Abstract
Background
The emergence and rapid spreading of multidrug-resistant Acinetobacter baumannii strains has become a major health threat worldwide. To better understand the genetic recombination related with the acquisition of drug-resistant elements during bacterial infection, we performed complete genome analysis on three newly isolated multidrug-resistant A. baumannii strains from Beijing using next-generation sequencing technology.
Methodologies/Principal Findings
Whole genome comparison revealed that all 3 strains share some common drug resistant elements including carbapenem-resistant bla OXA-23 and tetracycline (tet) resistance islands, but the genome structures are diversified among strains. Various genomic islands intersperse on the genome with transposons and insertions, reflecting the recombination flexibility during the acquisition of the resistant elements. The blood-isolated BJAB07104 and ascites-isolated BJAB0868 exhibit high similarity on their genome structure with most of the global clone II strains, suggesting these two strains belong to the dominant outbreak strains prevalent worldwide. A large resistance island (RI) of about 121-kb, carrying a cluster of resistance-related genes, was inserted into the ATPase gene on BJAB07104 and BJAB0868 genomes. A 78-kb insertion element carrying tra-locus and bla OXA-23 island, can be either inserted into one of the tniB gene in the 121-kb RI on the chromosome, or transformed to conjugative plasmid in the two BJAB strains. The third strains of this study, BJAB0715, which was isolated from spinal fluid, exhibit much more divergence compared with above two strains. It harbors multiple drug-resistance elements including a truncated AbaR-22-like RI on its genome. One of the unique features of this strain is that it carries both bla OXA-23 and bla OXA-58 genes on its genome. Besides, an Acinetobacter lwoffii adeABC efflux element was found inserted into the ATPase position in BJAB0715.
Conclusions
Our comparative analysis on currently completed Acinetobacter baumannii genomes revealed extensive and dynamic genome organizations, which may facilitate the bacteria to acquire drug-resistance elements into their genomes.
Introduction
Acinetobacter baumannii is an important opportunistic pathogen of hospital acquired infection, particularly in intensive care units, which is usually responsible for up to 10% of hospital-acquired infections and increases mortality up to 70% [1]–[4]. A. baumannii often causes outbreaks of infection and can survive for long periods in the hospital environment [5]. Moreover, A. baumannii shows a strong ability to acquire foreign DNA such as drug resistance and pathogenicity, which makes it to acquire genetic diversity and overcomes the antibiotic selection pressure [6]. The antimicrobial resistance in this nosocomial pathogen is mainly caused by inactivating enzymes such as β-lactamases, alteration of membrane porin channels, and mutations that change cellular functions.
Recently, increasing resistance to carbapenems in A. baumannii has emerged which severely limits the treatment options for this pathogen. The most important resistance mechanism is mediated by producing class D β-lactamases with carbapenemase activity, such as bla OXA-23-like, bla OXA-24-like, bla OXA-40-like, and bla OXA-58-like genes in A. baumannii [7]–[10]. Among them the bla OXA-23 gene, first identified in Scotland, has been found worldwide spread [11]–[20].
Next generation sequencing (NGS) technology provides an ability to evaluate resistance mechanisms, pathogenicity and evolution of bacterial pathogens on genome-wide level and has been proved to be useful to thoroughly understand the basic features of pathogens in order to ultimately control the spread of pathogen infections and to develop effective treatments. The whole genomes of many clinical important and prevalent A. baumannii representatives have been sequenced [21]–[32]. The identification of the genomic components of A. baumannii provides a scaffold to rapidly evaluate the genomic organization and epidemiological information of novel clinical A. baumannii isolates.
We reported here the genome sequences of three recently isolated multidrug-resistant (MDR) strains from Beijing, China (BJAB strains), including BJAB07104, BJAB0868, and BJAB0715, which were isolated from different clinical samples but all have bla OXA-23 gene. Genome comparison analysis was performed to determine how the differences of genomic organization and sequence divergence are related to the observed resistance and pathogenesis phenotypes.
Results and Discussion
Susceptibility Profiles and Multilocus Sequence Typing (MLST)
Three representative MDR A. baumannii strains, BJAB0715, BJAB0868 and BJAB07104, which were isolated from different clinical samples in Beijing during March 2007 and April 2008, were selected for whole-genome sequencing. The three strains were isolated from bloodstream (BJAB07104), ascites (BJAB0868) and cerebrospinal fluid (BJAB0715), respectively, and showed a similar susceptibility pattern. All of them are resistant to almost all currently available antibiotics including imipenem, amikacin, minocycline, ciprofloxacin, levofloxacin, piperacillin, piperacillin/tazobactam, ceftazidime, cefotaxime, cefepime, cefoperazone/sulbactam (1∶1) and meropenem; but susceptible to polymyxin B. The drug-susceptibility profiles were showed in Table 1.
Table 1. Susceptibility profiles of three MDR strains.
| Antibiotics | BJAB0715 | BJAB0868 | BJAB07104 | |||
| MIC(mg/L) | R/S | MIC(mg/L) | R/S | MIC(mg/L) | R/S | |
| amikacin | 256 | R | >256 | R | >256 | R |
| caftazidime | 16 | R | 128 | R | 128 | R |
| cefepime | 32 | R | 256 | R | 128 | R |
| cefotaxime | 64 | R | >256 | R | >256 | R |
| ciprofloxacin | 16 | R | 64 | R | 32 | R |
| imipenem | 64 | R | 128 | R | >64 | R |
| levofloxacin | 8 | R | 16 | R | 8 | R |
| meropenem | 64 | R | 64 | R | 64 | R |
| minocycline | 64 | R | 8 | S | 16 | R |
| piperacillin | >512 | R | >256 | R | >512 | R |
| tazobactam | >128 | R | >256 | R | >128 | R |
| polymyxin | 2 | S | 2 | S | 2 | S |
| Tetracyclines | >16 | R | >16 | R | >16 | R |
MLST was first performed for investigating the population structure of three A. baumannii clinical isolates [33]. An A. baumannii database (www.pasteur.fr/recherche/genopole/PF8/mlst/Abaumannii.html) was used to analyze sequences of the 7 housekeeping genes (cpn60, fusA, gltA, pyrG, recA, rpIB and rpoB). We found that the isolates of BJAB07104 and BJAB0868 show the same allelic profile (cpn60-2, fusA-2, gltA-2, pyrG-2, recA-2, rpIB-2 and rpoB-2), which corresponds to European clone II (also called global clone II (GC II)), and were recommended to be designated by ST2 or CC2 (where CC stands for clonal complex) for uniform nomenclature. BJAB0715 strain shows a different allelic profile (cpn60-1, fusA-3, gltA-10, pyrG-1, recA-4, rpIB-4 and rpoB-4), and was recommended to be designated by ST23 or CC10.
Whole Genome Sequencing of the ThreeA. baumannii Strains
Pair-end sequencing produced >9 million 75-bp nucleotide reads for each of the three strains. After de novo assembly and manual gap-closing by PCR and re-sequencing using Sanger sequencing method, the complete genomes of BJAB07104, BJAB0868 and BJAB0715 strains yield 4,022,090-bp, 3,976,962-bp, and 4,001,621-bp with a G+C content of 38.96%, 38.93 and 38.87% respectively (Fig. 1a, 1b and 1c). The characteristics of the three genomes are listed in Table 2. Using the NCBI Prokaryotic Genome Automatic Annotation Pipeline (PGAAP) and the genome of ACICU strain (CP000863.1) as a reference sequence, we predicted 3,869, 3,816, and 3,850 potential protein-coding genes from BJAB07104, BJAB0868, and BJAB0715 genomes respectively. Among them, 1,374 (35.51%), 1,325 (34.72%), and 1,360 (35.32%) genes in these three genomes respectively encode hypothetical proteins (Table 2). The number of genes is comparable with other previously sequenced A. baumannii strains [21]–[32].
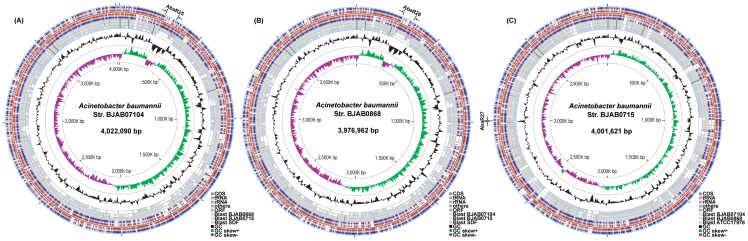
Figure 1. Circular representation of genomes of threeAcinetobacter baumannii strains.
