Abstract
Compelling evidence has documented the anxiolytic and mood-enhancing properties of cannabis. In susceptible users, however, consumption of this drug is conducive to panic, paranoia and dysphoria. We hypothesized that the up-regulation of CB1 receptors (CB1Rs) in select brain regions may enhance the vulnerability to cannabinoid-induced anxiety. To test this possibility, we assessed the behavioral impact of a potent cannabinoid agonist (CP55,940; 0.05–0.1 mg/kg, IP) on C57BL/6 male mice, respectively subjected to a prolonged pre-treatment of either the selective CB1R antagonist/inverse agonist AM251 (1 mg/kg/day IP, for 21 days, followed by a 3-day clearance period before testing) or its vehicle (VEH1). Anxiety-like responses were studied in the novel open field, elevated plus maze (EPM) and social interaction assays. While CP55,940 induced anxiolytic-like effects in the EPM in VEH1-exposed animals, it elicited opposite actions in AM251-exposed mice. In this last group, CP55,940 also reduced rearing and social interaction in comparison to its vehicle (VEH2). The divergent effects of CP55,940 in AM251- and VEH1-pretreated animals were confirmed in 129SvEv mice. Immunoblotting analyses on brain samples of C57BL/6 mice revealed that AM251 pre-treatment caused a significant up-regulation of CB1R expression in the prefrontal cortex and striatum, but also a down-regulation of these receptors in the hippocampus and midbrain. Notably, CB1R levels in the prefrontal cortex were negatively correlated with anxiolysis-related indices in the EPM; furthermore, midbrain CB1R expression was positively correlated with the total duration of social interaction. These results suggest that regional variations in brain CB1R expression may differentially condition the behavioral effects of cannabinoids with respect to anxiety-related responses.
1. Introduction
The widespread popularity of cannabis as a recreational substance is generally regarded as a consequence of its anxiolytic, mood-enhancing and euphorigenic properties (Green et al., 2003; SAMHSA, 2009); nevertheless, multiple anecdotal reports indicate that the psychological effects experienced by occasional marijuana smokers range from relaxation and heightened sociability to panic, paranoid ideation and dysphoria (Tambaro and Bortolato, 2012). This high variability is confirmed by several preclinical studies, which have shown that anxiety-like behaviors in rodents can be either attenuated or exacerbated by Δ9-tetrahydrocannabinol (Δ9-THC), the key psychoactive ingredient of hemp, or other cannabinoids (Bortolato and Piomelli, 2008; Bortolato et al., 2010).
The ability of natural and synthetic cannabinoids to influence anxiety responses is mostly mediated by the cannabinoid CB1 receptor (CB1R), a G-protein coupled receptor abundantly expressed in all the major brain regions implicated in emotional regulation, including the prefrontal cortex (PFC), amygdaloid complex, septo-hippocampal system and periaqueductal gray in the midbrain (Hajos and Freund, 2002; Herkenham et al., 1990; Herkenham et al., 1991; Katona et al., 2001). Differences in brain CB1R expression and/or sensitivity reflect the influence of multiple genetic and environmental factors (Kendler et al., 2003; Manzanares et al., 2004; Lazary et al., 2009) and may account for the polymorphous effects of cannabinoids on behavioral regulation. The role of CB1Rs in the modulation of anxiety, however, remains incompletely understood.
Prior evidence has shown that low doses of cannabinoids have anxiolytic-like properties in mice and rats (Berrendero and Maldonado, 2002; Braida et al., 2007; Haller et al., 2004; Patel and Hillard, 2006; Valjent et al., 2002), whereas higher concentrations of the same compounds elicit the opposite outcome (Celerier et al., 2006; Crippa et al., 2009; Genn et al., 2004; Marco et al., 2004; McGregor et al., 1996; Onaivi et al., 1990; Rodriguez de Fonseca et al., 1996). Building on these premises, we hypothesized that the behavioral response to the same dose of cannabinoids may depend on the expression of CB1Rs and that, specifically the up-regulation of these targets in specific brain regions may either abrogate or reverse the anxiolytic properties of low cannabinoid doses. To test this hypothesis, we endeavored to increase the expression of brain CB1Rs in C57BL/6 mice with a 3-week administration of AM251, a highly selective antagonist/inverse agonist of these targets (Lan et al., 1999). Following a 3-day washout period to allow for a full clearance of AM251, the behavioral effects of CP55,940 - a highly potent, synthetic analog of Δ9-THC with an analogous spectrum of pharmacological action – were studied across three complementary paradigms to test anxiety-related responses, namely the novel open field, elevated plus-maze and social interaction tests. Behavioral indices were then correlated with the regional expression of CB1Rs. Furthermore, in consideration of the role of the genetic background on anxiety responses and cannabinoid-mediated effects (Chakrabarti et al., 1998; Onaivi et al., 1995), all behavioral tests were repeated in 129SvEv mice, another murine line commonly used in preclinical experimentation, in consideration of the differences of these two genotypes with respect to anxiety-related behaviors (Pratte and Jamon, 2009).
2. Materials and Methods
2.1. Animals
Adult male C57BL/6 and 129SvEvTac (129S6) mice, weighing 25–30 g at the beginning of the study, were used. Animals were group-housed (3–4 for cage) with food and water available ad libitum. The room was maintained at 22 °C, on a 12-h light/dark cycle (with lights on at 06:00 AM). Experimental procedures were in compliance with the National Institute of Health guidelines and approved by the local Animal Use Committees of the Universities of Southern California and Cagliari. Each experimental group included 10–12 mice.
2.2. Drugs
AM251 [N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide] and CP55,940 [(−)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl) phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol] were purchased from Tocris Cookson (Bristol, UK). AM251 was dissolved in a vehicle (VEH1) of polyethylene glycol and saline solution (1:9, vol:vol). CP55,940 was dissolved in a vehicle (VEH2) of Tween 80, polyethylene glycol and saline (1:1:18). Both compounds were administrated intraperitoneally (i.p.) in an injection volume of 10 ml/kg.
