos Reis Junior FB, Simon MF, Gross E, et al. Nodulation and nitrogen fixation by Mimosa spp. in the Cerrado and Caatinga biomes of Brazil. New Phytologist. 2010;186:934–946. doi: 10.1111/j.1469-8137.2010.03267.x. [DOI] [PubMed] [Google Scholar]
- Elliott GN, Chen W-M, Chou J-H, et al. Burkholderia phymatum is a highly effective nitrogen-fixing symbiont of Mimosa spp. and fixes nitrogen ex planta. New Phytologist. 2007a;173:168–180. doi: 10.1111/j.1469-8137.2006.01894.x. [DOI] [PubMed] [Google Scholar]
- Elliott GN, Chen W-M, Bontemps C, et al. Nodulation of Cyclopia spp. (Leguminosae, Papilionoideae) by Burkholderia tuberum. Annals of Botany. 2007b;100:1403–1411. doi: 10.1093/aob/mcm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott GN, Chou J-H, Chen W-M, et al. Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N-limited conditions. Environmental Microbiology. 2009;11:762–778. doi: 10.1111/j.1462-2920.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Gamble JS. The Indian species of Mimosa. Bulletin of Miscellaneous Information, Kew. 1920;1920:1–6. [Google Scholar]
- Garau G, Yates RJ, Deiana P, Howieson JG. Novel strains of nodulating Burkholderia have a role in nitrogen fixation with papilionoid herbaceous legumes adapted to acid, infertile soils. Soil Biology and Biochemistry. 2009;41:125–134. [Google Scholar]
- Gehlot HS, Panwar D, Tak N, et al. Nodulation of legumes from the Thar desert of India and molecular characterization of their rhizobia. Plant and Soil. 2012;357:227–243. [Google Scholar]
- Graham PH. Ecology of the root-nodule bacteria of legumes. In: Dilworth MJ, James EK, Sprent JI, Newton WE, editors. Nitrogen-fixing legume symbioses. Dordrecht: Springer; 2008. pp. 23–58. [Google Scholar]
- Gyaneshwar P, Hirsch AM, Moulin L, et al. Legume-nodulating betaproteobacteria: diversity, host range and future prospects. Molecular Plant–Microbe Interactions. 2011;24:1276–1288. doi: 10.1094/MPMI-06-11-0172. [DOI] [PubMed] [Google Scholar]
- Haukka K, Lindström K, Young JPW. Three phylogenetic groups of nodA and nifH genes in Sinorhizobium and Mesorhizobium isolates from leguminous trees growing in Africa and Latin America. Applied and Environmental Microbiology. 1998;64:419–426. doi: 10.1128/aem.64.2.419-426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howieson JG, DeMeyer SE, Vivas-Marfisi A, Ratnayake S, Ardley JK, Yates RJ. Novel Burkholderia bacteria isolated from Lebeckia ambigua – A perennial suffrutescent legume of the fynbos. Soil Biology and Biochemistry. 2013;60:55–64. [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Klonowska A, Chaintreuil C, Tisseyre P, et al. Biodiversity of Mimosa pudica rhizobial symbionts (Cupriavidus taiwanensis, Rhizobium mesoamericanum) in New Caledonia and their adaptation to heavy metal-rich soils. FEMS Microbiology Ecology. 2012;81:618–635. doi: 10.1111/j.1574-6941.2012.01393.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Broughton WJ. Fine-tuning of symbiotic genes in rhizobia: flavonoid signal transduction cascade. In: Dilworth MJ, James EK, Sprent JI, Newton WE, editors. Nitrogen-fixing legume symbioses. Dordrecht: Springer; 2008. pp. 117–152. [Google Scholar]
- Kumar S, Sane PV. Legumes of South Asia: a check list. Kew: Royal Botanic Gardens; 2003. [Google Scholar]
- Liu XY, Wei W, Wang ET, Zhang B, Macdermott J, Chen WX. Phylogenetic relationships and diversity of beta-rhizobia associated with Mimosa spp. grown in Sishuangbanna, China. International Journal of Systematic and Evolutionary Microbiology. 2011;61:334–342. doi: 10.1099/ijs.0.020560-0. [DOI] [PubMed] [Google Scholar]