(a) BJAB07104. (b) BJAB0868. (c) BJAB0715. Circles display (from outside in order of) (i) coding regions in the clockwise direction; (ii-iii) open reading frames (>100 codons) in the clockwise and counterclockwise direction respectively; (iv) coding regions in the counterclockwise direction; (v-vi) comparison with three selected genomes by BLAST (BJAB0868, BJAB0715 and SDF for BJAB07104, BJAB07104, BJAB0715 and SDF for BJAB0868, BJAB07104, BJAB0868 and ATCC17978 for BJAB0715); (vii) GC content; and (viii) G-C skew. The plot was produced by CGView server (http://stothard.afns.ualberta.ca/cgview_server/index.html) [75]. The locus of AbaR-like resistance islands were marked beside the circular genomes.
Table 2. General features ofA. baumannii BJAB07104, BJAB0868 and BJAB0715 genomes.
| Characteristic | BJAB07104 | BJAB0868 | BJAB0715 | pBJAB07104 | p1BJAB0868 | p2BJAB0868 | pBJAB0715 |
| GenBank Accession No. | |||||||
| Main genome size | 4022090 | 3976962 | 4001621 | 20139 | 8721 | 20139 | 52268 |
| No. of plasmid | 1 | 2 | 1 | / | / | ||
| Whole genome size | 4042229 | 4005822 | 4053889 | 20139 | 8721 | 20139 | 52268 |
| G+C content (%) | 38.96 | 38.93 | 38.87 | 47.07 | 34.34 | 47.09 | 40.43 |
| No. of genes | 3933 | 3881 | 3926 | 20 | 10 | 20 | 60 |
| No. of protein-encoding genes | 3869 | 3816 | 3850 | 20 | 10 | 20 | 60 |
| No. of predicted genes | 1374 | 1325 | 1360 | 8 | 7 | 7 | 34 |
| No. of tRNAs | 74 | 75 | 73 | / | / | ||
| No. of rRNAs | 18 | 18 | 18 | / | / | ||
| No. of insertion sequences (ISAba1) | 21 (17) | 19 (13) | 26 (14) | 3 (0) | 0 | 3 (0) | 4 (0) |
Sequencing analysis also identified four plasmids from these three BJAB strains, of which two are from BJAB0868, one from BJAB07104, and one from BJAB0715 (Table 2). The BJAB0715 strain harbors a 52,268-bp plasmid (pBJAB0715) with little similarity to any published plasmids in NCBI database. It contains 60 protein coding genes including four antibiotic resistance genes bla OXA-58, aac3′-1, aphA6 and cm1A1 (Fig. 2a). For the two plasmids in BJAB0868 strain, p1BJAB0868 is 8,721-bp containing 10 protein coding genes and almost identical with the published plasmid pAB0057 (99.8%) [22]; p2BJAB0868 is 20,139-bp and near-perfectly identical with the plasmid pBJAB07104 from BJAB07104 strain, of which both share high similarity with the published plasmid pZJ06 (92%) [27]. Each of p2BJAB0868 and pBJAB07104 carries 20 protein-coding genes including six drug resistance genes on class I integron and aphA1 transposon (Fig. 2b). Except the plasmid p1BJAB0868 which was estimated having 15 copies in a cell, each of the rest three plasmids only have one copy based on the average sequencing coverage depth of NGS data. The genome sequences of the three A. baumannii strains and the four plasmids have been deposited into GenBank with accession numbers CP003846 (BJAB07104), CP003887 (pBJAB07104), CP003849 (BJAB0868), CP003850 (p1BJAB0868), CP003888 (p2BJAB0868), CP003847 (BJAB0715), and CP003848 (pBJAB0715).
Figure 2. The structures of representative plasmids containing drug resistance genes.
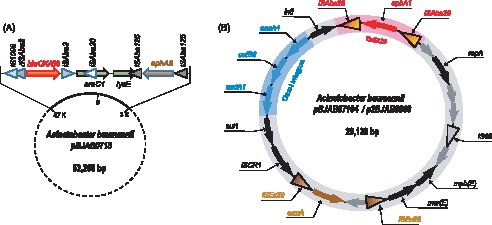
(a) pBJAB0715 contains bla OXA-58 flanked by two ISAba3 elements, and aphA6 flanked by two ISAba125 elements. (b) pBJAB07104 and p2BJAB0868 containing aphA1 in transposon Tn6210 (in red) and genes of class I integron (in blue).
Phylogenetic Analysis ofA. baumannii Genomes
Whole genome phylogenetic analysis was performed by using the conserved proteins among the three BJAB strains and ten other A. baumannii strains with complete genomes in GenBank. These include seven MDR strains (AYE, AB0057, ACICU, AB16562, ABTCDC0715, MDR-TJ, and MDR-ZJ06), two susceptible strains (ATCC17978 and AB307-0294) and a non-clinical strain SDF isolated from a human body louse. ADP1, a soil-living bacterium A. baylyi strain was used as outgroup for comparison. All clinical isolated A. baumannii strains contain a genetically highly homogeneous core genome which encodes proteins with functions involved in DNA replication, transcription, and translation, as well as many metabolic pathways. By using reciprocal best BLAST matches, we identified 1,119 conserved orthologous proteins among all 14 Acinetobacter isolates including A. baylyi ADP1 (Table S1). The number of conserved orthologous proteins increases to 1,331 among the 13 A. baumannii strains (exclude A. baylyi ADP1), and 3,115 among the three newly sequenced BJAB strains. The phylogenetic pattern within A. baumannii was investigated by neighbor-joining analysis of these 1,119 orthologous protein sequences with ADP1 as outgroup (Fig. 3a). Based on the phylogenetic data, the three strains (AYE, AB307-0294 and AB0057) which belong to global clone I (GC I) were grouped together. Two of the three BJAB strains (BJAB07104 and BJAB0868), along with 4 previously reported Asia strains, including MDR-ZJ06 (China), MDR-TJ (China), ABTCD0715 (Taiwan) and AB1656-2 (Korean), were grouped together with ACICU, a strain of global clone II (GC II) group. Interestingly, BJAB0715 is separated with all of the MDR strains (Fig. 3a), which may suggest BJAB0715 has a different origin comparing with other drug-resistant strains.
Figure 3. Sequence comparison of BJAB strains with otherA. baumannii strains.
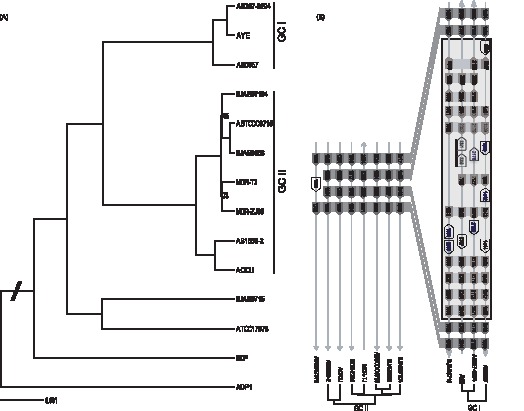
(a) Phylogenetic analysis of 13 A. baumannii strains with Acinetobacter baylyi ADP1 as outgroup. 1,119 orthologous proteins identified from 14 genomes were aligned by CLUSTALW and PHYLIP software package was used to construct the tree. 1000 replicates were used in bootstrap analysis. (b) An insert region in the genome of BJAB0715, was found in all three genomes of GC I strains but not in GC II strains.
