Abstract
Despite advances in early detection, prostate cancer remains the second highest cancer mortality in American men, and even successful interventions are associated with enormous health care costs as well as prolonged deleterious effects on quality of patient life. Prostate cancer chemoprevention is one potential avenue to alleviate these burdens. It is a regime whereby long-term treatments are intended to prevent or arrest cancer development, in contrast to more direct intervention upon disease diagnosis. Based on this intention, cancer chemoprevention generally focuses on the use of nontoxic chemical agents which are well-tolerated for prolonged usage that is necessary to address prostate cancer’s multistage and lengthy period of progression. One such nontoxic natural agent is the flavonoid silibinin, derived from the milk thistle plant (Silybum marianum), which has ancient medicinal usage and potent antioxidant activity. Based on these properties, silibinin has been investigated in a host of cancer models where it exhibits broad-spectrum efficacy against cancer progression both in vitro and in vivo without noticeable toxicity. Specifically in prostate cancer models, silibinin has shown the ability to modulate cell signaling, proliferation, apoptosis, epithelial to mesenchymal transition, invasion, metastasis, and angiogenesis, which taken together provides strong support for silibinin as a candidate prostate cancer chemopreventive agent.
Key words: cell cycle, chemoprevention, prostate cancer, signal transduction, silibinin
PROSTATE CANCER CHEMOPREVENTION
Cancer chemoprevention is a treatment regime centered on the use of chemical agents to reduce cancer risk. These agents may be derived either synthetically or from natural products and are intended for long-term use, generally limiting candidate agents to nontoxic compounds. The rationale for this modality is based on the multistage and often lengthy period of time required to accumulate the cellular damage necessary for carcinogenesis (i.e., dysfunctional proliferation, differentiation, apoptosis, etc.). This then provides an opportunity to inhibit or eliminate initiated cells or localized lesions prior to their development into a fully malignant tumor. This general concept of cancer chemoprevention can be subdivided into primary, secondary, and tertiary cancer chemoprevention depending on the stage of carcinogenesis that is being targeted. Primary chemoprevention focuses on the removal of the initiating cellular dysfunction/s to decrease or eliminate cancer incidence prior to cancer formation. This is an ideal clinical outcome as it has the greatest impact on reducing treatment costs, adverse effects to the patient, and ultimately, mortality due to cancer. Barring this outcome and premalignant lesions already formed, secondary chemoprevention seeks their arrest or elimination, thus slowing or reversing progression of these lesions into malignant ones. Finally, if the previous interventions have not or cannot be enacted and a primary tumor has formed, tertiary chemoprevention seeks to inhibit the progression of tumor into a metastatic cancer as well as to prevent the recurrence of this tumor if it has been treated. In short, a cancer chemoprevention strategy could be applicable to almost all stages of carcinogenesis including post-therapy.
Prostate cancer is well suited for a cancer chemoprevention scheme for several reasons. One reason is the number of people afflicted by this disease. It is the most common cancer diagnosed in men in the United States, and there were approximately 241,740 new prostate cancer cases in 2012 alone (1). As a result, prostate cancer is also the second leading cause of mortality with an estimated 28,170 deaths in 2012 (1). Prostate cancer, when detected in advanced and metastatic stage, results in high mortality with almost three fourths of those diagnosed dying within 5 years; however, advances in early detection have resulted in an increase in the treatment of prostate cancer as early and localized disease (1). But the counted success of early detection serves as a double-edged sword as it also comes with a concomitant increase in aggressive intervention where less aggressive treatment might have better served the patient. This is the second factor making prostate cancer a candidate for chemoprevention, situations where adverse side-effects develop in patients as a consequence of treating prostate cancer especially in cases where perhaps they were not necessary. There are a host of treatment options, and each to varying degrees carries the risk of adverse effects. Among these, the principle risks are urinary incontinence, bowel issues, and impotence. In one study, normalized by only reporting patients with normal function prior to treatment, upwards of 58% of patients reported minor to major urinary dysfunction and 27% reported bowel problems 3 years following radical prostatectomy (2). In addition, a whopping 94% reported some sexual dysfunction of which two thirds of these were reported as severe issues. In another study following the effects of radical prostatectomy 2 years following treatment, almost 49% of patients reported urinary issues ranging from complete lack of control to frequent or occasional leakage (3). Similarly, 60% of patients reported severe sexual dysfunction (3). In patients treated with radiation, it was found that urinary issues were only reported in 17% of cases but bowel issues increased in number to 66% of patients and sexual dysfunction remained high at 74% reporting mild or major issues 3 years following treatment (2). Finally, the enormous cost of treating prostate cancer highlights the benefits of the chemoprevention approach. In the USA, an estimated 11.85 billion dollars were spent in 2010 on direct health care costs alone (4). This cost is predicted to grow as a combination of increasing cancer incidence, improved early diagnosis, increasing life expectancy, and higher priced treatments gaining acceptance. In fact, this last factor is estimated to have increased prostate cancer health care costs by more than 350 million dollars from just 2002 to 2005 (5). As this sum only reflects the direct health care costs, it does not include lost worker productivity due to infirmity or death. In addition, the stress of diagnosis (whether it is accurate or a false positive) and the fear of disease recurrence place a heavy burden on the mental well-being of the patient. For these reasons, chemoprevention whereby prostate cancer might be prevented from developing or at least from progressing to a symptomatic level would be ideal to aid in lowering these costs while improving the quality of life of a large number of potential sufferers.
As there is such a clear benefit in reducing the burden of prostate cancer, both societal as well as individual, several classes of agents have been brought forth as potential chemopreventive agents. One agent of interest is finasteride, a synthetic type II 5-α-reductase (5αR) inhibitor used in the treatment of male pattern baldness. The mechanism of action of 5αR is to inhibit androgen receptor (AR) induced signaling by inhibiting the conversion of testosterone into dihydrotestosterone, a higher affinity ligand of AR. As androgen-AR signaling is an important factor in the development and progression of prostate cancer, this activity has the potential to reduce or reverse the development of the disease. In a large-scale study, finasteride was shown to reduce incidence of prostate cancer, but consistent with the long-term response of prostate cancers to other AR-ablating compounds, the tumors that did arise despite finasteride treatment more frequently had high Gleason scores, which is associated with high mortality (6,7). Other potential chemopreventive compounds have been derived from natural products often identified based on their historic usage and more specifically food products based partially on ease of clinical translation. Green tea is one such product, its active ingredient believed to be a mixture of catechins (polyphenols with antioxidant properties), most commonly derived from the plant Camellia sinensis (8,9). Green tea has been associated with decreased overall risk of cancer and a high intake was found to be associated with a lower incidence of prostate cancer in men (9,10). Oral administration of green tea catechins reduced PSA levels (9). Another natural product, soy, contains a mixture of isoflavones exerting antioxidant properties. Soy consumption has been associated with a decreased risk of prostate cancer (11), which might be a result of reported inhibition of signaling pathways including AR, Akt, NF-κB, mitogen-activated protein kinases (MAPKs), and Notch signaling (12,13). The tomato (Solanum lycopersicum) contains a compound called lycopene which is a carotenoid with strong antioxidant property. Elevated lycopene consumption is associated with low prostate cancer risk (10,14). Another fruit, pomegranate (Punica granatum) contains a mixture of polyphenolic compounds that act as antioxidants which have been shown to delay prostate cancer growth in patients diagnosed with prostate cancer (15). A specific polyphenolic antioxidant agent that has been extensively studied for its chemopreventive properties is silibinin.