2.3. Behavioral analyses
Experimental procedure
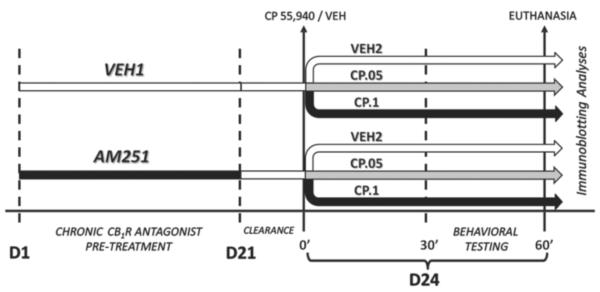
The experimental procedure is schematized in Fig.1. C57BL/6 mice were treated with daily injections of either AM251 (1 mg/kg/day) or its vehicle (VEH1) for 21 days. The dose of AM251 was selected in view of previous studies in mice (Chen et al.,2004; Zhou and Shearman, 2004), which showed its lack of significant effects on food intake and body weight. Injections were performed by expert personnel, so as to minimize pain or stress. Body weight was measured daily before the injections. At the end of the regimen, animals were left undisturbed in their cages for a period of 72 h (Fig.1), to ensure full clearance of AM251. The duration of this clearance period was based on preliminary studies in our laboratory, as well as previous data showing a normalization of food intake in rodents previously treated with chronic AM251 (Chambers et al., 2006). Subsequently, each group of mice was further subdivided into 3 subgroups and treated with either CP55,940 (0.05–0.1 mg/kg, i.p.) or its vehicle (VEH2). To avoid possible sources of bias due to cage-related effects, all cage mates received different treatment combinations. Thirty minutes after injection of CP55,940 or VEH2, mice were tested in a battery of paradigms for testing of anxiety-like behaviors (open field; elevated plus maze; social interaction) and cataleptic responses (bar test) (see below). The overall duration of the battery was 30 min, with 7-min intervals between subsequent tests (with the exception of the bar test, which was performed immediately after social interaction testing for 1 min). The experimental schedule for 129SvEv mice was identical to that used for C57BL/6 mice, but only one dose of CP55,940 (0.1 mg/kg, i.p.) was used. All behavioral testing took place between 10:00 AM and 3:00 PM.
Figure 1.
Synopsis of the experimental design, including treatment schedule, behavioral tests and immunobloting assays. For more details, see text.
Open field
Testing was conducted as previously indicated (Bortolato et al., 2011). The open field consisted of a Plexiglas square grey arena (40 × 40 cm) surrounded by 4 black walls (40 cm high). On the floor, two zones of equivalent areas were defined: a central square quadrant of 28.28 cm per side, and a concentric peripheral frame including the area within 11.72 cm from the walls. Light and sound were maintained at 20 lux and 70 dB, respectively. Mice were placed in the central zone and their behavior was monitored for 5 min. The distance travelled in the whole arena and the time spent in the center, as well as the number of rears, were measured with Ethovision software (Noldus Instruments, Wageningen, The Netherlands) for locomotor pattern analyses.
Elevated plus-maze
Testing was performed as previously described (Bortolato et al., 2009), in a black Plexiglas apparatus consisting of two open (25 × 5 cm) and two closed arms (25 × 5 × 5 cm), which extended from a central platform (5 × 5 cm) at 60 cm from the ground. Mice were individually placed on the central platform facing an open arm, and their behavior was recorded for 5 min. An arm entry was counted when all four paws were inside the arm. Behavioral measures included: number of entries and duration of time spent in each partition of the elevated plus-maze, number of stretch-attend postures and head dips, defined as described by Rodgers et al. (1992).
Social Interaction test
Mice were tested as previously described (Bortolato et al., 2011). Animals were introduced into a neutral, unfamiliar Makrolon cage (20 × 10 cm), with foreign strain-, age- and weight-matched male counterparts from separate litters and cages. To differentiate between the test mouse and the foreign conspecifics, the tail of the latter was colored with an odorless yellow acrylic paint marker. Testing sessions lasted 5 min and were video-recorded and later scored by observers blinded to the treatment. Social behaviors consisted of the frequency and duration of investigative sniffing of the conspecifics towards their facial, abdominal, and anogenital areas. Each of these behaviors was monitored in blind, with respect to its duration, number of events and latency to the first approach.
Bar test
Catalepsy was studied using the bar test, performed as previously described (Del Bel and Guimaraes, 2000). Briefly, the forepaws of each mouse were placed on a bar positioned 4 cm above a table, and the length of time during which the animal retained this position was recorded by an observer unaware of the treatment group. Each animal underwent three consecutive tests.
2.4. Neurochemical analyses
At the end of behavioral testing (60 min after VEH2 and CP55,940 treatment), C57BL/6 mice were deeply anesthetized with halothane, and sacrificed by cervical dislocation and quick decapitation. Skulls were promptly removed, and PFC, amygdala, striatum, hippocampus and midbrain were rapidly collected, snap-frozen in dry ice and stored at −80°C for further analyses.
Western Blotting
Protein extracts from tissue samples were prepared as previously described (Ramani et al., 2008) Protein concentrations were determined using Bradford protein assay reagent (Bio-Rad, Richmond, CA). 10ug of total protein were resolved on 10% SDS-PAGE gels (Criterion TGX, Bio-Rad, Hercules, CA) and transferred to nitrocellulose membrane (Trans-Blot, Bio-Rad) by electroblotting. Western blotting was performed following standard protocols (Amersham BioSciences, Piscataway, NJ) using primary antibodies for anti-CB1R (GTX100172S, GeneTex, Irvine, CA) and β-actin (Sigma-Aldrich, St. Louis, MO). Blots were developed using enhanced chemoluminescence (Millipore, Billerica, MA). Bands were quantified in arbitrary units using the NIH ImageJ Software, and normalized to β-actin levels.
2.5. Statistical analyses
Normality and homoscedasticity of data distribution were verified using the Kolmogorov-Smirnov and Bartlett's test. Parametric analyses were performed with two-way factorial ANOVA, followed by Newman-Keuls test for post-hoc comparisons. Analyses of stereotyped behaviors were performed using a three-way, repeated-measure ANOVA. Immunoblotting analyses were conducted on representative subpopulations of C57BL/6 mice (n=4–5/group). To minimize sampling errors, selection was performed with disproportionate stratification of the animals in each group, based on ranking of the average values of time spent in the open arms of the EPM and verification of the homoscedasticity of sampling strata. We ascertained that the time spent in open arms of the representative samples differed from the average time spent in open arms within each group by no more than 10% of the overall value of the population in each treatment group.
Correlations between behavioral and immunochemical parameters were studied by linear regression analyses. Alpha was set at P = 0.05.