Resistance Island (RI) Containingbla OXA-23 in Different A. baumannii Strains
Resistance islands (RIs) are large insertions containing a collection of horizontally transferred genes related to antibiotic inactivation and efflux. The RIs can be carried on bacterial chromosome or on plasmid, and antibiotic resistance genes are usually interspersed with mobile genetic elements such as IS and transposons [34]. bla OXA-23 containing RI was identified in the genome of all three BJAB strains. The bla OXA-23 gene is associated with carbapenems resistance and has been identified in clinical A. baumannii isolates around the world [11]–[20]. But the structure and genome location of bla OXA-23 containing RI is different among strains. In AB0057 and TCDC0715, the bla OXA-23 is carried by transposon Tn2006 (or a truncated form) in AbaR4 and inserted into the sulf gene region in AB0057 strain (Fig. 4a) [22], [30]. However, in the three BJAB strains, the bla OXA-23 resistance islands are different from AbaR4 by lack of uspA and sup genes but containing yeeB gene. These islands in three BJAB strains have the same structure as those in pABTJ1 and MDR-ZJ06 [27], [31], but the insertion positions are different. In MDR-ZJ06 and pABTJ1 (MDR-TJ), the bla OXA-23 is located in an 8,423-bp transposon (Tn2009) and inserted into either the pilus assembling gene cluster (Fig. 4b) on chromosome (MDR-ZJ06) or present on a plasmid (MDR-TJ). In the three BJAB strains, the bla OXA-23 are all located in an 8,426-bp transposon (designated as Tn6206) which has high sequence similarity (99.9%) with Tn2009 [27]. Tn6206 carries 8 protein coding genes including bla OXA-23, two copies of ATPase and DEAD/H, YeeB, YeeC and a few hypothetical proteins. There are two copies of the same-direction ISAba1 elements at both sides of the transposon and a 16-bp inverted repeat [5′-CTCTGTACACGA(T/C)AAA-3′] flanking the ISAba1 elements. In BJAB0715, the Tn6206 is inserted into the EGK48316 gene on chromosome and a 9-bp target site direct repeat sequence (5′-AAATATTTT-3′) was identified on both side of the insertion sequence (Fig. 4b and 4c). However, in BJAB07104 and BJAB0868, Tn6206 is inserted into the chromosome inside of tniB gene and interrupts it. Furthermore, we observed that a 78-kb insertion element containing Tn6206 and tra-locus in BJAB0868 and BJAB07104 strains could be either site-specifically integrated into chromosome, or excised as a circular plasmid which was confirmed by PCR amplification and Southern blot hybridization (Fig. S1–S2). Three types of plasmid could be formed from this 78-kb insertion sequence, the tra-locus alone, Tn6206 alone or tra-Tn6206 conjugation, indicating that the tra-locus and Tn6206 can transfer freely between chromosome and plasmid. When integrated into the chromosome, tra-locus and Tn6206 can be in two different orders (5′-Tn6206-tra-locus-3′, or 5′-tra-locus-Tn6206-3′), indicating that the plasmid containing tra-locus and Tn6206 is integrated into the chromosome by homologous recombination at different ISAba1 sites of this plasmid to form a 121-kb RI in BJAB07104 and BJAB0868. The 9-bp target site direct repeat sequences (DR) were found at both sides of the inserted sequences, but the DR sequences are different when this insertion is integrated in chromosome (ATTATTATT) or on plasmid (TAGATGTTC). Our data suggested that HGT mediated by plasmids is a key contributor for evolution of the clinical A. baumannii strains by vectoring ecologically important traits between strains and species. The transfer of the mobile genetic element between chromosome and plasmid may facilitate the rapid spreading of the resistant genes among A. baumannii strains [35].
Figure 4. Transposons containing drug resistance genebla OXA-23 in different A. baumannii strains.
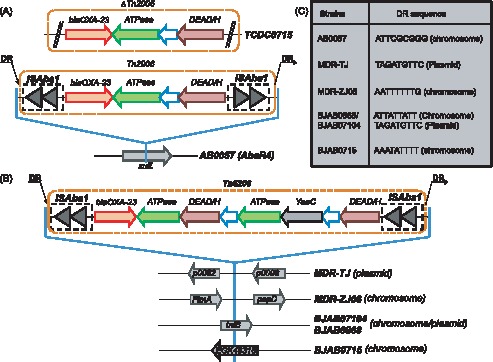
(a) Truncated Tn2006 in TCDC0715 and complete Tn2006 in AbaR4 reported in AB0057 strain; (b) Tn6206 found in three BJAB strains and Tn2009 in MDR-ZJ06 and MDR-TJ strains. (c) 9-bp target site direct repeat sequences (DR sequences) of ISAba1 elements (in bold font).
Novel AbaR-like Resistance Islands
The AbaR-like structure containing clusters of drug resistance genes has been reported in many drug-resistant A. baumannii strains [21]–[32], [36]. The largest AbaR-like RI in A. baumannii reported by far was the 86-kb AbaR1 in AYE, which harbored a cluster of 45 resistance-related genes [32]. In this study, we identified 3 novel AbaR-like resistance islands, designated as AbaR25, AbaR26 and AbaR27, from BJAB07104, BJAB0868 and BJAB0715, respectively (Fig. 5). The AbaR25 in BJAB07104 is about 121.7-kb containing 141 protein-coding genes including 7 antibiotic resistance genes (sul1, tetA(B), arsR, strB, strA, blaOXA-23 and sul2). AbaR25 is inserted into the ATPase (comM) gene position on chromosome with the identical 5-bp direct repeat (5′-accgc-3′) flanking both ends of the insertion sequence. The AbaR26 in BJAB0868 is almost identical to AbaR25 except that the 1,180-bp ISAba1 element on the right side of transposon Tn6208 is deleted in AbaR26 (Fig. 5a).
Figure 5. Structure of resistance islands AbaR25-27 containing drug resistant genetetA.
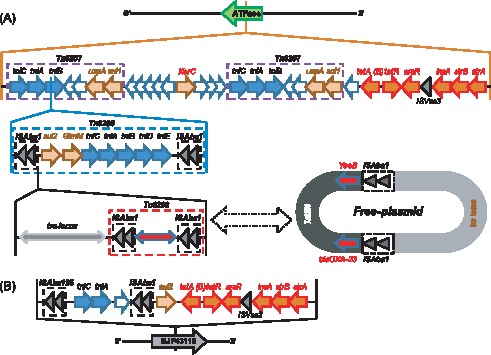
(a) AbaR25 in BJAB07104 and AbaR26 in BJAB0868 are both inserted into the ATPase gene. Transposons Tn6206-6209 are showed in dashed rectangles. While Tn6208 (in BJAB07104) is flanked by two ISAba1 elements, Tn6209 (in BJAB0868) only has the left flanking ISAba1 element. When being present in chromosome, Tn6206 and tra system are inserted into the gene tniB, while this region can also form free plasmids. (b) AbaR27 in BJAB0715 is inserted into gene EJP43116, which produces a protein 100% identical to a hypothetical protein found in A. baumannii OIFC032 strain.
Sequence analysis revealed that the backbone of the AbaR25/AbaR26 is a 34-kb insertion island which has similar structure as the AbaR22 in MDR-ZJ06 [27]. This 34-kb backbone consists of two copies of Tn6207, a tet island containing the tetracycline efflux pump and its regulator genes, tetA(B) and tetR, and a truncated Tn5393-like island containing aminoglycoside resistance genes strB and strA (Fig. 5a). An 87-kb fragment containing the tra-locus, Tn6206 and Tn6208 (Tn6209) are inserted into the tniB gene position in one of the Tn6207 locus in the backbone (Fig. 5a). This insertion sequence contains four or three ISAba1 elements flanking tra-locus, Tn6206, and Tn6208 (Tn6209) in AbaR25 (AbaR26), respectively.
AbaR27 in BJAB0715 is a truncated version of AbaR22 with the deletion of a big portion of the sequence between tniA and tetA(B), instead, an ISAba1 element and sul2 gene are inserted at the same location (Fig. 5b). Unlike the AbaR25/AbaR26 which are inserted inside of ATPase gene in BJAB0868 and BJAB07104, the 15.3-kb AbaR27 containing tetA(B), strA, strB, sul2 resistance genes is inserted inside of a hypothetical gene similar to EJP43116 in A. baumannii OIFC032 strain by using ISAba125 element in BJAB0715 (Fig. 5b). It should be noted that the novel AbaR27 is different from previously identified AbaR islands by lacking of uspA and sup genes, and not being inserted into the specific ATPase gene location. Also, no target site duplication was found in this resistance island in BJAB0715.