SILIBININ
Silibinin (Fig. 1a) is derived from the seeds (Fig. 1b) of milk thistle (Silybum marianum; Asteraceae) which has its origins in the Mediterranean region where for millennia it has been used as a remedy for a variety of ailments, particularly of the liver, gall bladder, and kidneys. More recently, milk thistle has been found to be effective in treating hepatic injury due to bile duct inflammation, cirrhosis, fatty liver, mushroom poisoning, and viral hepatitis (16). Perhaps as a consequence of this longstanding medicinal use, the characteristic purple-red flowers of the milk thistle (Fig. 1c) can now be found growing worldwide. In modern times, the usage of the whole milk thistle has been supplemented with a standardized extract of milk thistle seeds called silymarin. This extract is composed of a complex mixture of several flavonolignans and other compounds. The flavonoid silibinin is the principle active ingredient found in silymarin and is by far the most abundant component, along with the stereoisomers dihydrosilybin, isosilybin, silychristin, and silydianin. Silibinin, in turn, is composed of an approximately equimolar mixture of two diastereomers (silybin A and silybin B). As a polyphenolic compound, silibinin is fairly water insoluble and thus is often administered within capsules. Once in the GI tract, silibinin is absorbed, circulated, conjugated in the liver, and excreted, much of it in the bile (17). In mice, plasma concentrations of free silibinin peak at 30 min and in tissues at 60 min, whereupon it decays with a half-life of 57 to 127 min; however, the concentrations of conjugated silibinin peak at 1 h and decay with a half-life of 45 to 94 min (18). Silibinin exhibits low toxicity as reported in studies where animals were intravenously injected with silymarin. A 50% lethal dose (LD50) required high concentrations of silymarin, depending on specific experimental conditions: mice tolerate 400–1,050 mg/kg, rats 385–920 mg/kg, and rabbits and dogs 140–300 mg/kg (19–21). When silymarin was delivered orally, the required values of silymarin to achieve toxicity were, in some cases, over 10 g/kg (19–21). In two human trials, a commercial silibinin formulation, silibinin phytosome, was administered orally to prostate cancer patients at 13 g daily for a mean of 20 days and 2.5–20 g/daily for 28 days, respectively (22,23). Consistent with silibinin’s target organ in clinical usage, the most common adverse event at high doses was asymptomatic hepatotoxicity followed by low-grade hyperbilirubinemia (grades 1–2) and diarrhea (22,23). There was one case of a grade 4 postoperative thromboembolic event (out of 19 total treated patients within the two trials). Together these studies provide compelling evidence for well-tolerated administration of high doses of silibinin in human patients.
Fig. 1.
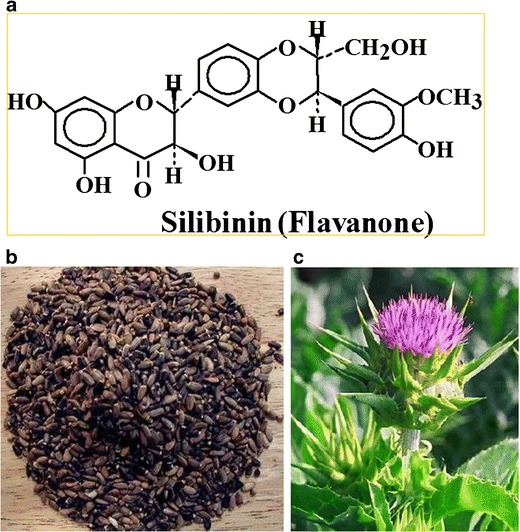
a Chemical structure of silibinin, b milk thistle seeds, and c flower of the S. marianum; Asteraceae
BROAD SPECTRUM CANCER CHEMOPREVENTIVE EFFICACY OF SILIBININ
Flavonoids possess antioxidant activity which has been reported to result in diverse biologically protective properties such as inhibiting inflammation, neoplasia, hepatic injury, and other ailments (24). Consistent with other flavonoids, silibinin has been found to be a very potent antioxidant, buttressing native cellular antioxidant mechanisms such as glutathione (GSH) and superoxide dismutase by scavenging free radicals, and reactive oxygen species (ROS) (25,26). This may in part explain silibinin’s effectiveness in addressing hepatic injury whether as a result of disease or exposure to toxins as this anti-oxidant activity may eliminate the oxidative stress associated with hepatic insults preventing the induction of lipid peroxidation (and thus cell death). As a consequence of the general anti-cancer properties associated with flavonoids collectively, as well as the strong antioxidant potential of silibinin specifically, there has been significant interest in adapting silibinin for use as a chemopreventive agent. In fact, silibinin has been widely investigated for anti-cancer efficacy in a broad range of cancers models.