- Liu XY, Wei S, Wang F, et al. Burkholderia and Cupriavidus spp. are the preferred symbionts of Mimosa spp. in Southern China. FEMS Microbiology Ecology. 2012;80:417–426. doi: 10.1111/j.1574-6941.2012.01310.x. [DOI] [PubMed] [Google Scholar]
- Lloret L, Ormeño-Orrillo E, Rincón R, Martinez-Romero J, Rogel-Hernandez MA, Martinez-Romero E. Ensifer mexicanus sp. nov. a new species nodulating Acacia angustissima (Mill.) Kuntze in Mexico. Systematic and Applied Microbiology. 2007;30:280–290. doi: 10.1016/j.syapm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Martinez-Romero E. Coevolution in Rhizobium–legume symbiosis? DNA and Cell Biology. 2009;28:361–370. doi: 10.1089/dna.2009.0863. [DOI] [PubMed] [Google Scholar]
- McInroy SG, Campbell CD, Haukka KE, et al. Characterisation of rhizobia from African acacias and other tropical woody legumes using Biolog and partial 16S rRNA sequencing. FEMS Microbiology Letters. 1999;170:111–117. doi: 10.1111/j.1574-6968.1999.tb13362.x. [DOI] [PubMed] [Google Scholar]
- Mishra RPN, Tisseyre P, Melkonian R, et al. Genetic diversity of Mimosa pudica rhizobial symbionts in soils of French Guiana: investigating the origin and diversity of Burkholderia phymatum and other beta-rhizobia. FEMS Microbiology Ecology. 2012;79:487–503. doi: 10.1111/j.1574-6941.2011.01235.x. [DOI] [PubMed] [Google Scholar]
- Moreira FMS, Haukka K, Young JPW. Biodiversity of rhizobia isolated from a wide range of forest legumes in Brazil. Molecular Ecology. 1998;7:889–895. doi: 10.1046/j.1365-294x.1998.00411.x. [DOI] [PubMed] [Google Scholar]
- Moulin L, Béna G, Boivin-Masson C, Stepkowski T. Phylogenetic analyses of symbiotic nodulation genes support vertical and lateral gene co-transfer within the Bradyrhizobium genus. Molecular Phylogenetic Evolution. 2004;30:720–732. doi: 10.1016/S1055-7903(03)00255-0. [DOI] [PubMed] [Google Scholar]
- Nick G, de Lajudie P, Eardly BD, et al. Sinorhizobium arboris sp. nov. and Sinorhizobium kostiense sp. nov., isolated from leguminous trees in Sudan and Kenya. International Journal of Systematic Bacteriology. 1999;49:1359–1368. doi: 10.1099/00207713-49-4-1359. [DOI] [PubMed] [Google Scholar]
- Parker MA, Wurtz AK, Paynter Q. Nodule symbiosis of invasive Mimosa pigra in Australia and in ancestral habitats: a comparative analysis. Biological Invasions. 2007;9:127–138. [Google Scholar]
- Perret X, Broughton WJ. Rapid identification of Rhizobium strains by targeted PCR fingerprinting. Plant and Soil. 1998;204:21–34. [Google Scholar]
- Pueppke SG, Broughton WJ. Rhizobium sp. strain NGR234 and USDA257 share exceptionally broad, nested host ranges. Molecular Plant–Microbe Interactions. 1999;12:293–318. doi: 10.1094/MPMI.1999.12.4.293. [DOI] [PubMed] [Google Scholar]
- Räsänen IA, Sprent JI, Lindström K. Symbiotic properties of sinorhizobia from Acacia and Prosopis nodules in Sudan and Senegal. Plant and Soil. 2001;235:193–210. [Google Scholar]
- Rincón-Rosales R, Lloret L, Ponce E, Martínez-Romero E. Rhizobia with different symbiotic efficiencies nodulate Acaciella angustissima in Mexico, including Sinorhizobium chiapanecum sp. nov. which has common symbiotic genes with Sinorhizobium mexicanum. FEMS Microbiology Ecology. 2009;67:103–117. doi: 10.1111/j.1574-6941.2008.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel MA, Ormeño-Orrillo E, Martinez Romero E. Symbiovars in rhizobia reflect bacterial adaptation to legumes. Systematic and Applied Microbiology. 2011;34:96–104. doi: 10.1016/j.syapm.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shetty BV, Singh V. Flora of Rajasthan. Vol. 1. Kolkata: Botanical Survey of India; 1987. [Google Scholar]