3. Results
3.1. Behavioral testing in C57BL/6 mice
Elevated Plus Maze
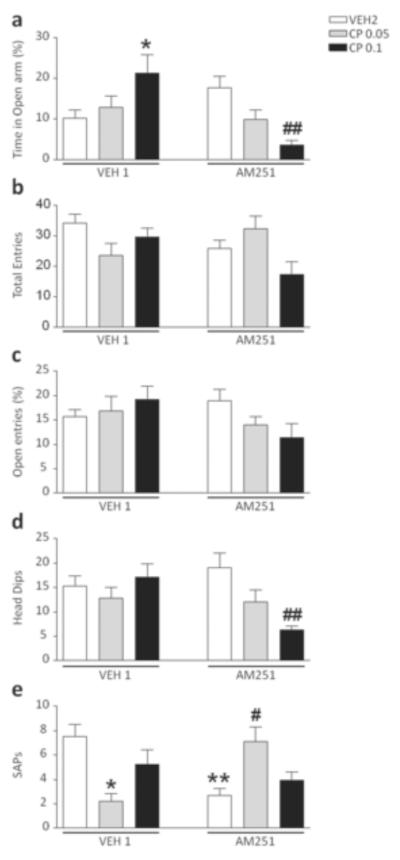
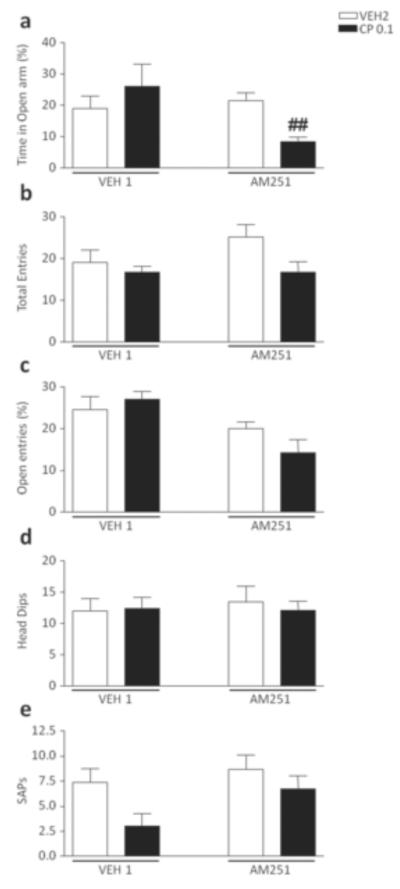
The analysis of the time spent in the open arms revealed a significant interaction between pre-treatment (VEH1 vs AM251) and treatment (VEH2 vs CP55,940) [F(2,56)=10.13, p<0.001, ANOVA]. Post-hoc comparisons revealed that the dose of 0.1 mg/kg of CP55,940 elicited an increase in time spent in the open arms in mice pre-exposed to VEH1 (P<0.05 for comparison between VEH1+VEH2 and VEH1+CP55,940 0.1; Newman-Keuls), while it exerted the opposite effect in animals treated with AM251 (P<0.01 for comparison between AM251+VEH2 and AM251+CP55,940 0.1; Newman-Keuls) (Fig. 2a). Conversely, no significant differences were identified for either the number of total arm entries (Fig. 2b) or the percentage of open-arm entries (Fig. 2c). A significant pre-treatment x treatment interaction was found for the head dips [F(2,58)=4.68, p<0.05] which was found to reflect a significant reduction induced by the highest dose of CP55,940 in AM251-pre-exposed mice (P<0.05 for comparison between AM251+VEH2 and AM251+CP55,940 0.1; Newman-Keuls] (Fig. 2d). A significant interaction was also identified in the stretch-attend postures (SAPs) [F(2,60)=10.20, P<0.001]. Newman-Keuls test revealed a significant decrease of SAPs in the mice treated with AM251 and VEH2 in comparison with VEH1 and VEH2 (P<0.01). In addition, the 0.05 mg/kg dose of CP reduced SAPs in VEH1-pretreated animals (P<0.05 for comparison between VEH1+VEH2 and VEH1+CP55,940 0.05), while exerting the opposite effects in AM251-exposed mice (P<0.05 for comparison between AM251+VEH2 and AM251+CP55,940 0.05) (Fig. 2e). No significant differences were found for the duration of the time spent in the central platform and in the closed arms (data not shown).
Figure 2.
Effects of CP55,940 on elevated plus maze test in C57BL/6 mice pre-exposed to chronic treatment of vehicle (polyethylene glycol: saline = 9:1) (VEH1) or AM251 (1 mg/kg/day, IP for 21 days). For all groups, n= 10–12. Doses are given in mg/kg (IP). Values represent mean ± SEM for each treatment. VEH2, vehicle of CP55,940 (Tween 80:polyethylene glycol: saline = 18:1:1); * P<0.05, ** P<0.01 compared with VEH1+VEH2; #P<0.05, ##P<0.01 compared with AM251+VEH2.
Open Field
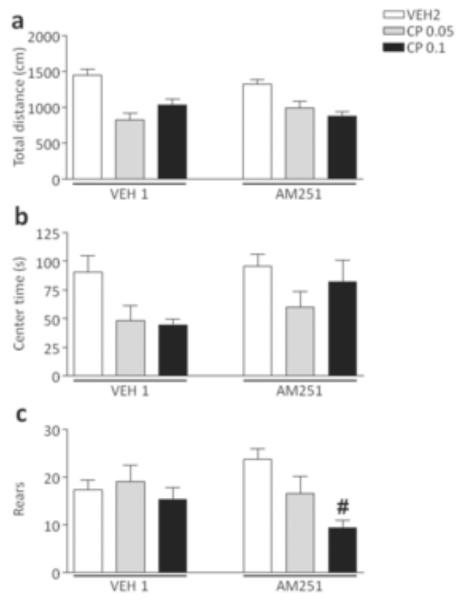
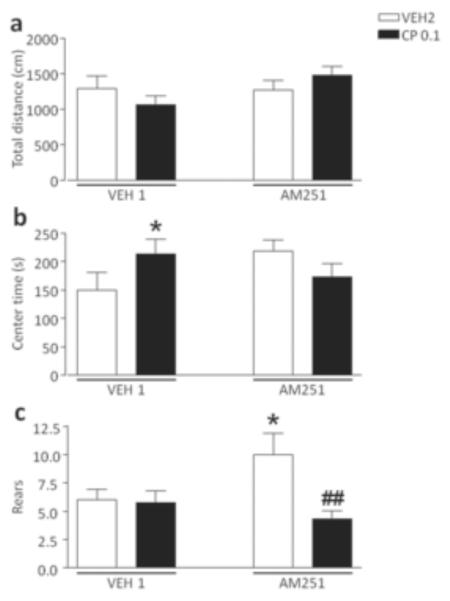
As shown in Fig. 3, no significant pre-treatment x treatment interactions were identified in the analysis of locomotor activity and trajectory patterns (Fig. 3a–b). The cannabinoid agonist, however, significantly decreased the number of rears in mice exposed to AM251 [F(2,54)=3.23, p<0.05, ANOVA; P<0.05 for comparisons between AM251+VEH2 and AM251+CP55,940 0.1; Newman-Keuls], but not VEH1 (Fig. 3c).
Figure 3.
Effects of CP55,940 on open-field behaviors in C57BL/6 mice pre-exposed to chronic treatment of vehicle (polyethylene glycol: saline = 9:1) (VEH1) or AM251 (1 mg/kg/day, IP for 21 days). For all groups, n= 10–12. Doses are given in mg/kg (IP). Values represent mean ± SEM for each treatment. VEH2, vehicle of CP55,940 (Tween 80:polyethylene glycol: saline = 18:1:1); #P<0.05 compared with AM251+VEH2..