Based on these factors, and the diseases silibinin has been historically used to treat, it is perhaps not a surprise that silibinin has been found to have an inhibitory effect on cancers of several digestive and excretory organs. Silibinin was found to inhibit cell proliferation and invasion in various hepatocarcinoma cell lines (27–30) as well as in a mouse xenograft model (31). These effects appear to be a consequence of inducing apoptosis as well as cell cycle arrest in hepatocarcinoma cells. Silibinin inhibited ERK1/2 signaling, downregulated survivin, highly expressed in cancer protein-1, the E2F1/DP1 complex, cyclin D1, cyclin D3, cyclin E, cyclin-dependent kinase (CDK)2, and CDK4 levels and induced CDK inhibitor (CDKI) Kip1/p27 in hepatocarcinoma HEPG2 cells (27,28). Furthermore, silibinin altered Akt signaling, downregulated phosphorylation of retinoblastoma (Rb), upregulated histone acetylation, and activated caspases 3 and 9 in hepatoma HuH7 cells (30). In addition, silibinin reduced vascular endothelial growth factor (VEGF) secretion, metalloproteinase-2 (MMP-2), and CD34 in hepatic cancer cells suggesting inhibition of angiogenesis in hepatic tumors (29,30). In pancreatic cancer cell lines, silibinin inhibited their proliferation in a dose- and time-dependent fashion which translated to a decrease in tumor volume in a mouse xenograft model (32). Again, this inhibition appeared to be a function of both increased apoptosis as well as cell cycle arrest by silibinin. In gastric cancer cells, silibinin dose-dependently inhibited TNF-α-induced secretion of metalloproteinase-9 (MMP-9) (33). Silibinin was found to be deliverable to the human colorectal mucosa in high amounts through ingestion of nontoxic doses of silibinin (34), and consistent for use as a chemopreventive agent, silibinin was found to be beneficial in early colon tumorigenesis (35), reducing loss of differentiation of carcinomas in mice (36), while also inhibiting colon cancer stem-like cells (37). Silibinin potently inhibited the growth of HT-29 and LoVo cells both in vitro as well as in xenograft models, strongly inducing G1 and more modestly G2-M cell cycle arrest (38,39). This was associated with decreased levels of cyclins (A, B1, D1, D3, and E), cell division cycle 25C (cdc25C), and Cdc2/p34; decreased activity of cyclin-dependent kinases (1,2,4,6); and phosphorylated Rb in conjunction with increased levels of CDKIs (Cip1/21 and Kip1/p27) (38,39). Silibinin also induced apoptosis associated with increased activation of caspases 3 and 9 as well as poly(ADP-ribose) polymerase (PARP) in LoVo cells (38); however, silibinin-induced apoptosis was independent of caspases activation in HT-29 cells (39). Furthermore, the invasive potential of LoVo cells was reduced by silibinin which was associated with a decrease in MMP-2 (40). Silibinin treatment also led to a decrease in polyp size and number in APCmin/+ mice, a model of familial adenomatous polyposis (41,42). This phenomenon was associated with decreased β-catenin, c-Myc, phospho-glycogen synthase kinase-3β, and phospho-Akt (41,42). Silibinin-mediated reduction in colorectal carcinoma proliferation and concomitant increase in apoptosis were associated with inhibition of ERK1/2 and Akt (43). Silibinin-mediated angiogenesis inhibition was associated with decreased VEGF, cyclooxygenase (COX), hypoxia-inducible factor-1α (HIF-1α), inducible nitric oxide synthase (iNOS), nitrotyrosine and nitrite levels, and an increased VEGFR-1 (Flt-1) expression (41,43–45). Silibinin inhibited CDK8 and β-catenin signaling which inhibited SW480 tumor growth (46) and initiated an autophagic-mediated survival response in SW480 and SW620 cells (47). Silibinin also suppressed 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis in rat models via modulating xenobiotic metabolizing enzymes and increasing enzymatic antioxidants to detoxify carcinogens (48,49). This action translated to decreased oxidative stress and subsequent lipid peroxidation, abrogating DMH-induced neoplasia (50).
Consistent with its effect in models of digestive organ cancers, silibinin was also found to inhibit excretory organ cancers. Silibinin treatment decreased renal cancer 786-O cell proliferation and invasiveness (51), while inhibiting proliferation and increasing apoptosis in renal cancer Caki-1 cells (52). This action was associated with inhibition of epidermal growth factor (EGF), ERK1/2, and survivin expression with concomitant upregulation of p53 expression and caspase cleavage (52). Silibinin feeding reduced the size of 786-O renal tumors in mice xenografts which was associated with decreased expression of MMP-2, MMP-9, and urokinase-type plasminogen activator (u-PA), and activation of p38 and ERK1/2 (51). Silibinin also enhanced the sensitivity of −786-O renal cell carcinoma cells towards 5-fluorouracil and paclitaxel (51). Treatment of SN12K1 cells with silibinin reduced cell viability and DNA synthesis resulting in apoptosis (53). Likewise, silibinin-fed SCID mice injected with SN12K1 cells exhibited a reduction in tumor size (54). Consistent with these results, several studies have shown that silibinin inhibits growth as well as induces apoptosis in several urinary bladder cancer cell lines which were associated with an increase in p53 expression, downregulation of survivin, cyclin D1, ERK1/2 phosphorylation and nuclear phospho-p65, cleavage of caspases, PARP, and Cip1/p21, and mitochondrial release of cytochrome c, Omi/HtrA2, and apoptosis-inducing factor (55–60). This silibinin-mediated inhibition was also observed in rat models of urinary bladder cancer reducing lesions (60).
Silibinin was also found to reduce oral cancer cell invasion as a consequence of decreased MMP-2 and u-Pa expression, decreased ERK1/2 activation, and increased tissue inhibitor of metalloproteinase-2 (TIMP-2), and plasminogen activator inhibitor-1 (PAI-1) expression (61). Likewise, in laryngeal squamous cell carcinoma SNU-46 cells, silibinin induced apoptosis (62). Furthermore, silibinin inhibited proliferation, invasion, and angiogenesis in lung carcinoma while simultaneously inducing apoptosis (63–65). Proliferation of Anip973 cells was inhibited by silibinin (66), which in non-small cell lung cancer cell lines corresponded to inhibition of CDK2, CDK4, and Rb phosphorylation, as well as induction of apoptosis by activation of the caspase cascade pathway (63,67). Similar to oral cancer, silibinin treatment concentration- and time-dependently decreased MMP-2 and u-Pa expression through inhibition of either ERK1/2 or Akt phosphorylation along with increasing TIMP-2 expression which together translated to an inhibition of invasiveness in the aggressive human lung adenocarcinoma A549 cells (68,69). Silibinin was reported to decrease expression of COX-2, iNOS, MMP-2, and MMP-9 and inhibit activation of ERK1/2, NF-κB, STAT-1, and STAT-3 in mouse lung epithelial LM2 cells (70). Reduction of iNOS elicited by silibinin treatment was also found in A549 cells (71). In the A/J mouse model of lung cancer, silibinin treatment reduced the number, growth, progression, and angiogenesis of induced tumors which was associated with downregulated VEGF, COX-2, iNOS, HIF-1α, STAT-3, and NF-κB, and increased Ang-2 and Tie-2 (64,65). Furthermore, silibinin enhanced sensitivity of A549 cells to doxorubicin through reduction of NFκ-B-mediated chemoresistance (72). In glioblastoma models, silibinin was shown to inhibit growth and invasiveness and induce apoptosis (73,74). Silibinin was also reported to inhibit EGFR activation in a rat glioma cell line stably expressing human EGFR (75). NF-κB-mediated stimulation of MMP-9 in glioblastoma U87 cells was found to be abrogated by silibinin treatment which served to attenuate invasiveness (74). Silibinin was found to induce caspase-mediated apoptosis by activating MAPKs as well as reverting sensitivity to TRAIL signaling in otherwise resistant glioma cells by modulating components of the death receptor-mediated apoptotic pathway (73,76). Interestingly, in the glioblastoma cell line, U87MG, silibinin appeared to partially synergize with arsenic trioxide treatments to increase apoptosis while inhibiting cell proliferation, metabolism, and mRNA expression of several proteinases (77) suggesting the possibility of combinatorial treatments to arrest cancer.
