Abstract
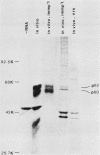
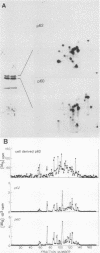
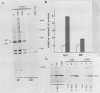
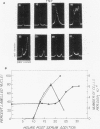
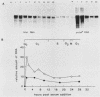
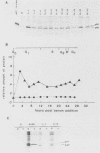
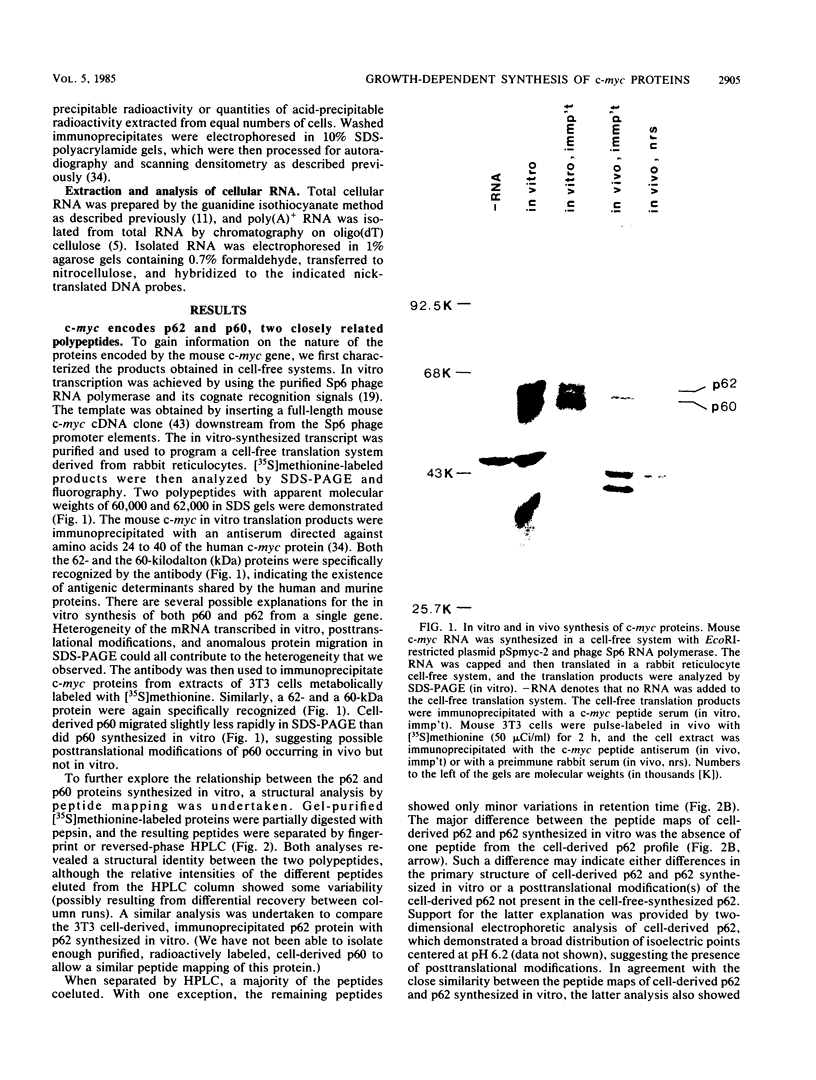
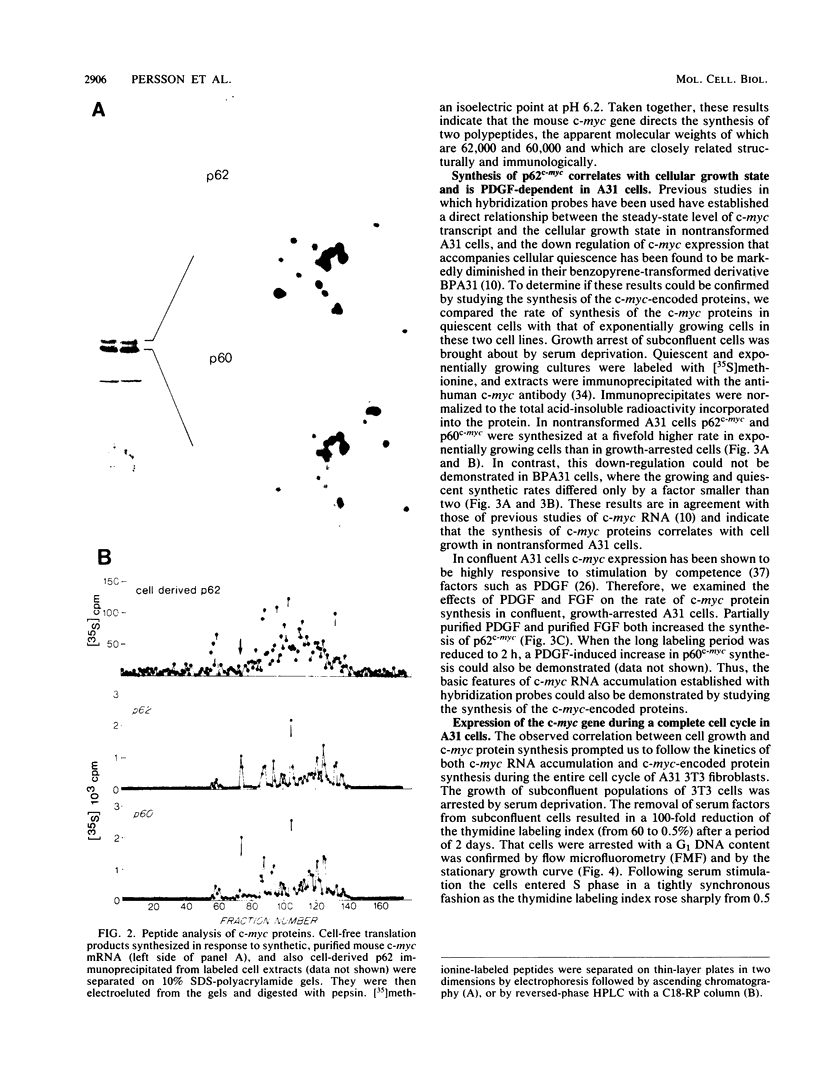
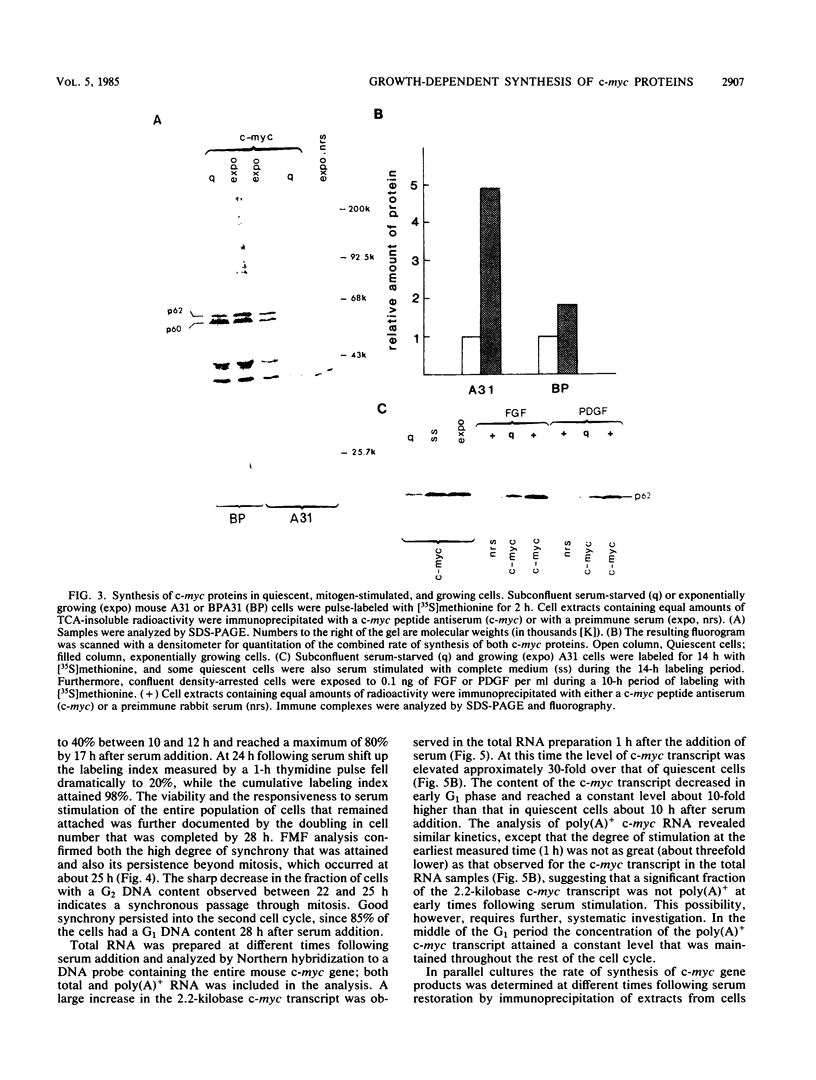
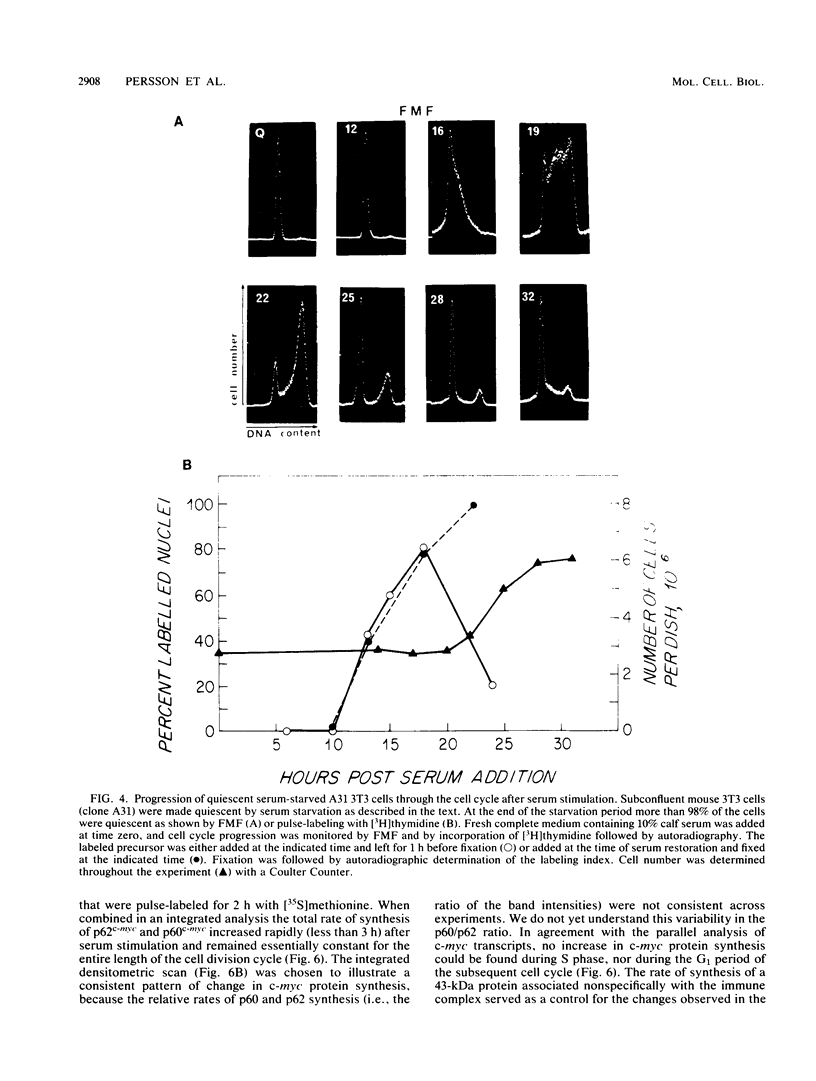
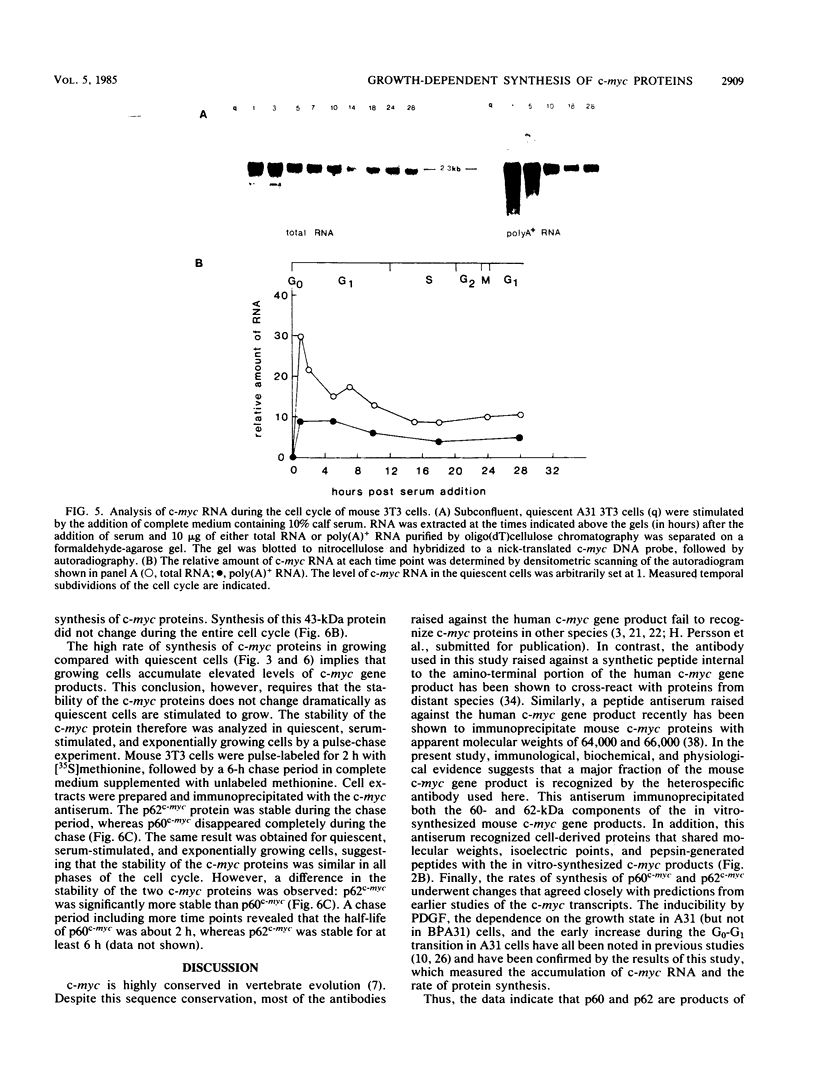
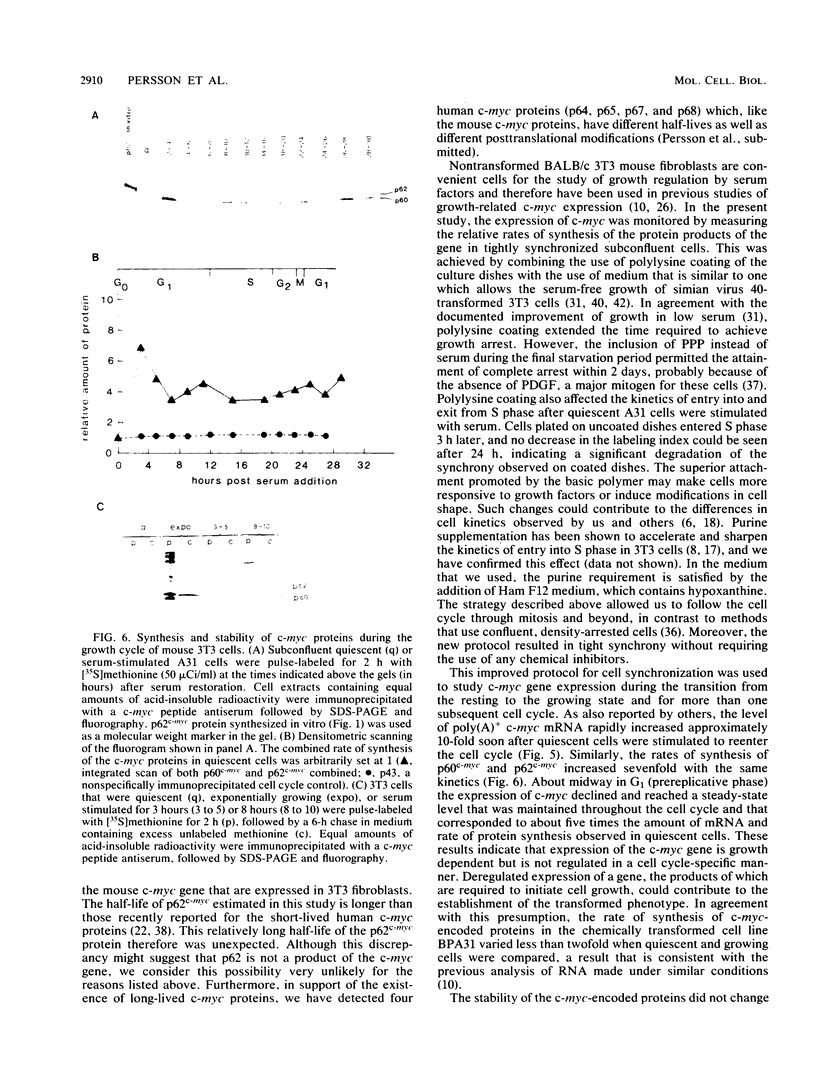
Synthesis of the c-myc gene product was measured during the entire cell cycle of subconfluent mouse 3T3 cells with an antibody raised against a human c-myc synthetic peptide. The antiserum recognized two mouse c-myc-encoded proteins with apparent molecular weights in sodium dodecyl sulfate-polyacrylamide gels of 62,000 and 60,000. Cell-derived p62 was compared with the mouse c-myc gene product synthesized in vitro. Immunoprecipitation, electrophoretic analyses, and peptide mapping provided evidence that p62 is encoded by the mouse c-myc gene. The rate of synthesis of the c-myc proteins was tightly coupled to the cellular growth state of nontransformed A31 3T3 