Social interaction
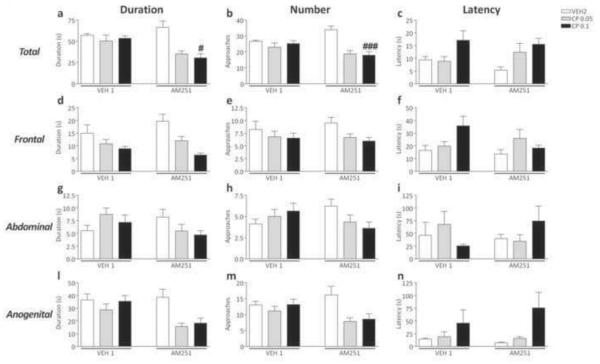
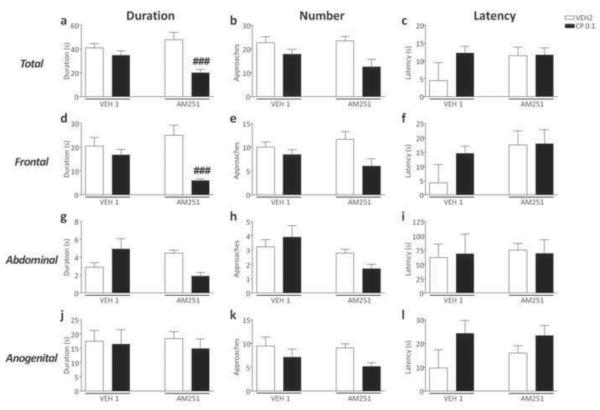
CP55,940 reduced the total duration [F(2,56)=6.14, p<0.01, ANOVA; P<0.05 for comparisons between AM251+VEH2 and AM251+CP55,940 0.1; Newman-Keuls] and number of social approaches [F(2,55)=6.14, p<0.01, ANOVA; P<0.001 for comparisons between AM251+VEH2 and AM251+CP55,940 0.1; Newman-Keuls] in AM251-exposed mice, but not in VEH1-treated counterparts (Fig.4a–b). Conversely, no significant differences were found in the latency to the first approach (Fig. 4c). No significant pre-treatment x treatment interactions were found in the analysis of frontal, abdominal and anogenital sniffing parameters (Fig. 4d–l).
Figure 4.
Effects of CP55,940 on overall duration, frequency and latency of social approaches in C57BL/6 mice pre-exposed to chronic treatment of vehicle (polyethylene glycol: saline = 9:1) (VEH1) or AM251 (1 mg/kg/day, IP, for 21 days). For all groups, n= 10–12. Doses are given in mg/kg (IP). Values represent mean ± SEM for each treatment. VEH2, vehicle of CP55,940 (Tween 80:polyethylene glycol: saline = 18:1:1); #P<0.05, ###P<0.001 compared with AM251+VEH2.
Catalepsy
The analysis of behavioral responses at the bar test revealed that CP55,940 did not induce catalepsy in either VEH1- or AM251-exposed mice (data not shown).
3.2. Behavioral testing in 129SvEv mice
Elevated plus maze
The analysis of the time spent in the open arms revealed a significant pre-treatment x treatment interaction [F(1,30)=11.66, P<0.01], which was found to depend on the effect of CP55,940 in AM251-pretreated mice (P<0.05 for comparison AM251+VEH2 vs AM251+CP55940, Newman-Keuls) (Fig. 5a). No significant difference was detected in the analysis of total entries, percent open entries, Head dips and SAPs (Fig. 5b–e).
Figure 5.
Effects of CP55,940 on elevated plus maze test in 129S6 mice pre-exposed to chronic treatment of vehicle (polyethylene glycol: saline = 9:1) (VEH1) or AM251 (1 mg/kg/day, IP for 21 days). For all groups, n= 10–12. Doses are given in mg/kg (IP). Values represent mean ± SEM for each treatment. VEH2, vehicle of CP55,940 (Tween 80:polyethylene glycol: saline = 18:1:1); ##P<0.01 compared with AM251+VEH2.
Open Field
As shown in Fig. 6, no group of mice exhibited significant changes of locomotor activity (Fig. 6a). However, CP55,940 significantly increased the time spent in the center of the arena by VEH1-exposed mice [F(1, 33)=9.64 p<0.01, ANOVA; P<0.01 for comparison between VEH1+CP 55,940 0.1 mg/kg and VEH1+VEH2; Newman-Keuls] (Fig. 6b). Mice pretreated with AM251 exhibited a significant enhancement in rears [F(1, 32)=5.54 p<0.05, ANOVA; P<0.05 for comparison between AM251+VEH2 and VEH1+VEH2; Newman-Keuls]; in addition, CP55,940 significantly reduced rears in AM251-exposed mice (P<0.01 for comparison between AM251+VEH2 and AM251+CP55,940; Newman-Keuls) (Fig. 6c).
Figure 6.
Effects of CP55,940 on open-field behaviors in 129S6 mice pre-exposed to chronic treatment of vehicle (polyethylene glycol: saline = 9:1) (VEH1) or AM251 (1 mg/kg/day, IP for 21 days). For all groups, n= 10–12. Doses are given in mg/kg (IP). Values represent mean ± SEM for each treatment. VEH2, vehicle of CP55,940 (Tween 80:polyethylene glycol: saline = 18:1:1); * P<0.05 compared with VEH1+VEH2; ##P<0.01 compared with AM251+VEH2.
Social interaction
CP55,940 reduced the duration of social interaction in AM251-, but not VEH1-exposed mice [F(1,34)=6.85, P<0.05; P<0.001 for comparison between AM251+VEH2 vs AM251+ CP55,940; Newman-Keuls] (Fig. 7a). Conversely, no significant pre-treatment x treatment interactions were found in the analysis of the total number of approaches and latency to the first approach (Fig. 7b–c). ANOVA also detected a reduction in the duration [F(1,33)=6.21, P<0.05] of frontal approaches, which was found to reflect a significant difference between AM251+VEH2 and AM251+CP55,940 (P<0.001; Newman-Keuls) (Fig. 7d). No significant differences in other parameters of social interaction were found (Fig. 7e–l).
Figure 7.
Effects of CP55,940 on overall duration, frequency and latency of social approaches in 129S6 mice mice pre-exposed to chronic treatment of vehicle (polyethylene glycol: saline = 9:1) (VEH1) (a–l) or AM251 (1 mg/kg/day, IP, for 21 days) (m–x). For all groups, n= 10–12. Doses are given in mg/kg (IP). Values represent mean ± SEM for each treatment. VEH2, vehicle of CP55,940 (Tween 80:polyethylene glycol: saline = 18:1:1); ###P<0.001 compared with AM251+VEH2.
Catalepsy
The analysis of behavioral responses at the bar test revealed that CP55,940 did not induce catalepsy in either VEH1- or AM251-treated mice (data not shown).