Several studies have also revealed that silibinin offers protection from photo-carcinogenesis in skin cancer models. A key mechanism by which silibinin mitigates UVA- and UVB-induced dysfunction is activation of the DNAPK-p53 pathway, inhibiting DNA synthesis, cellular proliferation, and apoptosis and inducing cell cycle arrest and repair in response to UV-induced DNA damage which together serves to inhibit tumor appearance and growth (78–81). This response is in part mediated by inhibition of ERK1/2, with concomitant increase of p53 and p21/Cip1 (82,83). Furthermore, silibinin was found to abrogate ATP and GSH depletion, ROS production, and lipid peroxidation in UVA-irradiated human keratinocytes, corresponding to inhibition of UVB-induced PARP and caspase 9 cleavage (84,85). These effects operated in conjunction with inhibition of inflammatory mediators such as COX-2, STAT-3, and NF-κB and angiogenic mediators such as HIF-1α and iNOS (86). In MG-63 cells, silibinin treatment reduced osteosarcoma invasiveness which was associated with inhibition of focal adhesion kinase, ERK1/2 activation, and uPA and MMP-2 expression (87). Similarly, in HT1080 cells, silibinin treatment activated p38 and JNK pathways and inhibited ERK and Akt pathways resulting in autophagy (88).
In breast cancer models, silibinin induced apoptosis in MCF-7 cells which synergized with inhibition of insulin growth factor receptor (IGFR) (89,90) and also inhibited metastasis of MDA-MB-231 cells (91). In addition, silibinin dose-dependently decreased expression of EGFR ligand-induced CD44, 12-O-tetradecanoylphorbol-13-acetate-induced MMP-9 and VEGF, as well as activation of ERK1/2 (92–94). Interestingly, silibinin induced reactive nitrogen species and ROS generation in MCF-7 cells (95). These phenomena translated to induction of tumor growth arrest and apoptosis in silibinin-treated HER-2/neu transgenic mice (96). In accordance with these findings, silibinin increased apoptosis and induced G2-M cell cycle arrest of A2780/taxol cells, enhancing their sensitivity to paclitaxel, which was associated with the downregulation of survivin and P-glycoproteins (97). In turn, mice xenografts with A2780 cells exhibited a reduction in angiogenic activity in response to silipide (silibinin phytosomes) treatment as a consequence of downregulation of VEGF receptor 3 and upregulation of Ang-2 (98). Together, the abovementioned studies clearly demonstrated the broad spectrum chemopreventive and anticancer efficacy of silibinin. Next, we have focused on silibinin efficacy and mechanism of its action against prostate cancer cells.
MOLECULAR MECHANISMS FOR SILIBININ CHEMOPREVENTIVE EFFICACY AGAINST PROSTATE CANCER
Silibinin has been shown to potently inhibit prostate cancer through targeting multiple cell signaling pathways, decreasing proliferation, inducing apoptosis, and inhibiting invasion, metastasis, and angiogenesis. The specific molecular targets of silibinin that induce broad-spectrum efficacy against prostate cancer are summarized in Fig. 2.
Fig. 2.
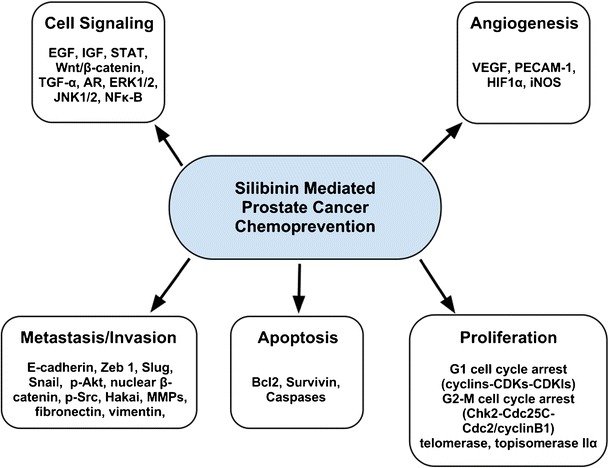
Schematic representation of the molecular mechanisms for silibinin-mediated prostate cancer chemoprevention
SILIBININ EFFECTS ON CELL SIGNALING IN PROSTATE CANCER CELLS
Silibinin has been shown to disrupt several signaling pathways known to be important in the development and progression of prostate cancer. Treatment of prostate cancer cells with silibinin abrogated constitutive activation of STAT-3 in DU145 cells (99), disrupted EGFR signaling in LNCaP and DU145 cells (100,101), targeted IGFR signaling in PC3 cells (102), the Wnt/β-catenin pathway in PC3 and DU145 cells (103), and AR signaling in LNCaP cells both directly by reducing nuclear localization of the receptor (104) and indirectly through downregulation of a co-activator, prostate epithelium-derived Ets transcription factor (105,106). Disruption of EGF signaling by silibinin in prostate cancer cells was associated with a decrease in secreted transforming growth factor-α and modulation of MAPK activity of both ERK1/2 and JNK1/2 (101). Disruption of the Wnt/β-catenin pathway involved modulation of a co-receptor, the low-density lipoprotein receptor-related protein-6 (LRP6) (103). Silibinin inhibited the promoter activity, mRNA, basal expression, as well as phosphorylation of LRP6 (103). Silibinin also dose-dependently induced mRNA for insulin-like growth factor-binding protein-3 (IGFBP-3) which translated into higher concentrations of IGFBP-3 in PC3 conditioned medium (102). In accordance with this finding, silibinin feeding of mice was found to upregulate both circulating plasma and tumor levels of IGFBP-3 and a decreased loss of differentiation in their tumors (36,107,108). Finally, silibinin was found in several studies to broadly alter NF-κB signaling (109,110). It inhibited the constitutive activation of NF-κB found in prostate carcinoma DU145 cells, decreasing IKKα kinase activity, the resultant ratio of phospho-IκBα to IκBα, and ultimately, the translocation of p50 and p65 NF-κB subunits to the nucleus (109).
SILIBININ INHIBITS PROLIFERATION OF PROSTATE CANCER CELLS
Multiple studies have shown that silibinin inhibits prostate cancer cell proliferation (111–114). In addition, mice fed with silibinin exhibited decreased tumor growth both in xenograft as well as transgenic models of prostate cancer (107,108,115–117). These phenomena were in part due to potent cell cycle arrest induced by silibinin in prostate cancer cells (111). Silibinin mediated G1 arrest in prostate cancer cells by modulating a plethora of elements in the cyclins–CDKs–CDKIs pathway: decreasing protein levels of cyclin D1, cyclin D3, cyclin E, CDK4, CDK6, and CDK2, and kinase activity of CDK2 and CDK4, increasing CDKIs Kip1/p27 and Cip1/p21, and sequestering cyclin D1 and CDK2 in the cytoplasm (108,111,113,118,119). In addition, silibinin induced a marked increase in Rb levels, principally in the hypophosphorylated retinoblastoma Rb/p107 and Rb2/p130, as well as a marked decrease in levels of the transcription factors, E2F3, E2F4, and E2F5 which altogether serves to inhibit cell cycle progression (113,118). Furthermore, silibinin mediated G2-M arrest by modulating the Chk2–Cdc25C–Cdc2/cyclin B1 pathway and decreasing levels of cyclin A, cyclin B1, both total and phosphorylated Cdc2, Cdc25B, and Cdc25C phosphatases, and inhibiting Cdc2 kinase activity (111,120,121). The inhibition of Cdc25C phosphatases combined with increased checkpoint kinase-2 phosphorylation resulted in the translocation of nuclear Cdc25C to the cytoplasm as a result of increased phosphorylation (111). This was accompanied by an increased binding with 14–3-3β (111). In addition, silibinin has been reported to inhibit both telomerase as well as DNA topoisomerase IIα activity in LNCaP and DU145 cells, respectively (105,122). Interestingly, both mitoxantrone and doxorubicin were found to synergize with silibinin in inhibiting prostate cancer cell proliferation (121,123), and cisplatin and carboplatin were found to synergize with silibinin in inducing G2-M arrest corresponding to potent downregulation of Cdc2, cyclin B1, and Cdc25c (124). Together, these findings suggest the potential for combinatorial treatments to arrest prostate cancer progression.