cells, but not to that of their benzo(a)pyrene-transformed derivative (BPA31). Furthermore, the synthesis of the c-myc proteins was stimulated by the exposure of confluent, density-arrested A31 cells to platelet-derived growth factor or fibroblast growth factor. Tightly synchronized cell populations were obtained on the addition of serum factors to subconfluent, serum-deprived A31 cells, and c-myc expression could be monitored for more than one complete cell cycle. One hour after stimulation the steady-state level of the 2.2 kilobase c-myc transcript increased 30-fold relative to that of quiescent cells and decreased thereafter to the level observed during exponential growth. The rate of synthesis of c-myc-encoded proteins was determined by immunoprecipitation after a 2-h labeling period. After an initial sevenfold increase detectable 2 h after serum addition, the rate of synthesis remained constant throughout the rest of the cell cycle. No further changes associated with the late prereplicative period, S phase, G2, or mitosis could be demonstrated. Pulse-chase and long-term labeling experiments revealed different half-lives for the two c-myc-encoded proteins. The half-lives of the c-myc proteins, however, were independent of the cellular growth state. The sustained expression observed throughout the cell cycle suggests that the growth-related function of c-myc may be required during the G0-G1 transition and in all phases of the cycle of the growing cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams H. D., Rohrschneider L. R., Eisenman R. N. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell. 1982 Jun;29(2):427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Ramsay G., Bishop J. M., Pfeifer S. O., Colby W. W., Levinson A. D. Identification of nuclear proteins encoded by viral and cellular myc oncogenes. Nature. 1983 Nov 17;306(5940):274–277. doi: 10.1038/306274a0. [DOI] [PubMed] [Google Scholar]
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980 Sep;21(2):365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. The kinetics of serum-induced initiation of DNA synthesis in BHK 21/C13 cells, and the influence of exogenous adenosine. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):369–377. doi: 10.1002/jcp.1040860409. [DOI] [PubMed] [Google Scholar]