3.3. Western Blotting
Animal pretreated with AM251 exhibited a significant increase of CB1R expression in the PFC [F(1, 13)=8.60, P<0.05] and striatum [F(1, 13)=5.95, P<0.05] as compared to VEH1-pretreated controls (Fig. 8a, c). No significant variation of CB1R expression was detected in amygdala between mice pretreated with VEH1 and AM251 (Fig. 8b). AM251-pretreated animals showed a significant decrease of CB1Rs in the hippocampus [F(1, 10)=19.04, P<0.01] and midbrain [F(1, 14)=6.79, P<0.05], (Fig. 8d, e). These effects were not affected by CP55,940 injection.
Figure 8.
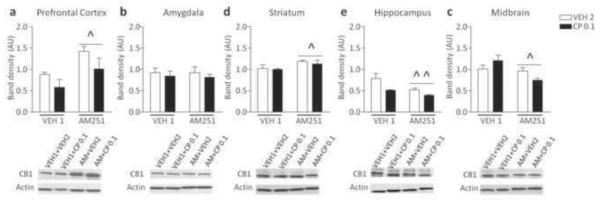
Western blot analyses of CB1 receptor protein expression in C57BL/6 mice pretreated with vehicle (VEH) and AM251-exposed treated with CP55,940 (0.1 mg/kg; IP) or vehicle. Data are expressed as average ± S.E.M. ^P < 0.05, ^^ P < 0.01 in comparison with VEH-pre-exposed mice (Main effects). For further details, see text.
3.4. Correlations between behavioral indices and brain CB1R expression
We then evaluated whether the anxiogenic-like behavior effects of CP55,940 (0.1mg/kg) on AM251-pretreated mice were correlated with CB1R expression (Table 1). In the PFC, CB1R expression was inversely correlated with the number of head dips in elevated plus maze paradigm [r2=0.92, B coefficient (slope)= −47.89 ± 9.72; β= −0.96 ± 0.20; Y intercept: 53.32 ± 7.17; P<0.05] and duration of center time (r2=0.89, B= −17.01 ± 4.13; β= −0.95 ± 0.23; Y intercept: 22.69 ± 3.05; P<0.05). In addition, a marginal statistical trend was detected for an inverse correlation between CB1R levels in the PFC and time spent in the open arms (r2=0.88, B= −54.51 ± 14.31; β= −0.94 ± 0.23; Y intercept: 57.15 ± 10.55; P=0.06). CB1R expression in PFC was not correlated with any of the behavioral indices in open field and social interaction paradigms.
Table 1.
Analyses of correlation between CB1 receptor levels (X) in the prefrontal cortex (PFC), amygdala (AMY), striatum (STR), hippocampus (HIP) and midbrain (MID) and anxiety-related behavioral indices (Y) in the elevated plus maze (EPM), open field (OF) and social interaction (SI) in C57BL/6. For further details, see text.
| X | Y | r2 | p | Slope | Intercept-Y axis | |
|---|---|---|---|---|---|---|
|
| ||||||
| PFC | EPM | Time in open arm (s) | 0.88 | 0.06 | −54.51 ± 14.31 | 57.15 ± 10.55 |
| Time in center (s) | 0.89 | 0.05 | −17.01 ± 4.14 | 22,69 ± 3.05 | ||
| Head Dips (fz) | 0.92 | 0.04 | −47.89 ± 9.73 | 53.32 ± 7.18 | ||
|
| ||||||
| OF | Total movement (cm) | 0.79 | 0.11 | −696.5 ± 252.2 | 1199 ± 186.0 | |
| Time in center (s) | 0.50 | 0.29 | −148.3 ± 104.2 | 170.0 ± 76.83 | ||
| Rearing (fz) | 0.40 | 0.36 | −15.34 ± 13.16 | 16.02 ± 6.83 | ||
|
| ||||||
| SI | Total sniffing (s) | 0.33 | 0.42 | −35.97 ± 36.02 | 38.12 ± 18.69 | |
| Frontal sniffing (s) | 0.74 | 0.14 | −21.35 ± 8.87 | 18.42 ± 8.86 | ||
| Abdominal sniffing (s) | 0.36 | 0.40 | −7.77 ± 7.35 | 6.43 ± 3.81 | ||
| Anogenital sniffing (s) | 0.01 | 0.93 | −3.59 ± 34.89 | 12.83 ± 18.10 | ||
|
| ||||||
| AMY | EPM | Time in open arm (s) | 0.38 | 0.39 | 66.07 ± 60.02 | −8.80 ± 21.22 |
| Time in center (s) | 0.65 | 0.19 | 26.69 ± 13.74 | −1.64 ± 4.86 | ||
| Head Dips (fz) | 0.42 | 0.55 | 47.96 ± 56.38 | −2.18 ± 20.39 | ||
|
| ||||||
| OF | Total movement (cm) | 0.12 | 0.57 | −566.6 ± 882.0 | 1098 ± 307.5 | |
| Time in center (s) | 0.13 | 0.55 | −261.4 ± 384.3 | 185.0 ± 134.0 | ||
| Rearing (fz) | 0.37 | 0.27 | −31.90 ± 23.94 | 22.11 ± 8.346 | ||
|
| ||||||
| SI | Total sniffing (s) | 0.10 | 0.60 | −43.37 ± 74.82 | 51.49 ± 26.08 | |
| Frontal sniffing (s) | 0.03 | 0.79 | 3.97 ± 13.57 | 6.49 ± 4.73 | ||
| Abdominal sniffing (s) | 0.07 | 0.66 | 9.11 ± 18.69 | 1.40 ± 6.51 | ||
| Anogenital sniffing (s) | 0.15 | 0.52 | −52.64 ± 72.11 | 42.75 ± 25.14 | ||
|
| ||||||
| STR | EPM | Time in open arm (s) | 0.57 | 0.25 | 343.6 ± 212.8 | −167.5 ± 118.1 |
| Time in center (s) | 0.45 | 0.33 | 106.6 ± 83.83 | −47.79 ± 46.52 | ||
| Head Dips (fz) | 0.61 | 0.22 | 312.9 ± 177.7 | −150.6 ± 98.62 | ||
|
| ||||||
| OF | Total movement (cm) | 0.52 | 0.17 | 1545 ± 858.8 | 5.795 ± 516.1 | |
| Time in center (s) | 0.05 | 0.73 | 158.9 ± 416.2 | 25.29 ± 250.1 | ||
| Rearing (fz) | 0.14 | 0.53 | −17.46 ± 24.81 | 19.77 ± 14.91 | ||
|
| ||||||
| SI | Total sniffing (s) | 0.23 | 0.41 | −35.55 ± 37.07 | 64.72 ± 22.28 | |
| Frontal sniffing (s) | 0.01 | 1.00 | 0.09± 35.87 | 9.95 ± 21.56 | ||
| Abdominal sniffing (s) | 0.11 | 0.59 | 12.35 ± 20.54 | −1.14 ± 12.35 | ||
| Anogenital sniffing (s) | 0.15 | 0.52 | −49.85 ± 68.99 | 57.41 ± 41.46 | ||
|
| ||||||
| HIP | EPM | Time in open arm (s) | 0.50 | 0.29 | −135.8 ± 96.22 | 70.62 ± 39.66 |
| Time in center (s) | 0.64 | 0.20 | −54.95 ± 28.87 | 31.47 ± 11.90 | ||
| Head Dips (fz) | 0.58 | 0.24 | −135.6 ± 82.30 | 72.47 ± 33.92 | ||
|
| ||||||
| OF | Total movement (cm) | 0.01 | 0.88 | 322.4 ± 1949 | 752.3 ± 803.3 | |
| Time in center (s) | 0.03 | 0.82 | −109.5 ± 419.0 | 108.6 ± 172.7 | ||
| Rearing (fz) | 0.37 | 0.39 | 144.8 ± 134.2 | −33.90 ± 55.31 | ||
|
| ||||||
| SI | Total sniffing (s) | 0.49 | 0.30 | −105.1 ± 76.00 | 57.53 ± 31.32 | |
| Frontal sniffing (s) | 0.42 | 0.35 | −44.90 ± 37.40 | 26.70 ± 15.42 | ||