Genes Related with MDR in ThreeA. baumannii BJAB Strains
Antimicrobial susceptibility testing showed that the 3 BJAB strains are resistant to almost all commonly used antibiotics (Table 1). The genetic variations responsible for resistance to most of the antibiotics have been identified from all BJAB strains (Table 3 and Table S2). Among the 26 drug-resistance-related genes and mutations identified from BJAB genomes, 9 of them are shared by all 3 strains and 20 are common between BJAB07104 and BJAB0868. The common drug-resistance genes shared by all 3 strains include the strA and strB (resistance to streptomycin), tetA/B (resistance to tetracycline), blaADC and bla OXA-23 (resistance to carbapenems), as well as ade genes (adeABC, adeIJK, adeM) encoding for efflux pumps. A mutation (Ser83Leu) in gyrA gene which encodes for DNA gyrase and is responsible for resistance to fluoroquinolones was also identified in all 3 BJAB strains. BJAB07104 and BJAB0868 shared all drug resistant genes except blaTEM-1 (encoding beta-lactamase class A) which is unique in BJAB0868 and is flanked by two IS26 elements [37]. BJAB0715 harbors 14 drug-resistance genes, 9 of them are shared with the other two stains and 5 are unique to BJAB0715, including bla OXA58, aac3′-1, aphA6, cm1A1 and bla OXA-10. Interestingly, four of these unique genes in BJAB0715 are carried on its 52-kb plasmid (Fig. 2a and Table 3). Most of the drug-resistance genes are clustered on mobile genetic elements such as RI, transposons and plasmid, and therefore are transferable among different strains during the infection. As stated previously, AbaR25 and AbaR26 in BJAB07104 and BJAB0868 harbor 7 resistance genes and AbaR27 in BJAB0715 carries 5 resistance genes (sul2, tetA(B), arsR, strB, strA) (Fig. 5). A 20-kb plasmid identified from both BJAB0868 and BJAB07104 strains carried a group of resistance genes (aphA1, sul1, armA, msrE, mphE) and class I integron (aadA1, aacA4 and catB8) (Fig. 2b). This plasmid shares 92% sequence similarity with the plasmid pZJ06 which contained all the described drug resistance genes except catB8. A 52-kb plasmid in BJAB0715 also carries some unique drug-resistance genes such as in aphA6, bla OXA-58, aac3′-I and cm1A1 (Fig. 2a). In addition, the drug-resistance gene clusters are always accompanied by multiple insertion elements, including ISAba1, ISAba3, IS26, ISAba125. These insertion elements may mediate the integration of resistant islands into chromosome and therefore, facilitate the transfer of drug-resistance genes among strains. On the other hand, IS elements may also enhance drug-resistance activity by promoting drug resistance gene expression [7], [38].
Table 3. Genes associated with Antimicrobial resistance in BJAB07104, BJAB0868 and BJAB0715.
| Genes | Products | Drug-resistant function | Protein Locus Tag on BJAB genome | ||
| BJAB07104 | BJAB0868 | BJAB0715 | |||
| aac A4 | AAC (3)-I aminoglycoside acetyltransferase | Aminoglycoside-modifying enzymes | BJAB07104_p0002 | BJAB0868_p0013 | |
| aac 3′-I | Aminoglycoside N3′-acetyltransferase | Aminoglycoside-modifying enzymes | BJAB0715_p0027 | ||
| aph A1-Iab | Aminoglycoside phosphotransferase | Aminoglycoside-modifying enzymes | BJAB07104_p0020 | BJAB0868_p0011 | |
| aphA6 | Aminoglycoside phosphotransferase | Aminoglycoside-modifying enzymes | BJAB0715_p0002 | ||
| aad A1 | ANT (3″)-I aminoglycoside adenylyltransferase | Aminoglycoside-modifying enzymes | BJAB07104_p0004 | BJAB0868_p0015 | |
| adeT | RND (resistance-nodulation-division) family efflux pump | Efflux pumps | BJAB07104_01909 | BJAB0868_02074 | |
| adeIJK | RND (resistance-nodulation-division) family efflux pump | Efflux pumps | BJAB07104_03177-79 | BJAB0868_03059-61 | BJAB0715_03116-18 |
| adeABC | RND (resistance-nodulation-division) family efflux pump | Efflux pumps | BJAB07104_01911-15 | BJAB0868_02068-72 | BJAB0715_00260-64 |
| abeM | MATE (multidrug and toxic compound extrusion) family efflux pump | Efflux pumps | BJAB07104_00448 | BJAB0868_00548 | BJAB0715_00431 |
| arm A | 16S rRNA methylase | Aminoglycoside-modifying enzymes | BJAB07104_p0008 | BJAB0868_p0019 | |
| str A | Streptomycin resistance protein A | Aminoglycoside-modifying enzymes | BJAB07104_00282 | BJAB0868_00382 | BJAB0715_02883 |
| Str B | Streptomycin resistance protein B | Aminoglycoside-modifying enzymes | BJAB07104_00281 | BJAB0868_00381 | BJAB0715_02882 |
| tet A(B) | MFS (major facilitator superfamily) familyefflux pump | Ttetracycline resistance protein | BJAB07104_00277 | BJAB0868_00377 | BJAB0715_02878 |
| TEM-1 | Beta-lactamase class A | β-lactamases | BJAB0868_01360 | ||
| ADC | Beta-lactamase class C | β-lactamases | BJAB07104_02829 | BJAB0868_02710 | BJAB0715_02760 |
| blaOXA-23 | Beta-lactamase class D | β-lactamases | BJAB07104_02733 | BJAB0868_00355 | BJAB0715_03039 |
| blaOXA-10 | Beta-lactamase class D (OXA-51like) | β-lactamases | BJAB0715_01734, | ||
| blaOXA-66 | Beta-lactamase class D (OXA-51like) | β-lactamases | BJAB07104_02182 OXA-66 | BJAB0868_01795OXA-66 | |
| blaOXA-58 | Beta-lactamase class D | β-lactamases | BJAB0715_p00053 | ||
| cat B8 | Chloramphenicol acetyltransferase | Chloramphenicol resistance | BJAB07104_p0003 | BJAB0868_p0014 | |
| cm1A1 | Chloramphenicol resistance protein | Chloramphenicol resistance | BJAB0715_p00013 | ||
| mph (E) | macrolide 2′-phosphotransferase | Macrolide resistance | BJAB07104_p0012 | BJAB0868_p0023 | |
| msr E | macrolide efflux protein | Macrolide resistance | BJAB07104_p0011 | BJAB0868_p0022 | |
| sul 1 | Dihydropteroate synthase | Sulphonamides | BJAB07104_p0005 | BJAB0868_p0016 | |
| gyr A a | DNA gyrase subunit A | Fluoroquinolones | BJAB07104_03067, R | BJAB0868_02946, R | BJAB0715_02991, R |
| par C b | Topoisomerase IV subunit A | BJAB07104_00229, S | BJAB0868_00235, R | BJAB0715_00241, S | |
R: Ser-Leu mutation at 83, S: no mutation at 83; b: R: Ser-Leu mutation at 84, S: no mutation at 84.
An important group of drug-resistance genes identified from BJAB strains are the genes related to efflux pump function, including resistance-nodulation-cell division (RND) family, major facilitator superfamily (MFS) and multidrug and toxic efflux (MATE) family (Table S2). All three strains carry adeABC, adeIJK, and abeM genes which are important efflux pumps for multiple drug resistance in A. baumannii [39]–[41]. Sequence comparison revealed that these efflux genes (adeABC, adeIJK, and abeM) were conserved with almost 100% sequence similarity in all 13 A. baumannii strains with the exception of adeABC in BJAB0715 which showed 90% amino acid sequence similarity to that of A. lwoffii and inserted into ATPase (comM gene) position. The adeABC efflux pump belongs to a member of the resistance-nodulation-cell division family and can pump out multiple antibiotics and the overexpression of adeABC efflux pump may confer high-level resistance to carbapenems. A mechanism that controls the expression of this pump was elucidated as a two-step regulator (adeR) and sensor (adeS) system [39]. The adeABC efflux pump together with its regulatory proteins adeR and adeS are present in all BJAB strains, however, the mutations in adeR and adeS genes which were reported to be associated with MDR phenotype in other A. baumannii strains were not detected in the three BJAB strains.
Another efflux pump system identified from the BJAB strains is tetA(B) which drives the efflux of tetracycline (Fig. 5). The upstream of tetA(B) is the regulation gene tetR. The tetR-tetA(B) operon is located in the AbaR-like islands (AbaR25/AbaR26/AbaR27), same as that in other MDR A. baumannii strains (such as MDR-ZJ06).
gyrA and parC are intrinsic genes and point mutations in these genes confer resistance to fluoroquinolones [42], [43]. The Ser83Leu mutation in gyrA was detected in all three BJAB strains, but Ser84Leu mutation in parC was only detected in BJAB0868.