- Bunte T., Donner P., Pfaff E., Reis B., Greiser-Wilke I., Schaller H., Moelling K. Inhibition of DNA binding of purified p55v-myc in vitro by antibodies against bacterially expressed myc protein and a synthetic peptide. EMBO J. 1984 Aug;3(8):1919–1924. doi: 10.1002/j.1460-2075.1984.tb02068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Eisenman R. N., Tachibana C. Y., Abrams H. D., Hann S. R. V-myc- and c-myc-encoded proteins are associated with the nuclear matrix. Mol Cell Biol. 1985 Jan;5(1):114–126. doi: 10.1128/mcb.5.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström W., Zetterberg A. The relationship between purines, pyrimidines, nucleosides, and glutamine for fibroblast cell proliferation. J Cell Physiol. 1984 Aug;120(2):233–241. doi: 10.1002/jcp.1041200218. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Abrams H. D., Rohrschneider L. R., Eisenman R. N. Proteins encoded by v-myc and c-myc oncogenes: identification and localization in acute leukemia virus transformants and bursal lymphoma cell lines. Cell. 1983 Oct;34(3):789–798. doi: 10.1016/0092-8674(83)90535-4. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Eisenman R. N. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol. 1984 Nov;4(11):2486–2497. doi: 10.1128/mcb.4.11.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S. R., Thompson C. B., Eisenman R. N. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. 1985 Mar 28-Apr 3Nature. 314(6009):366–369. doi: 10.1038/314366a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H., Kiernan J. A., Messmer T. O. Control of growth of benzo(a)pyrene-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3229–3232. doi: 10.1073/pnas.73.9.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., Ham R. G. Stimulation of clonal growth of normal fibroblasts with substrata coated with basic polymers. J Cell Biol. 1976 Dec;71(3):727–734. doi: 10.1083/jcb.71.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Persson H., Hennighausen L., Taub R., DeGrado W., Leder P. Antibodies to human c-myc oncogene product: evidence of an evolutionarily conserved protein induced during cell proliferation. Science. 1984 Aug 17;225(4663):687–693. doi: 10.1126/science.6431612. [DOI] [PubMed] [Google Scholar]
- Persson H., Leder P. Nuclear localization and DNA binding properties of a protein expressed by human c-myc oncogene. Science. 1984 Aug 17;225(4663):718–721. doi: 10.1126/science.6463648. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G., Evan G. I., Bishop J. M. The protein encoded by the human proto-oncogene c-myc. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7742–7746. doi: 10.1073/pnas.81.24.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Rockwell G. A., Sato G. H., McClure D. B. The growth requirements of SV40 virus transformed Balb/c-3T3 cells in serum-free monolayer culture. J Cell Physiol. 1980 May;103(2):323–331. doi: 10.1002/jcp.1041030218. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Multiplication of Swiss 3T3 cells in a serum-free medium. Exp Cell Res. 1983 Jul;146(2):249–260. doi: 10.1016/0014-4827(83)90127-1. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. Autoradiography. Methods Enzymol. 1979;58:279–292. doi: 10.1016/s0076-6879(79)58143-9. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Bellvé A. R., Leder P. Transcription and promoter usage of the myc gene in normal somatic and spermatogenic cells. Science. 1984 Nov 9;226(4675):707–710. doi: 10.1126/science.6494906. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]