| Abdominal sniffing (s) | 0.43 | 0.34 | −23.37 ± 18.99 | 13.09 ± 7.82 | ||
| Anogenital sniffing (s) | 0.57 | 0.25 | −37.01 ± 22.88 | 18.55 ± 9.42 | ||
|
| ||||||
| MID | EPM | Time in open arm (s) | 0.72 | 0.07 | 211.9 ± 76.17 | −97.52 ± 42.73 |
| Time in center (s) | 0.67 | 0.09 | 69.01 ± 27.91 | −27.96 ± 15.66 | ||
| Head Dips (fz) | 0.64 | 0.11 | 174.4 ± 76.03 | −76.54 ± 42.65 | ||
|
| ||||||
| OF | Total movement (cm) | 0.02 | 0.80 | 508.1 ± 1879 | 611.0 ± 1054 | |
| Time in center (s) | 0.30 | 0.34 | 517.2 ± 452.1 | −199.5 ± 253.6 | ||
| Rearing (fz) | 0.44 | 0.23 | −197.6 ± 129.7 | 130.1 ± 72.77 | ||
|
| ||||||
| SI | Total sniffing (s) | 0.77 | 0.04 | 185.4 ± 57.80 | −83.26 ± 32.43 | |
| Frontal sniffing (s) | 0.13 | 0.54 | 31.43 ± 46.02 | −9.67 ± 25.82 | ||
| Abdominal sniffing (s) | 0.11 | 0.58 | 14.24 ± 23.45 | −4.514 ± 13.16 | ||
| Anogenital sniffing (s) | 0.75 | 0.06 | 142.5 ± 47.93 | −69.63 ± 26.89 | ||
Furthermore, CB1R levels in the midbrain were positively correlated with the total sniffing time in the social interaction test (r2=0.77, B= 185.40 ± 57.80; β= 0.88 ± 0.27; Y intercept: −83.26 ± 32.43; P<0.05). Furthermore, statistical trends were found for positive correlations between midbrain CB1R levels and the time spent in the open arms (r2=0.77, B= 211.9 ± 76.17; β= 0.85 ± 0.31; Y intercept: −97.52 ± 42.73; P=0.07) and in the central platform (r2=0.67, B= 69.01 ± 27.91; β= 0.82 ± 0.33; Y intercept: −27.96 ± 15.66; P=0.09) of the EPM. No statistical correlations were found between any of the behavioral indices and CB1R levels across striatum, amygdala and hippocampus.
4. Discussion
The results of the present study show that the prolonged administration of the selective CB1R antagonist/inverse agonist AM251 in mice induced a significant up-regulation of CB1Rs in the PFC and striatum, all the while reducing the expression of these targets in the hippocampus and midbrain; in addition, this regimen did not induce any specific variations of CB1R expression in the amygdala.
These neurochemical changes were accompanied by marked modifications of the behavioral reactivity to acute administration of CP55,940, a potent synthetic analog of Δ9-THC, across different behavioral paradigms aimed at capturing complementary aspects of anxiety-related responsiveness. In particular, the intraperitoneal dose of 0.1 mg/kg of this cannabinoid agonist increased the proclivity of VEH1-pretreated mice to explore the unprotected arms of the EPM, a phenomenon generally associated with anxiolytic drugs; conversely, the same dose elicited anxiogenic-like effects in AM251-exposed animals in the same paradigm, as signified by a decrease of open-arm duration, head dips and SAPs. The bidirectional effects of CP55,940 are reminiscent of previous data, indicating the ability of low and high doses of this compound to respectively reduce or increase anxiety-like behaviors in the EPM (Marco et al., 2004). Previous studies have shown that the infusion of low doses of CB1R agonists in the PFC induce an anxiolytic-like response in rats, whereas higher doses elicit opposite effects (Rubino et al., 2008), suggesting a key role of this brain region in mediating the biphasic role of cannabinoids in EPM-related anxiety. In support of this finding, we showed that, in AM251-pretreated mice, CB1R levels in the PFC, but not in other regions, were inversely correlated with the numbers of head dips and the overall center duration, as well as a statistical trend (P=0.06) for the open-arm duration. This finding suggests that higher levels of prefrontal CB1Rs may shift cannabinoid-induced effects from anxiolytic to anxiogenic, in a fashion akin to that elicited by increasing CB1R agonist doses.
The implication of the PFC in this process is in keeping with the well-known role of this region as a key player in the cognitive modulation of anxiety and other emotional states (Davidson, 2002), as well as in cannabinoid-mediated anxiety (Rubino et al., 2008). Several studies have documented that the PFC mediates the spatio-temporal integration of multiple signals from different brain areas and engaging limbic circuitries that regulate emotional responsiveness (Fuster, 2001; Gray et al., 2002; Miller and Cohen, 2001). These processes allow for the appraisal of potential threats (Bishop et al., 2004) and the attribution of emotional valences to environmental cues (Davidson, 2002; Ochsner et al., 2005; Phillips et al., 2003), which play a key role in triggering anxiety responses.
Recently, Rey and colleagues (2012) have shown that the bimodal actions of CP55,940 on anxiety regulation subtend the activation of distinct populations of forebrain CB1Rs: specifically, the anxiolytic-like effects of the low dose of cannabinoids are mediated via the CB1Rs receptor on cortical glutamatergic terminals, while the anxiogenic responses reflect the activation of CB1Rs in GABAergic neurons. This background suggests that prolonged antagonism of CB1Rs may differentially affect these receptors in pyramidal cells (glutamatergic) and interneurons (GABAergic) in the PFC. Future immunolabeling studies will be needed to evaluate the nature of the adaptive changes in CB1R across different neuronal groups in this and other regions.