Genes Related to Pathogenesis in BJAB Strains
O-glycosylation plays an important role in bacterial pathogenesis such as adhesion, motility, DNA uptake, protein stability, immune evasion, and animal colonization and has been reported in A. baumannii ATCC 17978 and other clinical isolates [44]. In BJAB strains, the presence of a general O-glycosylation system including seven glycoproteins genes as well as other pathogenesis genes related with pilus formation, hemin utilization, iron metabolism, biofilm formation, capsule formation and some putative virulence factors were verified (Table S2). Besides, the genes of phospholipase D and penicillin-binding protein 7/8 which promote the proliferation of bacteria in blood and resistance to bactericidal activity [45], [46], and outer membrane protein ompA which induces cytotoxicity [47] were also identified in all three BJAB strains (Table S2). Most of the pathogenesis-related genes are the same as their orthologous genes in other A. baumannii isolates, with the exceptions of fimA which is silent in BJAB0715 due to a G to A mutation.
In addition, the virulence genes encoding type IV secretary pathway such as virB4 and virD4 are only present in BJAB0868 and BJAB07104 with high sequence similarity to the corresponding sequence from pABTJ1 [31], but not in BJAB0715 (Table S2). It is reported that VirB4 and VirD4 are required at an early stage of the bacterial infection and these T4SS-associated virulence genes could be important virulence factors [48], [49]. Our data further confirmed that the virB4/virD4 T4SS secretion system is prevalent in the epidemic A. baumannii clones in China. The type IV secretion system conjugation TrbI family proteins which were reported in AYE, ACICU and AB0057 were not found in the three BJAB strains. Furthermore, the CRISPR (clustered regularly interspaced short palindromic repeats) repeat elements, which were identified in the genomes of three GC I strains (AYE, AB0057 and AB307-0294) with a function to degrade exogenous DNA by Cas (CRISPR-associated) proteins [50], were not present in the BJAB strains by CRISPRFinder [51].
Furthermore, the pathogenesis islands (PIs) were predicted by PIPs software in three BJAB strains with length of 6 kb to 79 kb (Table S3). Six PIs were identified in BJAB07104, seven in BJAB0715, and four in BJAB0868. Most of the PIs are related to cell wall biogenesis, fatty acid or amino acid metabolism, drug resistance, and transport system.
Insertion Sequence (IS) in BJAB Strains
Genome analysis of published MDR strains had identified more than 10 IS elements, including ISAba1, ISAba125, ISAba2 and IS26, but very few IS elements were found in susceptible strains. Most of the reported IS elements were also found in the genome of BJAB strains. For example, there are 14 ISAba1 and 8 ISAba125 in BJAB0715, 13 ISAba1 and 4 IS26 in BJAB0868, and 17 ISAba1 and 2 IS26 in BJAB07104. These IS elements might mediate the insertion of genetic elements into certain positions in the genome and therefore play an important role for the transition of drug resistance genes among strains. Furthermore, it has been reported that ISAba1 has promoter activity and can enhance the gene expression when located at the upstream of a gene [52]. Indeed, the ISAba1 elements were identified in the upstream of blaOXA-23 and other RIs in all 3 BJAB strains, which could increase the expression of the downstream drug resistance genes. Besides, an ISAba1 element was found at the upstream of blaADC in both BJAB0868 and BJAB07104, which can enhance the resistance to cephalosporins by potentially upregulating the expression of blaADC in A. baumannii [53]. However, no IS element was identified at the upstream of blaADC in BJAB0715, which may explain why the resistant levels to cephalosporin [ceftazidime (CAZ) and cefotaxime (CTX)] are higher in BJAB0868 and BJAB07104 (both MICs>128 µg/ml) than that in BJAB0715 (MIC 16 and 64 µg/ml for CAZ and CTX respectively).
Genomic Variants in Three BJAB Strains
Although all three BJAB strains share high similarity in their genome, through comparative genomics analysis, we identified many genomic variants in three BJAB strains, with the scales from large structural genome re-arrangements to single nucleotide polymorphism (SNP).
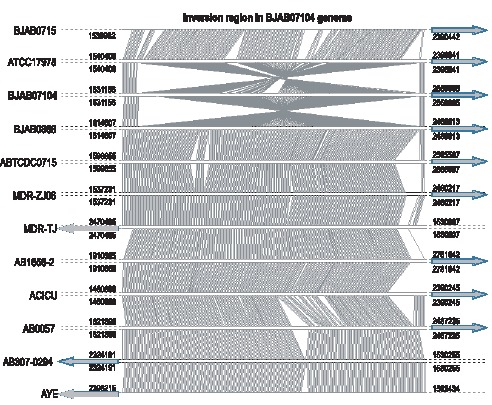
Genome comparison among 12 A. baumannii strains identified a large inverted fragment in the genome of BJAB07104 (Fig. 6) which also was verified by PCR amplification and Sanger sequencing (Fig. S3). This 800-kb inversion contains multiple transporter-related proteins. In the scope of our knowledge, this is the first report of large genomic inversion region in A. baumannii genome and it may represent an evolution event of clinical isolates.
Figure 6. The alignment of a 800-kb inversion region in BJAB07104 genome with the genomes of other 11A. baumannii strains.
By comparing genome of BJAB0715 with other whole-genome sequenced A. baumannii strains, we found a 10-Kb region in BJAB0715 which shares high similarity with genomic regions of three GC I strains: AB0057, AB307-0294, and AYE, but has no similarity with genomes of any GC II strains. 7 of 12 genes in this 10 kb BJAB0715 genomic region share very high protein sequence similarity in all three GC I strains (93%∼100%). For the rest 5 genes, 3 have high similarity with proteins in two of the three GC I strains, and the rest 2 genes are unique in BJAB0715 (Fig. 3b). This genome re-arrangement points out that genomic DNA transferring among different strains may not be limited by GC groups.
Genomic islands (GIs) are the most important element for acquiring foreign genes by horizontal gene transfer (HGT) [54]. We identified 16, 21 and 16 GIs by screening the genomes of BJAB07104, BJAB0715 and BJAB0868, respectively (Table S4). BJAB0868 and BJAB07104 share all of the common GIs except blaTEM-1 which is absent in BJAB07104. Most of GIs identified from these two strains are also present in most of the other reported MDR strains (Table S4), suggesting that most of the prevalent MDR strains (in GC I or GC II groups) are from the same epidemic lineage. However, BJAB0715 harbors not only more number of GIs on its genome (21 vs. 16), but also contains some unique GIs which are not present on the genomes of other drug-resistant A. baumannii strains (Table 4 and Table S4). The existing of multiple GIs in the BJAB genome explains the rapid spreading of drug-resistance under antimicrobial selection by HGT.
Table 4. Major Resistant Islands (RI) in threeA. baumannii strains and unique genomic islands (GI) in BJAB0715.
| GI | BJAB0715 | BJAB0868 | BJAB07104 | Length(bp) | G+C content | Function | |||
| Start | End | Start | End | Start | End | ||||
| GI-715-1 | 275447 | 294778 | / | / | / | / | 19332 | 35.47 | Acinetobacter lwoffii adeABC |
| GI-715-2 | 630027 | 657071 | / | / | / | / | 27045 | 35.29 | Phage related |
| GI-715-3 | 2115447 | 2135523 | / | / | / | / | 20077 | 30.67 | Acinetobacter johnsonii hypothetical proteins |
| GI-715-4 | 2561321 | 2588970 | / | / | / | / | 27650 | 39.28 | Unknown |
| GI-715-5 | 3272335 | 3294027 | / | / | / | / | 21693 | 34.87 | Acinetobacter junii and A. lwoffii hypothetical proteins |
| GI-715-6 | 3771627 | 3791134 | / | / | / | / | 19508 | 34.71 | Unknown |
| GI-715-7 | 1061467 | 1075377 | / | / | / | / | 13911 | 35.79 | OXA-23 island |
| GI-868-1 | / | / | 260642 | 381202 | / | / | 120561 | 35.68 | OXA-23 island, tra system, tetA(B) island |
| GI-7104-1 | / | / | / | / | 263697 | 385450 | 121754 | 35.68 | OXA-23 island, tra system, tetA(B) island |
Another important source of genome variation which contributes to drug resistance and pathogenesis in A. baumannii is single nucleotide polymorphism (SNP) [55], [56]. Table S5 listed the SNPs between ACICU and the three BJAB strains using SNPFinder. BJAB07104 and BJAB0868 contain much less SNPs (10,274 and 10,766, respectively) than BJAB0715 (52,439), further indicating that BJAB0715 is much more divergent from other MDR A. baumannii strains. The SNP analysis result is consistent with the phylogenetic analysis using core genomes as shown in Figure 3a.