SILIBININ INDUCES APOPTOSIS IN PROSTATE CANCER CELLS
Studies have shown that silibinin also initiates apoptosis in prostate cancer cells under certain treatment conditions (99,107,108,124,125). The mechanism appeared to be a consequence of decreased Bcl-2 and survivin levels, caspase activation (caspases 3, 9, and 7), subsequent cytochrome c release from mitochondria, and ultimately apoptosis (99,107,108,124). Interestingly, mitoxantrone, doxorubicin, cisplatin, and carboplatin were each found to synergize with silibinin in inducing apoptosis in prostate cancer cells (121,123,124).
SILIBININ INHIBITS INVASION AND METASTASIS OF PROSTATE CANCER CELLS
Multiple studies have revealed that silibinin initiates a shift of treated advanced prostate cancer cells back into an epithelial phenotype and inhibits metastasis (110,116,117,126). It was reported that in PC3, PC3MM2, and C4-2B cells, silibinin upregulated E-cadherin on their cell surface, significantly inhibiting their migratory and invasive potential (126). This phenomenon appeared to be a result of downregulation of epithelial to mesenchymal transition (EMT) regulatory molecules Slug, Snail, phospho-Akt (ser473), nuclear β-catenin, phospho-Src (tyr419), and Hakai (126). This silibinin-induced increase in E-cadherin was also found in a transgenic adenocarcinoma of the mouse prostate (TRAMP) model in which silibinin decreased levels of MMPs, Snail, fibronectin, and vimentin translating into a reduction in cancer metastasis (116,117). Other studies found ARCaPM cells treated with silibinin exhibited decreased expression of major EMT regulators, the transcription factors ZEB1 and Slug, corresponding with decreased expression of EMT markers, vimentin and MMP-2, together translating into dose- and time-dependent reduction of invasion, motility, and migration (110,127). Along with MMP-2, silibinin has been found to inhibit MMP-9 expression in human prostate carcinoma cell lines (116,117).
SILIBININ EXHIBITS STRONG ANTI-ANGIOGENIC EFFICACY AGAINST PROSTATE CANCER CELLS
Targeting angiogenesis is considered an important element in preventing the growth and progression of solid tumors including prostate cancer. Silibinin was reported to inhibit angiogenesis, decreasing VEGF expression levels and tumor microvessel density in prostate tumors (107,108,116). This anti-angiogenic potential was supported in a study of TRAMP mice where silibinin feeding resulted in decreasing expression of platelet endothelial cell adhesion molecule-1 (PECAM1)/CD-31, VEGF, VEGFR2, HIF-1α, and iNOS (117). This expression pattern corresponded to an increase in glucose and citrate use along with a concomitant decrease in lactate, cholesterol, and phosphatidylcholine levels in prostatic tumors of silibinin-fed TRAMP mice (128). Silibinin treatment of LNCaP and PC3 prostate cancer cells also inhibited their synthesis of HIF-1α both basally as well as induced by hypoxia (129).
CONCLUSIONS
Silibinin, a flavonoid antioxidant derived from the milk thistle has been used for millennia to treat a diverse set of ailments. In more recent times, as a product of this long-term historical usage and aforementioned antioxidant chemistry along with protective properties identified in several other flavonoids, silibinin has been investigated in a host of cancer models. In these studies, silibinin has been found to possess multifactorial anti-cancer efficacy, operating on a broad array of signaling and regulatory mechanisms in diverse milieus. Specifically in regards to prostate cancer, silibinin has been shown to alter cell proliferation, apoptosis, EMT, invasion, metastasis, and angiogenesis. These effects of silibinin have the potential to impact prostate cancer progression encompassing the full range of clinical disease presentation from initial cellular dysfunctions in incipient lesions to advanced metastatic tumors. However, further investigations to confirm the mechanisms of silibinin effect on the prostate cancer microenvironment, as well as to elucidate its efficacious delivery and clinical usage are still needed. Taken together, the evidence provides strong support for the promise of silibinin as a candidate prostate cancer chemopreventive agent.
Acknowledgment
This work was supported by NCI RO1 grant CA102514.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol. 2009;27(24):3916–22. doi: 10.1200/JCO.2008.18.6486. [DOI] [PubMed] [Google Scholar]
- 3.Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283(3):354–60. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 4.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States. J Natl Cancer Inst. 2010–2020;103:117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen PL, Gu X, Lipsitz SR, Choueiri TK, Choi WW, Lei Y, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29(12):1517–24 [DOI] [PMC free article] [PubMed]
- 6.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. New Engl J Med. 2003;349(3):215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 7.Koochekpour S. Androgen receptor signaling and mutations in prostate cancer. Asian J Androl. 2010;12(5):639–57 [DOI] [PMC free article] [PubMed]
- 8.Fujiki H. Green tea: health benefits as cancer preventive for humans. Chem Rec. 2005;5(3):119–32. doi: 10.1002/tcr.20039. [DOI] [PubMed] [Google Scholar]
- 9.Khan N, Adhami VM, Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutr Cancer. 2009;61(6):836–41. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jian L, Lee AH, Binns CW. Tea and lycopene protect against prostate cancer. Asia Pac J Clin Nutr. 2007;16(Suppl 1):453–7. [PubMed] [Google Scholar]
- 11.Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. Int J Cancer. 2005;117(4):667–9. doi: 10.1002/ijc.21266. [DOI] [PubMed] [Google Scholar]
- 12.Hsu A, Bray TM, Ho E. Anti-inflammatory activity of soy and tea in prostate cancer prevention. Exp Biol Med (Maywood). 2010;235(6):659–67 [DOI] [PMC free article] [PubMed]
- 13.Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem. 2008;283(41):27707–16. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87(23):1767–76. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 15.Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12(13):4018–26. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 16.Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124(5):491–504. [PubMed] [Google Scholar]