Notably, the dichotomy observed in the EPM was not found in the OF in the C57BL/6 mice. Furthermore, in the social interaction test, AM251 pre-exposure led to the exacerbation of anxiety-related response to CP55,940. The different outcomes observed across the three behavioral paradigms are likely reflective of the divergent endophenotypes and facets of anxiety captured by these paradigms.
It is worth noting that the levels of CB1Rs in the midbrain of AM251-exposed mice were significantly correlated with the total duration of social interaction, and yielded a statistical correlation trend (P<0.10) with open-arm duration in the EPM, suggesting a dichotomic action of CB1Rs between PFC and midbrain with respect to the circuitry of anxiety.
The regional differences in the plastic adaptive changes of CB1Rs may reflect the complex role of these targets in promoting the down-regulation of multiple neurochemical systems with opposite functions, such as GABA and glutamate. Interestingly, previous studies have shown that CP55,940 and other potent cannabimimetic drugs can dynamically regulate CB1R trafficking and internalization through clathrin-coated pits (Hsieh et al., 1999). Although the present set of results cannot explain the differential responsiveness of multiple brain regions to CB1R antagonism, these phenomena may reflect the existence of variable cannabinoid homeostatic balances (including the existence of cannabinergic tones in select brain structures), and distinct region-specific processes of receptor internalization and degradation.
An intriguing corollary of our findings is that the anxiogenic shift ensuing AM251 pre-treatment may be shared by other biological processes leading to prefrontal up-regulation (or midbrain down-regulation) of CB1Rs. Accordingly, CB1R agonists have been shown to exert anxiolytic or anxiogenic properties in relation to the pre-exposure to chronic stress (Hill and Gorzalka, 2004), a condition associated with CB1R up-regulation in the PFC and down-regulation in the midbrain (Bortolato et al., 2007). Furthermore, exposure to other substances of abuse, such as entactogens or opioids may affect the psychological outcomes of cannabinoids by altering their CB1R distribution (Bortolato et al., 2010; Gonzalez et al., 2002).
Although the responses to AM251 and CP55,940 in 129SvEv mice were analogous to those observed in C57BL/6 counterparts, the latter animals also exhibited an exquisite sensitivity to the modulatory effects of cannabinoids to the anxiety-like responses evoked by the center of a novel open field. Specifically, the same dose of CP55,940 either attenuated or augmented thigmotactic behaviors, related to the instinctual tendency of rodents to avoid the center of an open arena and remain near its walls (Treit and Fundytus, 1988; Wilson et al., 1976). It is likely that the strain differences may reflect specific changes in baseline emotional state and/or different regional distributions of brain CB1Rs. Accordingly, the ability of cannabinoids to exert different influences on emotional reactivity has been shown to be governed by genetic factors (Bortolato et al., 2010; Verweij et al., 2010).
Several limitations of this study should be acknowledged. First, AM251 was recently shown to bind also to μ opioid receptors (Seely et al., 2012), raising the possibility that some of the observed effects may be partially related to these targets. This possibility, however, is partially challenged by the fact that the affinity of AM251 is markedly lower (> 30 fold) than that for CB1 receptors and by the observation that the observed behavioral profile does not appear to fully overlap with the behavioral alterations induced by systemic μ receptor ligands (Anseloni et al., 1999). Future studies, however, should ascertain whether the observed changes can be replicated with other CB1 receptor antagonists with no affinity for μ opioid receptors, such as AM281 (Seely et al., 2012). Second, the correlations between CB1R expression in the PFC and defensive postures in the EPM do not necessarily imply a causal nexus between the two phenomena. Indeed, because the up-regulation of CB1Rs was pharmacologically induced as a compensatory response to a chronic pre-treatment with a CB1R antagonist, our data cannot ultimately rule out that the observed behavioral and neurochemical changes may be unrelated, but both engendered by other common causes. Alternative additional approaches, such as regional injections with CB1R-carrying lentiviral vectors, will be needed to study the direct role of CB1R overexpression in influencing the outcome of cannabinoids on anxiety. Third, our experimental design does not allow to rule out the possibility that the behavioral effects observed in our study may be contributed by potential carryover effects of stress (due to repeated testing in the same mouse) as well as differences in pharmacokinetic effects (due to different time intervals between the drug injection and testing). Fourth, several key factors affecting the role of cannabinoids on anxiety regulation, such as gender and age, were not examined in the present study. The expression and responsiveness of CB1Rs is subject to genetic and age-dependent variations (Berrendero et al., 1999; McLaughlin et al., 1994; Ortiz et al., 2004). Fifth, our analyses were only limited to anxiety-related responses and did not evaluate other behavioral domains subjected to bimodal modulatory effects of cannabinoids, including novelty seeking (Maldonado and Rodriguez de Fonseca, 2002) and motivation (Maldonado, 2002).
Irrespective of these caveats, the present results point to the possibility that the variable responsiveness to acute consumption of cannabis among different individuals may reflect differential expressions of CB1Rs across multiple brain regions.
5. Conclusions
The identification of different relations between the levels of CB1Rs in PFC and midbrain and the effects of cannabinoids in anxiety regulation is extremely intriguing in view of its potential translational implications for the identification of vulnerability factors to cannabis-induced behavioral effects. Marijuana consumption with therapeutic purposes is relatively high among patients affected by certain anxiety-spectrum disorders, such as social phobia, obsessive-compulsive disorder and post-traumatic stress disorder (Crippa et al., 2009). Considering that the state of wellness and the improved sociability induced by cannabis are arguably the main incentives to the consumption of this drug, the present results highlight the possibility that different levels of CB1R expression may serve as biomarkers for vulnerability to various psychological effects of cannabis. This finding may have important repercussions on the development of novel strategies aimed at harnessing the anxiolytic potential of cannabinoids in some patients and curb cannabis dependence in susceptible subjects.