Divergence of BJAB0715 with the Other MDR Strains
We found BJAB0715 strain shows clear divergence with other MDR strains in comparative genomics analysis. First, it is separated with other MDR strains in phylogentic analysis (Fig. 3a). Second, it is the only strain having both blaOXA-23 and blaOXA-58 genes. Third, BJAB0715 genome has six unique genomic islands (GI-715-1 through GI-715-6) which are not found in the genomes of other two BJAB strains (Table 4). These genomic islands (GIs) have varied sizes from 19-kb to 30-kb and they all have different G+C contents from the core genome of A. baumannii strains. Some of these genomic islands (GIs) shared the sequence similarity with GIs in other Acinetobacter species. For example, GI-715-1 contains an adeABC system which is similar to the one identified in A. lwoffii and inserted into a specific ATPase (comM gene) position which is usually an insertion hotspot for AbaR-like island in GC I and GC II clones. GI-715-3 and GI-715-5 harbor genes which have high similarity to those in A. johnsonii and A junii. GI-715-2 carries some phage-related genes. It is not clear how and why the BJAB0715 acquires various GIs from other Acinetobacter species. However, the divergence of BJAB0715 with other drug-resistant A. baumannii strains suggests that BJAB0715 is probably a newly emerged MDR strain in China.
In conclusion, in this study, we analyzed the genome of three drug resistant A. baumannii isolates from Beijing, China. The BJAB07104 and BJAB0868, isolated from blood and ascites samples, are genetically closest to ABTCDC0715 among whole genome sequenced A. baumannii strains. However, BJAB0715 is genetically more divergent to GC I and GC II strains. The identification of a 121-kb large resistance island containing transposons from several different origins and multiple drug resistance genes provided a new insight on the acquirement of drug resistance. Plasmid and insertion sequence plays an important role on HGT by direct insertion or integration into the chromosome. The evolution of A. baumannii clinical strains is mainly mediated by gene rearrangement such as inversion, deletion and transfer besides HGT.
Materials and Methods
Bacterial Isolates and Antimicrobial Susceptibility Testing (AST)
All clinical isolates of A. baumannii were from General Hospital of People’s Liberation Army in Beijing, China and characterized in the Clinical Microbiology Laboratory of the General Hospital of People’s Liberation Army by standard biochemical tests [20]. BJAB0715 strain was isolated from cerebrospinal fluid (CSF) sample of a patient with cerebrospinal rhinorrhea in March 2007. BJAB0868 was isolated from ascites sample of a patient with mesenteric venous thrombosis (MVT) and BJAB07104 was isolated from blood sample of a patient with liver cirrhosis in April 2008 and January 2007 respectively. All isolates were identified to the species level by the Vitek GNI system (bioMérieux, France). The MICs of several antibiotics were determined for three isolates by the agar dilution method with Müeller-Hinton agar with an inoculum of 104 CFU per spot [57]. The antibiotics include imipenem (IPM), meropenem (MEM), minocycline (MNO), ciprofloxacin (CIP), levofloxacin (LVX), polymyxin, piperacillin (PIP), piperacillin/tazobactam (TZP), caftazidime, cefotaxime (CTX), cefepime (FEP) and amikacin (AMK). All protocols associated with the collection and storage of these isolates from human subjects were approved by the Hospital Review Board of the General Hospital of People’s Liberation Army. Written consent was obtained from patients for their information to be stored in the hospital database and used for research.
Multilocus Sequence Typing (MLST)
MLST was performed based on the protocols as previously described [58]. PCR reactions were carried out in a Peltier PTC225 thermal cycler (MJ Research Inc., Watertown, MA). Sequencing reactions were performed with the ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit (PerkinElmer Applied Biosystems). Data analysis and sequence alignments were carried out with the MegAlign software (DNASTAR). Sequence allele typing was performed with the multiple locus query tool at the publicly available A. baumannii MLST database at the Pasteur Institute’s MLST website (www.pasteur.fr/recherche/genopole/PF8/mlst/Abaumannii.html).
Genome Sequencing and Assembling
Paired-end libraries (300–500 bp fragments) were constructed by using the Illumina ® TruSeq™ DNA Sample Preparation Kit (Illumina). Then each library was deposited onto a HiSeq Flow Cell and sequenced using an Illumina HiSeq-2000 next-generation DNA sequencer.
The Illumina short reads were assembled by VELVET to construct the contigs for each strain. Then the scaffolds and large contigs from each assembly were ordered and oriented by using the Mauve contig mover [59] and in-house script with the finished ACICU genome (GenBank accession number CP000863) as a reference. We also wrote scripts to identify un-assembled reads to fill the gaps in super-contigs and scaffolds. PCR amplification and Sanger sequencing are used to solve the ambiguity of the order and orientation of scaffolds (primer sequences and part of gel electrophoresis results were listed in Table S6 and Fig. S4–5).
Genome Annotation
The assembled genome sequence was annotated by the NCBI Prokaryotic Genome Automatic Annotation Pipeline (PGAAP) which uses Glimmer 3.0 for identification of protein-coding genes [60], tRNAscan-SE for tRNA genes [61], and RNAmmer for rRNA genes [62]. ISs were identified using the IS Finder database (www-is.biotoul.fr) [63]. The origin of replication (oriC) and putative DnaA boxes were identified by using Ori-Finder [64]. The regions with abnormal G+C contents in the genomic sequence were obtained by using the GC-Profile program [65] to identify the genomic islands and screened in the horizontal gene transfer database (HGT-DB) [66].
Comparative Genomics Analysis
Data used in comparative analysis were downloaded from the NCBI database (ftp://ftp.ncbi.nlm.nih.gov/GenBank/genomes/Bacteria/), including complete genome sequences and annotation of A. baumannii isolates MDR-ZJ06 (CP001937.1), MDR-TJ (CP003500.1), ABTCDC0715 (CP002522.1), AB1656-2 (CP001921.1), AB0057 (CP001182), AB307-0294 (CP001172), ATCC 17978 (CP000521), ACICU (CP000863), AYE (CU459141), SDF (CU468230), and ADP1 (CR543861.1). Multiple sequence alignments and comparison analysis of these genomes were performed with Mauve [67], [68] and ACT (http://www.sanger.ac.uk/Software/ACT) [69]. BLASTP was used to compare proteins from each pair of genomes to identify the best reciprocal matches with cutoff of >50% amino acid similarity and >80% coverage in length. PHYLIP package (ver. 3.69) was used to construct the phylogram of the 1,119 orthologous proteins with 1000 replicates in bootstrap. Mauve, IslandViewer and in-house-developed Perl scripts were used to identify the potential genomic islands [67], [70]. SNPFinder and Mauve were used to identify SNPs [67], [71]. PIPs [72] was used to predict the potential pathogenicity islands with SDF as a reference strain.
Southern Blot Analysis and Location ofbla OXA-23 Gene
To determine the location of the bla OXA-23 gene and tra-locus, chromosomal and plasmid DNA in two isolates of BJAB07104 and BJAB0868 were evaluated by Southern blot analysis. Genomic DNA was prepared with Wizard Genomic DNA Purification Kit (Promega, Madison, Wis.). Extraction of plasmid DNA was performed using the Kieser method as described previously [73]. Genomic and plasmid DNAs were digested using BamHI/BglII, separated by electrophoresis on 0.8% agarose gels, and transferred onto Hybond N+ membranes (Amersham International, Buckinghamshire, England) as described by Sambrook and Russell [74]. Labeling of probes (522-bp of bla OXA-23 amplicon generated with primers OXA-23-L and OXA-23-R, and 920-bp of virD4 amplicon generated with primers virD4-L and virD4-R) were performed with digoxigenin as described by the manufacturer (Roche Diagnostics GmbH, Mannheim, Germany). Southern hybridization was performed at 68°C with the buffers recommended in the instructions included in the digoxigenin kit from Roche.
Nucleotide Sequence Accession Numbers
The complete genome sequences of Acinetobacter baumannii strains BJAB07104, BJAB0715 and BJAB0868 and plasmids pBJAB07104, p1BJAB0868, p2BJAB0868, pBJAB0715 reported in this paper have been deposited in the GenBank (http://www.ncbi.nlm.nih.gov/genbank/) under accession numbers CP003846, CP003849, CP003847, CP003887, CP003850, CP003888, and CP003848 respectively. In addition, the sequences of AbaR25 and AbaR26 have been deposited in the GenBank under accession numbers CP003907 and CP003908 respectively.
Supporting Information
Gel electrophoresis of the sequencing assembly of Tn6206 and tra-locus in chromosome and free plasmids verified by PCR amplification in BJAB07104 (a) and in BJAB0868 (b). All the expected PCR products were sequenced by DNA Sanger sequencing.