- Simon MF, Proença C. Phytogeographic patterns of Mimosa (Mimosoideae, Leguminosae) in the Cerrado biome of Brazil: an indicator genus of high altitude centers of endemism? Biological Conservation. 2000;96:279–296. [Google Scholar]
- Simon MF, Grether R, Queiroz LP, Sarkinen TE, Dutra VF, Hughes CE. The evolutionary history of Mimosa (Leguminosae): towards a phylogeny of the sensitive plants. American Journal of Botany. 2011;98:1201–1221. doi: 10.3732/ajb.1000520. [DOI] [PubMed] [Google Scholar]
- Sprent JI. Legume nodulation. A global perspective. Chichester, UK: Wiley-Blackwell; 2009. [Google Scholar]
- Sprent JI, Gehlot HS. Nodulated legumes in arid and semi-arid environments: are they important? Plant Ecology and Diversity. 2010;3:211–219. [Google Scholar]
- Talbi C, Delgado MJ, Girard L, Ramirez-Trujillo A, Caballero-Mellado J, Bedmar EJ. Burkholderia phymatum strains capable of nodulating Phaseolus vulgaris are present in Moroccan soils. Applied and Environmental Microbiology. 2010;76:4587–4591. doi: 10.1128/AEM.02886-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX–windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo I, Lloret L, Martinez-Romero E. Sinorhizobium americanus sp. nov., a new Sinorhizobium species nodulating native Acacia spp. in Mexico. Systematic and Applied Microbiology. 2003;26:54–64. doi: 10.1078/072320203322337317. [DOI] [PubMed] [Google Scholar]
- Verma SC, Chowdhury SP, Tripathi AK. Phylogeny based on 16S rDNA and nifH sequences of Ralstonia taiwanensis strains isolated from nitrogen-fixing nodules of Mimosa pudica in India. Canadian Journal of Microbiology. 2004;50:313–322. doi: 10.1139/w04-020. [DOI] [PubMed] [Google Scholar]
- Vincent JM. A manual for the practical study of root nodule bacteria. Oxford: Blackwell Scientific Publications; 1970. [Google Scholar]
- Wang ET, Rogel MA, García-de los Santos A, Martínez-Romero J, Cevallos MA, Martínez-Romero E. Rhizobium etli bv. mimosae, a novel biovar isolated from Mimosa affinis. International Journal of Systematic Bacteriology. 1999;49:1479–1491. doi: 10.1099/00207713-49-4-1479. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang ET, Wu LJ, Sui XH, Li Y, Jr, Chen WX. Rhizobium vallis sp. nov., isolated from nodules of three leguminous species. International Journal of Systemtaic and Evolutionary Microbiology. 2011;61:2582–2588. doi: 10.1099/ijs.0.026484-0. [DOI] [PubMed] [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K, Sessitsch A, Corbo JC, Giller KE, Akkermans ADL, Jefferson RA. β-Glucuronidase (GUS) transposons for ecological and genetic studies of rhizobia and other Gram-negative bacteria. Microbiology. 1995;141:1691–1705. doi: 10.1099/13500872-141-7-1691. [DOI] [PubMed] [Google Scholar]
- Wolde-Meskel E, Terefework Z, Frostegård A, Lindström K. Genetic diversity and phylogeny of rhizobia isolated from agroforestry legume species in southern Ethiopia. International Journal of Systemtatic and Evolutionary Microbiology. 2005;55:1439–1452. doi: 10.1099/ijs.0.63534-0. [DOI] [PubMed] [Google Scholar]
- Xu KW, Penttinen P, Chen YX, Chen Q, Zhang X. Symbiotic efficiency and phylogeny of the rhizobia isolated from Leucaena leucocephala in arid–hot river valley area in Panxi, Sichuan, China. Applied Microbiology and Biotechnology. 2013;97:783–793. doi: 10.1007/s00253-012-4246-2. [DOI] [PubMed] [Google Scholar]
- Young JPW. The phylogeny and evolution of nitrogenases. In: Palacios R, Newton WE, editors. Genomes and genomics of nitrogen-fixing organisms. Dordrecht: Springer; 2005. pp. 221–241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.