Acknowledgments
The present study was supported by grants from the National Institute of Health (R21HD070611, to M.B.), Tourette Syndrome Association (to M.B.), and the Sardinia Region Government “Master and Back” fellowship (to S.T.). We would like to thank Caleb Finch and Todd Morgan for their precious help and suggestions. We are grateful to Sean Godar, Tatevik Kirakosian and Reyna Pulliam for their technical assistance. None of the institutions had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- Anseloni VC, Coimbra NC, Morato S, Brandão ML. A comparative study of the effects of morphine in the dorsal periaqueductal gray and nucleus accumbens of rats submitted to the elevated plus-maze test. Exp Brain Res. 1999;129:260–268. doi: 10.1007/s002210050896. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Bini V, Tambaro S. Vulnerability Factors for the Psychiatric and Behavioral Effects of Cannabis. Pharmaceuticals. 2010;3:2799–2820. doi: 10.3390/ph3092799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Godar SC, Chen G, Wu W, Rebrin I, Farrell MR, Scott AL, Wellman CL, Shih JC. Social Deficits and Perseverative Behaviors, but not Overt Aggression, in MAO-A Hypomorphic Mice. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Davarian S, Chen K, Shih JC. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology. 2009;34:2746–2757. doi: 10.1038/npp.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Piomelli D. The endocannabinoid system and anxiety responses Handbook of Behavioral Neuroscience 17: Handbook of anxiety and fear. Chapter 4.5. Elsevier; Amsterdam, Netherlands: 2008. pp. 303–324. [Google Scholar]
- Braida D, Limonta V, Malabarba L, Zani A, Sala M. 5-HT1A receptors are involved in the anxiolytic effect of Delta9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague-Dawley rats. Eur J Pharmacol. 2007;555:156–163. doi: 10.1016/j.ejphar.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Celerier E, Ahdepil T, Wikander H, Berrendero F, Nyberg F, Maldonado R. Influence of the anabolic-androgenic steroid nandrolone on cannabinoid dependence. Neuropharmacology. 2006;50:788–806. doi: 10.1016/j.neuropharm.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Ekuta JE, Onaivi ES. Neurobehavioral effects of anandamide and cannabinoid receptor gene expression in mice. Brain Res Bull. 1998;45:67–74. doi: 10.1016/s0361-9230(97)00291-8. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Koopmans HS, Pittman QJ, Sharkey KA. AM 251 produces sustained reductions in food intake and body weight that are resistant to tolerance and conditioned taste aversion. Br J Pharmacol. 2006;147:109–116. doi: 10.1038/sj.bjp.0706439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Huang RR, Shen CP, MacNeil DJ, Fong TM. Synergistic effects of cannabinoid inverse agonist AM251 and opioid antagonist nalmefene on food intake in mice. Brain Res. 2004;999:227–230. doi: 10.1016/j.brainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Martin-Santos R, Bhattacharyya S, Atakan Z, McGuire P, Fusar-Poli P. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515–523. doi: 10.1002/hup.1048. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Del Bel EA, Guimaraes FS. Sub-chronic inhibition of nitric-oxide synthesis modifies haloperidol-induced catalepsy and the number of NADPH-diaphorase neurons in mice. Psychopharmacology (Berl) 2000;147:356–361. doi: 10.1007/s002130050003. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Genn RF, Tucci S, Marco EM, Viveros MP, File SE. Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharmacol Biochem Behav. 2004;77:567–573. doi: 10.1016/j.pbb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci U S A. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B, Kavanagh D, Young R. Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev. 2003;22:453–460. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Enhancement of anxiety-like responsiveness to the cannabinoid CB(1) receptor agonist HU-210 following chronic stress. Eur J Pharmacol. 2004;499:291–295. doi: 10.1016/j.ejphar.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Hsieh C, Brown S, Derleth C, Mackie K. Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem. 1999;73:493–501. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Hunyady L, Juhasz G, Bagdy G. Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1118–1127. doi: 10.1002/ajmg.b.31024. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther. 2002;95:153–164. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodriguez de Fonseca F. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci. 2002;22:3326–3331. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J, Uriguen L, Rubio G, Palomo T. Role of endocannabinoid system in mental diseases. Neurotox Res. 2004;6:213–224. doi: 10.1007/BF03033223. [DOI] [PubMed] [Google Scholar]
- Marco EM, Perez-Alvarez L, Borcel E, Rubio M, Guaza C, Ambrosio E, File SE, Viveros MP. Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in male rats. Behav Pharmacol. 2004;15:21–27. doi: 10.1097/00008877-200402000-00003. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Issakidis CN, Prior G. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav. 1996;53:657–664. doi: 10.1016/0091-3057(95)02066-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin CR, Martin BR, Compton DR, Abood ME. Cannabinoid receptors in developing rats: detection of mRNA and receptor binding. Drug Alcohol Depend. 1994;36:27–31. doi: 10.1016/0376-8716(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D'Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Chakrabarti A, Gwebu ET, Chaudhuri G. Neurobehavioral effects of delta 9-THC and cannabinoid (CB1) receptor gene expression in mice. Behav Brain Res. 1995;72:115–125. doi: 10.1016/0166-4328(96)00139-8. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990;253:1002–1009. [PubMed] [Google Scholar]
- Ortiz S, Oliva JM, Perez-Rial S, Palomo T, Manzanares J. Differences in basal cannabinoid CB1 receptor function in selective brain areas and vulnerability to voluntary alcohol consumption in Fawn Hooded and Wistar rats. Alcohol Alcohol. 2004;39:297–302. doi: 10.1093/alcalc/agh063. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Gregory LJ, Cullen S, Coen S, Ng V, Andrew C, Giampietro V, Bullmore E, Zelaya F, Amaro E, Thompson DG, Hobson AR, Williams SC, Brammer M, Aziz Q. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain. 2003;126:669–684. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- Pratte M, Jamon M. Detection of social approach in inbred mice. Behav Brain Res. 2009;203:54–64. doi: 10.1016/j.bbr.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Ramani K, Yang H, Xia M, Ara AI, Mato JM, Lu SC. Leptin's mitogenic effect in human liver cancer cells requires induction of both methionine adenosyltransferase 2A and 2beta. Hepatology. 2008;47:521–531. doi: 10.1002/hep.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, Lutz B. Biphasic Effects of Cannabinoids in Anxiety Responses: CB1 and GABA(B) Receptors in the Balance of GABAergic and Glutamatergic Neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Lee C, Shepherd JK. Effects of diazepam on behavioural and antinociceptive responses to the elevated plus-maze in male mice depend upon treatment regimen and prior maze experience. Psychopharmacology (Berl) 1992;106:102–110. doi: 10.1007/BF02253596. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, Petrosino S, Perletti G, Maccarrone M, Di Marzo V, Parolaro D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- Seely KA, Brents LK, Franks LN, Rajasekaran M, Zimmerman SM, Fantegrossi WE, Prather PL. AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies. Neuropharmacology. 2012;63:905–915. doi: 10.1016/j.neuropharm.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2008 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD, USA: 2009. (NSDUH Series H-36). HHS Publication No. SMA 09-4434. [Google Scholar]
- Tambaro S, Bortolato M. Cannabinoid-related agents in the treatment of anxiety disorders: current knowledge and future perspectives. Recent Pat CNS Drug Discov. 2012;7:25–40. doi: 10.2174/157488912798842269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij KJ, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, Boomsma DI, Vink JM. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010;105:417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Vacek T, Lanier DL, Dewsbury DA. Open-field behavior in muroid rodents. Behav Biol. 1976;17:495–506. doi: 10.1016/s0091-6773(76)90901-9. [DOI] [PubMed] [Google Scholar]
- Zhou D, Shearman LP. Voluntary exercise augments acute effects of CB1-receptor inverse agonist on body weight loss in obese and lean mice. Pharmacol Biochem Behav. 2004;77:117–125. doi: 10.1016/j.pbb.2003.10.015. [DOI] [PubMed] [Google Scholar]