(PPTX)
Identification of the localization of Tn6206 and tra-locus in chromosomal DNA and plasmid DNA by Southern blot. (a) Hybridization of the BamHI/BglII-fragments with a bla OXA-23 probe. The chromosome-integrated fragment (Tn6206->tra) produced one band (11337 bp for BJAB07104, and 11336 bp for BJAB0868); and the chromosome-integrated fragment (tra->Tn6206) produced one band (7943 bp for BJAB07104 and BJAB0868); the free plasmid produced one band (7943 bp for a plasmid containing tra+Tn6206, and 7245 bp for a plasmid containing only Tn6206). (b) Hybridization of the BamHI/BglII-fragments with a virD4 probe. Both chromosome-integrated fragments (Tn6206->tra, tra->Tn6206) and the free plasmids (containing Tn6206+tra, or containing only tra) produced a 1418-bp fragment.
(PPTX)
Gel electrophoresis of the large inversion verified by PCR amplification in BJAB07104. All the expected PCR products were confirmed by Sanger sequencing.
(PPTX)
Gel electrophoresis of gap-closing PCR in BJAB07104. All the expected PCR products were confirmed by Sanger sequencing.
(PPTX)
Gel electrophoresis of gap-closing PCR in BJAB0715. All the expected PCR products were confirmed by Sanger sequencing.
(PPTX)
List of 1119 conserved genes among all 14 Acinetobacter baumannii strains.
(XLSX)
The genes associated with resistance and pathogenesis in three BJ strains.
(XLSX)
The predicted pathogenicity islands in three BJAB strains.
(XLSX)
The genomic islands and their functions in three BJAB strains.
(XLSX)
SNPs analysis in three A. baumannii strains.
(XLSX)
Primer sequences for gap-closing PCR.
(XLSX)
Acknowledgments
We would like to acknowledge Nancy Liu and Yun Lian in University of Texas Southwestern Medical Center Microarray Core Facility for assistance in next-generation sequencing and data collection. We would also like to thank Dr. Lawrence Reitzer, Dr. Juan Gonzalez, and Dr. Stephen Spiro for their advice and suggestions in plasmid DNA extraction.
Funding Statement
This work was supported by Capital Medical Development Research Fund, Beijing, China (No. 2009-1023), UT Dallas start-up fund for ZYX and National Institutes of Health grants P50 CORT AR055503, R03 AR055778. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kwon KT, Oh WS, Song JH, Chang HH, Jung SI (2007) Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother 59: 525–530. [DOI] [PubMed] [Google Scholar]
- 2. Sunenshine RH, Wright MO, Maragakis LL (2007) Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 13: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lockhart SR, Abramson MA, Beekmann SE (2007) Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol 45: 3352–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21: 538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falagas ME, Karveli EA (2007) The changing global epidemiology of Acinetobacter baumannii infections: a development with major public health implications. Clin Microbiol Infect 13: 117–119. [DOI] [PubMed] [Google Scholar]
- 6. Domingues S, Harms K, Fricke WF (2012) Natural transformation facilitates transfer of transposons, integrons and gene cassettes between bacterial species. PLoS Pathog 8: e1002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poirel L, Nordmann P (2006) Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12: 826–836. [DOI] [PubMed] [Google Scholar]
- 8. Bogaerts P, Naas T, Wybo I (2006) Outbreak of infection by carbapenem-resistant Acinetobacter baumannii producing the carbapenemase OXA-58 in Belgium. J Clin Microbiol 44: 4189–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez-Otsoa F, Gallego L, Towner KJ (2002) Endemic carbapenem resistance associated with OXA-40 carbapenemase among Acinetobacter baumannii isolates from a hospital in northern Spain. J Clin Microbiol 40: 4741–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bou G, Oliver A, Martinez-Beltran J (2000) OXA-24, a novel class D beta-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob Agents Chemother 44: 1556–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scaife W, Young HK, Paton RH (1995) Transferable imipenem-resistance in Acinetobacter species from a clinical source. J Antimicrob Chemother 36: 585–586. [DOI] [PubMed] [Google Scholar]
- 12. Stoeva T, Higgins PG, Bojkova K (2008) Clonal spread of carbapenem-resistant OXA-23-positive Acinetobacter baumannii in a Bulgarian university hospital. Clin Microbiol Infect 14: 723–727. [DOI] [PubMed] [Google Scholar]
- 13. Zhou H, Pi BR, Yang Q (2007) Dissemination of imipenem-resistant Acinetobacter baumannii strains carrying the ISAba1 bla OXA-23 genes in a Chinese hospital. J Med Microbiol 56: 1076–1080. [DOI] [PubMed] [Google Scholar]
- 14. Carvalho KR, Carvalho-Assef AP, Peirano G (2009) Dissemination of multidrug-resistant Acinetobacter baumannii genotypes carrying bla OXA-23 collected from hospitals in Rio de Janeiro, Brazil. Int J Antimicrob Agents 34: 25–28. [DOI] [PubMed] [Google Scholar]
- 15. Calhoun JH, Murray CK, Manring MM (2008) Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res 466: 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naas T, Levy M, Hirschauer C (2005) Outbreak of carbapenem-resistant Acinetobacter baumannii producing the carbapenemase OXA-23 in a tertiary care hospital of Papeete, French Polynesia. J Clin Microbiol 43: 4826–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mugnier PD, Poirel L, Naas T (2010) Worldwide dissemination of the bla OXA-23 carbapenemase gene of Acinetobacter baumannii . Emerg Infect Dis 16: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu Y, Zhou J, Zhou H (2010) Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemother 65: 644–650. [DOI] [PubMed] [Google Scholar]
- 19. Lin MF, Kuo HY, Yeh HW (2011) Emergence and dissemination of bla OXA-23-carrying imipenem-resistant Acinetobacter sp in a regional hospital in Taiwan. J Microbiol Immunol Infect 44: 39–44. [DOI] [PubMed] [Google Scholar]
- 20. Yan ZQ, Shen DX, Cao JR (2010) Susceptibility patterns and molecular epidemiology of multidrug-resistant Acinetobacter baumannii strains from three military hospitals in China. Int J Antimicrob Agents 35: 269–273. [DOI] [PubMed] [Google Scholar]
- 21. Adams MD, Chan ER, Molyneaux ND (2010) Genomewide analysis of divergence of antibiotic resistance determinants in closely related isolates of Acinetobacter baumannii . Antimicrob Agents Chemother 54: 3569–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adams MD, Goglin K, Molyneaux N (2008) Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii . J Bacteriol 190: 8053–8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahl JW, Johnson JK, Harris AD (2011) Genomic comparison of multi-drug resistant invasive and colonizing Acinetobacter baumannii isolated from diverse human body sites reveals genomic plasticity. BMC Genomics 12: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao F, Wang Y, Liu YJ (2011) Genome sequence of Acinetobacter baumannii MDR-TJ. J Bacteriol 193: 2365–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vallenet D, Nordmann P, Barbe V (2008) Comparative analysis of Acinetobacters: three genomes for three lifestyles. PLoS One 3: e1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith MG, Gianoulis TA, Pukatzki S (2007) New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev 21: 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou H, Zhang T, Yu D (2011) Genomic analysis of the multidrug-resistant Acinetobacter baumannii strain MDR-ZJ06 widely spread in China. Antimicrob Agents Chemother 55: 4506–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iacono M, Villa L, Fortini D (2008) Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob Agents Chemother 52: 2616–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park JY, Kim S, Kim SM (2011) Complete genome sequence of multidrug-resistant Acinetobacter baumannii strain 1656-2, which forms sturdy biofilm. J Bacteriol 193: 6393–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen CC, Lin YC, Sheng WH (2011) Genome sequence of a dominant, multidrug-resistant Acinetobacter baumannii strain, TCDC-AB0715. J Bacteriol 193: 2361–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang H, Yang ZL, Wu XM (2012) Complete genome sequence of Acinetobacter baumannii MDR-TJ and insights into its mechanism of antibiotic resistance. J Antimicrob Chemother 67: 2825–2832. [DOI] [PubMed] [Google Scholar]
- 32. Fournier PE, Vallenet D, Barbe V (2006) Comparative genomics of multidrug resistance in Acinetobacter baumannii . PLoS Genet 2: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maiden MC (2006) Multilocus sequence typing of bacteria. Annu Rev Microbiol 60: 561–588. [DOI] [PubMed] [Google Scholar]
- 34. Martinez JL, Baquero F (2002) Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance. Clin Microbiol Rev 15: 647–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harrison E, Brockhurst MA (2012) Plasmid-mediated horizontal gene transfer is a coevolutionary process. Trends Microbiol 20: 262–267. [DOI] [PubMed] [Google Scholar]