- 17.Wu JW, Lin LC, Hung SC, Lin CH, Chi CW, Tsai TH. Hepatobiliary excretion of silibinin in normal and liver cirrhotic rats. Drug Metab Dispos. 2008;36(3):589–96. doi: 10.1124/dmd.107.017004. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Agarwal R. Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention. Carcinogenesis. 1999;20(11):2101–8. doi: 10.1093/carcin/20.11.2101. [DOI] [PubMed] [Google Scholar]
- 19.Desplaces A, Choppin J, Vogel G, Trost W. The effects of silymarin on experimental phalloidine poisoning. Arzneimittelforschung. 1975;25(1):89–96. [PubMed] [Google Scholar]
- 20.Vogel G, Trost W, Braatz R, Odenthal KP, Brusewitz G, Antweiler H, et al. Pharmacodynamics, site and mechanism of action of silymarin, the antihepatoxic principle from Silybum mar. (L) Gaertn. 1. Acute toxicology or tolerance, general and specific (liver-) pharmacology. Arzneimittelforschung. 1975;25(1):82–9. [PubMed] [Google Scholar]
- 21.Lecomte J. Pharmacologic properties of silybin and silymarin. Rev Med Liege. 1975;30(4):110–4. [PubMed] [Google Scholar]
- 22.Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25(2):139–46. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 23.Flaig TW, Glode M, Gustafson D, van Bokhoven A, Tao Y, Wilson S, et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate. 2010;70(8):848–55 [DOI] [PubMed]
- 24.Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65(4):337–53. doi: 10.1016/S0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 25.Ligeret H, Brault A, Vallerand D, Haddad Y, Haddad PS. Antioxidant and mitochondrial protective effects of silibinin in cold preservation–warm reperfusion liver injury. J Ethnopharmacol. 2008;115(3):507–14. doi: 10.1016/j.jep.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 26.de Groot H, Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam Clin Pharmacol. 1998;12(3):249–55. doi: 10.1111/j.1472-8206.1998.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 27.Momeny M, Khorramizadeh MR, Ghaffari SH, Yousefi M, Yekaninejad MS, Esmaeili R, et al. Effects of silibinin on cell growth and invasive properties of a human hepatocellular carcinoma cell line, HepG-2, through inhibition of extracellular signal-regulated kinase 1/2 phosphorylation. Eur J Pharmacol. 2008;591(1–3):13–20. doi: 10.1016/j.ejphar.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Varghese L, Agarwal C, Tyagi A, Singh RP, Agarwal R. Silibinin efficacy against human hepatocellular carcinoma. Clin Cancer Res. 2005;11(23):8441–8. doi: 10.1158/1078-0432.CCR-05-1646. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Maceira P, Mateo J. Silibinin inhibits hypoxia-inducible factor-1alpha and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: implications for anticancer therapy. Oncogene. 2009;28(3):313–24. doi: 10.1038/onc.2008.398. [DOI] [PubMed] [Google Scholar]
- 30.Lah JJ, Cui W, Hu KQ. Effects and mechanisms of silibinin on human hepatoma cell lines. World J Gastroenterol. 2007;13(40):5299–305. doi: 10.3748/wjg.v13.i40.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui W, Gu F, Hu KQ. Effects and mechanisms of silibinin on human hepatocellular carcinoma xenografts in nude mice. World J Gastroenterol. 2009;15(16):1943–50. doi: 10.3748/wjg.15.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nambiar D, Prajapati V, Agarwal R, Singh RP. In vitro and in vivo anticancer efficacy of silibinin against human pancreatic cancer BxPC-3 and PANC-1 cells. Cancer Lett. 2013;(in press). [DOI] [PubMed]
- 33.Kim S, Choi MG, Lee HS, Lee SK, Kim SH, Kim WW, et al. Silibinin suppresses TNF-alpha-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway. Molecules. 2009;14(11):4300–11. doi: 10.3390/molecules14114300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoh C, Boocock D, Marczylo T, Singh R, Berry DP, Dennison AR, et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin Cancer Res. 2006;12(9):2944–50. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]
- 35.Velmurugan B, Singh RP, Tyagi A, Agarwal R. Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male Fisher 344 rats. Cancer Prev Res (Phila) 2008;1(5):376–84. doi: 10.1158/1940-6207.CAPR-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verschoyle RD, Greaves P, Patel K, Marsden DA, Brown K, Steward WP, et al. Evaluation of the cancer chemopreventive efficacy of silibinin in genetic mouse models of prostate and intestinal carcinogenesis: Relationship with silibinin levels. Eur J Cancer. 2008;44(6):898–906. doi: 10.1016/j.ejca.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Wang JY, Chang CC, Chiang CC, Chen WM, Hung SC. Silibinin suppresses the maintenance of colorectal cancer stem-like cells by inhibiting PP2A/AKT/mTOR pathways. J Cell Biochem. 2012;113(5):1733–43 [DOI] [PubMed]
- 38.Kaur M, Velmurugan B, Tyagi A, Deep G, Katiyar S, Agarwal C, et al. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol Cancer Ther. 2009;8(8):2366–74. doi: 10.1158/1535-7163.MCT-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22(51):8271–82. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 40.Lin CM, Chen YH, Ma HP, Wang BW, Chiu JH, Chua SK, et al. Silibinin inhibits the invasion of IL-6-stimulated colon cancer cells via selective JNK/AP-1/MMP-2 modulation in vitro. J Agric Food Chem. 2012;60(51):12451–7 [DOI] [PubMed]
- 41.Rajamanickam S, Kaur M, Velmurugan B, Singh RP, Agarwal R. Silibinin suppresses spontaneous tumorigenesis in APCmin/+ mouse model by modulating beta-catenin pathway. Pharm Res. 2009;26(12):2558–67. doi: 10.1007/s11095-009-9968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R. Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin. Cancer Res. 2010;70(6):2368–78 [DOI] [PMC free article] [PubMed]
- 43.Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68(6):2043–50. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- 44.Yang SH, Lin JK, Huang CJ, Chen WS, Li SY, Chiu JH. Silibinin inhibits angiogenesis via Flt-1, but not KDR, receptor up-regulation. J Surg Res. 2005;128(1):140–6. doi: 10.1016/j.jss.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 45.Yang SH, Lin JK, Chen WS, Chiu JH. Anti-angiogenic effect of silymarin on colon cancer LoVo cell line. J Surg Res. 2003;113(1):133–8. doi: 10.1016/S0022-4804(03)00229-4. [DOI] [PubMed] [Google Scholar]
- 46.Kaur M, Velmurugan B, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling. Neoplasia. 2010;12(5):415–24 [DOI] [PMC free article] [PubMed]
- 47.Kauntz H, Bousserouel S, Gosse F, Raul F. Silibinin triggers apoptotic signaling pathways and autophagic survival response in human colon adenocarcinoma cells and their derived metastatic cells. Apoptosis. 2011;16(10):1042–53 [DOI] [PubMed]