- 36. Hamidian M, Hall RM (2011) AbaR4 replaces AbaR3 in a carbapenem-resistant Acinetobacter baumannii isolate belonging to global clone 1 from an Australian hospital. J Antimicrob Chemother 66: 2484–2491. [DOI] [PubMed] [Google Scholar]
- 37. Chen CH, Young TG, Huang CC (2006) Predictive biomarkers for drug-resistant Acinetobacter baumannii isolates with bla TEM-1, AmpC-type bla and integrase 1 genotypes. J Microbiol Immunol Infect 39: 372–379. [PubMed] [Google Scholar]
- 38. Poirel L, Nordmann P (2006) Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene bla OXA-58 in Acinetobacter baumannii . Antimicrob Agents Chemother 50: 1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marchand I, Damier-Piolle L, Courvalin P (2004) Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother 48: 3298–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hou PF, Chen XY, Yan GF (2012) Study of the correlation of imipenem resistance with efflux pumps AdeABC, AdeIJK, AdeDE and AbeM in clinical isolates of Acinetobacter baumannii . Chemotherapy 58: 152–158. [DOI] [PubMed] [Google Scholar]
- 41. Coyne S, Courvalin P, Perichon B (2011) Efflux-mediated antibiotic resistance in Acinetobacter spp . Antimicrob Agents Chemother 55: 947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vila J, Ruiz J, Goni P (1995) Mutation in the gyrA gene of quinolone-resistant clinical isolates of Acinetobacter baumannii . Antimicrob Agents Chemother 39: 1201–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vila J, Ruiz J, Goni P (1997) Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii . J Antimicrob Chemother 39: 757–762. [DOI] [PubMed] [Google Scholar]
- 44. Iwashkiw JA, Seper A, Weber BS (2012) Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog 8: e1002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Russo TA, MacDonald U, Beanan JM (2009) Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J Infect Dis 199: 513–521. [DOI] [PubMed] [Google Scholar]
- 46. Jacobs AC, Hood I, Boyd KL (2010) Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect Immun 78: 1952–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choi CH, Hyun SH, Lee JY (2008) Acinetobacter baumannii outer membrane protein A targets the nucleus and induces cytotoxicity. Cell Microbiol 10: 309–319. [DOI] [PubMed] [Google Scholar]
- 48. Schulein R, Dehio C (2002) The VirB/VirD4 type IV secretion system of Bartonella is essential for establishing intraerythrocytic infection. Mol Microbiol 46: 1053–1067. [DOI] [PubMed] [Google Scholar]
- 49. Atmakuri K, Cascales E, Christie PJ (2004) Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol Microbiol 54: 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Horvath P, Barrangou R (2010) CRISPR/Cas, the immune system of bacteria and archaea. Science 327: 167–170. [DOI] [PubMed] [Google Scholar]
- 51. Grissa I, Vergnaud G, Pourcel C (2007) CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res 35: W52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heritier C, Poirel L, Nordmann P (2006) Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii . Clin Microbiol Infect 12: 123–130. [DOI] [PubMed] [Google Scholar]
- 53. Lopes BS, Amyes SG (2012) Role of ISAba1 and ISAba125 in governing the expression of bla ADC in clinically relevant Acinetobacter baumannii strains resistant to cephalosporins. J Med Microbiol 61: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 54. Dobrindt U, Hochhut B, Hentschel U (2004) Genomic islands in pathogenic and environmental microorganisms. Nat Rev Microbiol 2: 414–424. [DOI] [PubMed] [Google Scholar]
- 55. Lewis T, Loman NJ, Bingle L (2010) High-throughput whole-genome sequencing to dissect the epidemiology of Acinetobacter baumannii isolates from a hospital outbreak. J Hosp Infect 75: 37–41. [DOI] [PubMed] [Google Scholar]
- 56. Zarrilli R, Pournaras S, Giannouli M (2013) Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 41: 11–19. [DOI] [PubMed] [Google Scholar]
- 57.National Committee for Clinical Laboratory Standards (2003) Performance standards for antimicrobial disk susceptibility tests; approved standard. NCCLS, Wayne, PA.
- 58. Bartual SG, Seifert H, Hippler C (2005) Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 43: 4382–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rissman AI, Mau B, Biehl BS (2009) Reordering contigs of draft genomes using the Mauve aligner. Bioinformatics 25: 2071–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Delcher AL, Bratke KA, Powers EC (2007) Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23: 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25: 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lagesen K, Hallin P, Rodland EA (2007) RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 35: 3100–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siguier P, Perochon J, Lestrade L (2006) ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34: D32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gao F, Zhang CT (2008) Ori-Finder: a web-based system for finding oriCs in unannotated bacterial genomes. BMC Bioinformatics 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gao F, Zhang CT (2006) GC-Profile: a web-based tool for visualizing and analyzing the variation of GC content in genomic sequences. Nucleic Acids Res 34: W686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Garcia-Vallve S, Guzman E, Montero MA (2003) HGT-DB: a database of putative horizontally transferred genes in prokaryotic complete genomes. Nucleic Acids Res 31: 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Darling AC, Mau B, Blattner FR (2004) Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14: 1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Darling AE, Mau B, Perna NT (2010) progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5: e11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carver TJ, Rutherford KM, Berriman M (2005) ACT: the Artemis Comparison Tool. Bioinformatics 21: 3422–3423. [DOI] [PubMed] [Google Scholar]
- 70. Langille MG, Brinkman FS (2009) IslandViewer: an integrated interface for computational identification and visualization of genomic islands. Bioinformatics 25: 664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Song J, Xu Y, White S (2005) SNPsFinder–a web-based application for genome-wide discovery of single nucleotide polymorphisms in microbial genomes. Bioinformatics 21: 2083–2084. [DOI] [PubMed] [Google Scholar]
- 72. Soares SC, Abreu VA, Ramos RT (2012) PIPS: pathogenicity island prediction software. PLoS One 7: e30848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kieser T (1984) Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid (12) 19–36. [DOI] [PubMed] [Google Scholar]
- 74.Sambrook J, Russell DW (2001) Extraction and purification of plasmid and screening of bacterial colonies by hybridization, p. 1.40–1.92 Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 75. Grant JR, Stothard P (2008) The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res 36: w181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gel electrophoresis of the sequencing assembly of Tn6206 and tra-locus in chromosome and free plasmids verified by PCR amplification in BJAB07104 (a) and in BJAB0868 (b). All the expected PCR products were sequenced by DNA Sanger sequencing.
(PPTX)
Identification of the localization of Tn6206 and tra-locus in chromosomal DNA and plasmid DNA by Southern blot. (a) Hybridization of the BamHI/BglII-fragments with a bla OXA-23 probe. The chromosome-integrated fragment (Tn6206->tra) produced one band (11337 bp for BJAB07104, and 11336 bp for BJAB0868); and the chromosome-integrated fragment (tra->Tn6206) produced one band (7943 bp for BJAB07104 and BJAB0868); the free plasmid produced one band (7943 bp for a plasmid containing tra+Tn6206, and 7245 bp for a plasmid containing only Tn6206). (b) Hybridization of the BamHI/BglII-fragments with a virD4 probe. Both chromosome-integrated fragments (Tn6206->tra, tra->Tn6206) and the free plasmids (containing Tn6206+tra, or containing only tra) produced a 1418-bp fragment.
(PPTX)
Gel electrophoresis of the large inversion verified by PCR amplification in BJAB07104. All the expected PCR products were confirmed by Sanger sequencing.
(PPTX)
Gel electrophoresis of gap-closing PCR in BJAB07104. All the expected PCR products were confirmed by Sanger sequencing.
(PPTX)
Gel electrophoresis of gap-closing PCR in BJAB0715. All the expected PCR products were confirmed by Sanger sequencing.
(PPTX)
List of 1119 conserved genes among all 14 Acinetobacter baumannii strains.
(XLSX)
The genes associated with resistance and pathogenesis in three BJ strains.
(XLSX)
The predicted pathogenicity islands in three BJAB strains.
(XLSX)
The genomic islands and their functions in three BJAB strains.
(XLSX)
SNPs analysis in three A. baumannii strains.
(XLSX)
Primer sequences for gap-closing PCR.
(XLSX)