- 48.Sangeetha N, Viswanathan P, Balasubramanian T, Nalini N. Colon cancer chemopreventive efficacy of silibinin through perturbation of xenobiotic metabolizing enzymes in experimental rats. Eur J Pharmacol. 2011;674(2,3):430–8. [DOI] [PubMed]
- 49.Sangeetha N, Felix AJ, Nalini N. Silibinin modulates biotransforming microbial enzymes and prevents 1,2-dimethylhydrazine-induced preneoplastic changes in experimental colon cancer. Eur J Cancer Prev. 2009;18(5):385–94. doi: 10.1097/CEJ.0b013e32832d1b4f. [DOI] [PubMed] [Google Scholar]
- 50.Sangeetha N, Aranganathan S, Nalini N. Silibinin ameliorates oxidative stress induced aberrant crypt foci and lipid peroxidation in 1,2 dimethylhydrazine induced rat colon cancer. Invest New Drugs. 2009;28(3):225–33. [DOI] [PubMed]
- 51.Chang HR, Chen PN, Yang SF, Sun YS, Wu SW, Hung TW, et al. Silibinin inhibits the invasion and migration of renal carcinoma 786-O cells in vitro, inhibits the growth of xenografts in vivo and enhances chemosensitivity to 5-fluorouracil and paclitaxel. Mol Carcinog. 2011;50(10):811–23. [DOI] [PubMed]
- 52.Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking EGFR-ERK activation in renal cell carcinoma. Cancer Lett. 2008;272(1):61–9. [DOI] [PubMed]
- 53.Cheung CW, Vesey DA, Nicol DL, Johnson DW. Silibinin inhibits renal cell carcinoma via mechanisms that are independent of insulin-like growth factor-binding protein 3. BJU Int. 2007;99(2):454–60. doi: 10.1111/j.1464-410X.2007.06571.x. [DOI] [PubMed] [Google Scholar]
- 54.Cheung CW, Taylor PJ, Kirkpatrick CM, Vesey DA, Gobe GC, Winterford C, et al. Therapeutic value of orally administered silibinin in renal cell carcinoma: manipulation of insulin-like growth factor binding protein-3 levels. BJU Int. 2007;100(2):438–44. doi: 10.1111/j.1464-410X.2007.07012.x. [DOI] [PubMed] [Google Scholar]
- 55.Tyagi A, Singh RP, Agarwal C, Agarwal R. Silibinin activates p53-caspase 2 pathway and causes caspase-mediated cleavage of Cip1/p21 in apoptosis induction in bladder transitional-cell papilloma RT4 cells: evidence for a regulatory loop between p53 and caspase 2. Carcinogenesis. 2006;27(11):2269–80. doi: 10.1093/carcin/bgl098. [DOI] [PubMed] [Google Scholar]
- 56.Tyagi A, Raina K, Singh RP, Gu M, Agarwal C, Harrison G, et al. Chemopreventive effects of silymarin and silibinin on N-butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male ICR mice. Mol Cancer Ther. 2007;6(12 Pt 1):3248–55. doi: 10.1158/1535-7163.MCT-07-2006. [DOI] [PubMed] [Google Scholar]
- 57.Singh RP, Tyagi A, Sharma G, Mohan S, Agarwal R. Oral silibinin inhibits in vivo human bladder tumor xenograft growth involving down-regulation of survivin. Clin Cancer Res. 2008;14(1):300–8. doi: 10.1158/1078-0432.CCR-07-1565. [DOI] [PubMed] [Google Scholar]
- 58.Tyagi A, Agarwal C, Harrison G, Glode LM, Agarwal R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis. 2004;25(9):1711–20. doi: 10.1093/carcin/bgh180. [DOI] [PubMed] [Google Scholar]
- 59.Tyagi AK, Agarwal C, Singh RP, Shroyer KR, Glode LM, Agarwal R. Silibinin down-regulates survivin protein and mRNA expression and causes caspases activation and apoptosis in human bladder transitional-cell papilloma RT4 cells. Biochem Biophys Res Commun. 2003;312(4):1178–84. doi: 10.1016/j.bbrc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 60.Zeng J, Sun Y, Wu K, Li L, Zhang G, Yang Z, et al. Chemopreventive and chemotherapeutic effects of intravesical silibinin against bladder cancer by acting on mitochondria. Mol Cancer Ther. 2011;10(1):104–16 [DOI] [PubMed]
- 61.Chen PN, Hsieh YS, Chiang CL, Chiou HL, Yang SF, Chu SC. Silibinin inhibits invasion of oral cancer cells by suppressing the MAPK pathway. J Dent Res. 2006;85(3):220–5. doi: 10.1177/154405910608500303. [DOI] [PubMed] [Google Scholar]
- 62.Bang CI, Paik SY, Sun DI, Joo YH, Kim MS. Cell growth inhibition and down-regulation of survivin by silibinin in a laryngeal squamous cell carcinoma cell line. Ann Otol Rhinol Laryngol. 2008;117(10):781–5. doi: 10.1177/000348940811701014. [DOI] [PubMed] [Google Scholar]
- 63.Sharma G, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23(3B):2649–55. [PubMed] [Google Scholar]
- 64.Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, Dwyer-Nield LD, et al. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3. Cancer Prev Res (Phila) 2009;2(1):74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98(12):846–55. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 66.Li W, Mu D, Song L, Zhang J, Liang J, Wang C, et al. Molecular mechanism of silymarin-induced apoptosis in a highly metastatic lung cancer cell line anip973. Cancer Biother Radiopharm. 2011;26(3):317–24 [DOI] [PubMed]
- 67.Mateen S, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin inhibits human nonsmall cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol Carcinog. 2010;49(3):247–58. [DOI] [PMC free article] [PubMed]
- 68.Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog. 2004;40(3):143–9. doi: 10.1002/mc.20018. [DOI] [PubMed] [Google Scholar]
- 69.Chen PN, Hsieh YS, Chiou HL, Chu SC. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact. 2005;156(2–3):141–50. doi: 10.1016/j.cbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Tyagi A, Agarwal C, Dwyer-Nield LD, Singh RP, Malkinson AM, Agarwal R. Silibinin modulates TNF-alpha and IFN-gamma mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol Carcinog. 2011;51(10):832–42. [DOI] [PubMed]
- 71.Chittezhath M, Deep G, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits cytokine-induced signaling cascades and down-regulates inducible nitric oxide synthase in human lung carcinoma A549 cells. Mol Cancer Ther. 2008;7(7):1817–26. doi: 10.1158/1535-7163.MCT-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh RP, Mallikarjuna GU, Sharma G, Dhanalakshmi S, Tyagi AK, Chan DC, et al. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin Cancer Res. 2004;10(24):8641–7. doi: 10.1158/1078-0432.CCR-04-1435. [DOI] [PubMed] [Google Scholar]
- 73.Son YG, Kim EH, Kim JY, Kim SU, Kwon TK, Yoon AR, et al. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res. 2007;67(17):8274–84. doi: 10.1158/0008-5472.CAN-07-0407. [DOI] [PubMed] [Google Scholar]
- 74.Momeny M, Malehmir M, Zakidizaji M, Ghasemi R, Ghadimi H, Shokrgozar MA, et al. Silibinin inhibits invasive properties of human glioblastoma U87MG cells through suppression of cathepsin B and nuclear factor kappa B-mediated induction of matrix metalloproteinase 9. Anticancer Drugs. 2010;21(3):252–60. [DOI] [PubMed]
- 75.Qi L, Singh RP, Lu Y, Agarwal R, Harrison GS, Franzusoff A. Epidermal growth factor receptor mediates silibinin-induced cytotoxicity in a rat glioma cell line. Cancer Biol Ther. 2003;2(5):526–31. doi: 10.4161/cbt.2.5.452. [DOI] [PubMed] [Google Scholar]
- 76.Kim KW, Choi CH, Kim TH, Kwon CH, Woo JS, Kim YK. Silibinin inhibits glioma cell proliferation via Ca2+/ROS/MAPK-dependent mechanism in vitro and glioma tumor growth in vivo. Neurochem Res. 2009;34(8):1479–90. doi: 10.1007/s11064-009-9935-6. [DOI] [PubMed] [Google Scholar]
- 77.Dizaji MZ, Malehmir M, Ghavamzadeh A, Alimoghaddam K, Ghaffari SH. Synergistic effects of arsenic trioxide and silibinin on apoptosis and invasion in human glioblastoma U87MG cell line. Neurochem Res. 2012;37(2):370–80. [DOI] [PubMed]
- 78.Singh RP, Dhanalakshmi S, Mohan S, Agarwal C, Agarwal R. Silibinin inhibits UVB- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappaB in mouse epidermal JB6 cells. Mol Cancer Ther. 2006;5(5):1145–53. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- 79.Mallikarjuna G, Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004;64(17):6349–56. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- 80.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53–p21/Cip1 in epidermis. Carcinogenesis. 2004;25(8):1459–65. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 81.Gu M, Singh RP, Dhanalakshmi S, Mohan S, Agarwal R. Differential effect of silibinin on E2F transcription factors and associated biological events in chronically UVB-exposed skin versus tumors in SKH-1 hairless mice. Mol Cancer Ther. 2006;5(8):2121–9. doi: 10.1158/1535-7163.MCT-06-0052. [DOI] [PubMed] [Google Scholar]
- 82.Gu M, Dhanalakshmi S, Singh RP, Agarwal R. Dietary feeding of silibinin prevents early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1344–9. doi: 10.1158/1055-9965.EPI-04-0664. [DOI] [PubMed] [Google Scholar]
- 83.Singh RP, Tyagi AK, Zhao J, Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002;23(3):499–510. doi: 10.1093/carcin/23.3.499. [DOI] [PubMed] [Google Scholar]
- 84.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Dual efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis. 2004;25(1):99–106. doi: 10.1093/carcin/bgg188. [DOI] [PubMed] [Google Scholar]
- 85.Svobodova A, Zdarilova A, Walterova D, Vostalova J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J Dermatol Sci. 2007;48(3):213–24. doi: 10.1016/j.jdermsci.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 86.Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007;67(7):3483–91. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 87.Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis. 2007;28(5):977–87. doi: 10.1093/carcin/bgl221. [DOI] [PubMed] [Google Scholar]
- 88.Duan WJ, Li QS, Xia MY, Tashiro S, Onodera S, Ikejima T. Silibinin activated p53 and induced autophagic death in human fibrosarcoma HT1080 cells via reactive oxygen species-p38 and c-Jun N-terminal kinase pathways. Biol Pharm Bull. 2011;34(1):47–53. [DOI] [PubMed]
- 89.Noh EM, Yi MS, Youn HJ, Lee BK, Lee YR, Han JH, et al. Silibinin enhances ultraviolet B-induced apoptosis in mcf-7 human breast cancer cells. J Breast Cancer Mar. 2011;14(1):8–13. [DOI] [PMC free article] [PubMed]
- 90.Wang HJ, Tashiro S, Onodera S, Ikejima T. Inhibition of insulin-like growth factor 1 receptor signaling enhanced silibinin-induced activation of death receptor and mitochondrial apoptotic pathways in human breast cancer MCF-7 cells. J Pharmacol Sci. 2008;107(3):260–9. doi: 10.1254/jphs.08054FP. [DOI] [PubMed] [Google Scholar]
- 91.Dastpeyman M, Motamed N, Azadmanesh K, Mostafavi E, Kia V, Jahanian-Najafabadi A, et al. Inhibition of silibinin on migration and adhesion capacity of human highly metastatic breast cancer cell line, MDA-MB-231, by evaluation of beta1-integrin and downstream molecules, Cdc42, Raf-1 and D4GDI. Med Oncol. 2011;29(4):2512–8. [DOI] [PubMed]
- 92.Kim S, Han J, Kim JS, Kim JH, Choe JH, Yang JH, et al. Silibinin suppresses EGFR ligand-induced CD44 expression through inhibition of EGFR activity in breast cancer cells. Anticancer Res. 2011;31(11):3767–73. [PubMed]
- 93.Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, et al. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine. 2009;16(6–7):573–80. doi: 10.1016/j.phymed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 94.Kim S, Kim SH, Hur SM, Lee SK, Kim WW, Kim JS, et al. Silibinin prevents TPA-induced MMP-9 expression by down-regulation of COX-2 in human breast cancer cells. J Ethnopharmacol. 2009;126(2):252–7. doi: 10.1016/j.jep.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 95.Wang HJ, Wei XF, Jiang YY, Huang H, Yang Y, Fan SM, et al. Silibinin induces the generation of nitric oxide in human breast cancer MCF-7 cells. Free Radic Res. 2010;44(5):577–84. [DOI] [PubMed]
- 96.Provinciali M, Papalini F, Orlando F, Pierpaoli S, Donnini A, Morazzoni P, et al. Effect of the silybin-phosphatidylcholine complex (IdB 1016) on the development of mammary tumors in HER-2/neu transgenic mice. Cancer Res. 2007;67(5):2022–9. doi: 10.1158/0008-5472.CAN-06-2601. [DOI] [PubMed] [Google Scholar]
- 97.Zhou L, Liu P, Chen B, Wang Y, Wang X, Chiriva Internati M, et al. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Res. 2008;28(2A):1119–27. [PubMed] [Google Scholar]
- 98.Gallo D, Giacomelli S, Ferlini C, Raspaglio G, Apollonio P, Prislei S, et al. Antitumour activity of the silybin-phosphatidylcholine complex, IdB 1016, against human ovarian cancer. Eur J Cancer. 2003;39(16):2403–10. doi: 10.1016/S0959-8049(03)00624-5. [DOI] [PubMed] [Google Scholar]
- 99.Agarwal C, Tyagi A, Kaur M, Agarwal R. Silibinin inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis. 2007;28(7):1463–70. doi: 10.1093/carcin/bgm042. [DOI] [PubMed] [Google Scholar]
- 100.Sharma Y, Agarwal C, Singh AK, Agarwal R. Inhibitory effect of silibinin on ligand binding to erbB1 and associated mitogenic signaling, growth, and DNA synthesis in advanced human prostate carcinoma cells. Mol Carcinog. 2001;30(4):224–36. doi: 10.1002/mc.1032. [DOI] [PubMed] [Google Scholar]
- 101.Tyagi A, Sharma Y, Agarwal C, Agarwal R. Silibinin impairs constitutively active TGFalpha-EGFR autocrine loop in advanced human prostate carcinoma cells. Pharm Res. 2008;25(9):2143–50. doi: 10.1007/s11095-008-9545-z. [DOI] [PubMed] [Google Scholar]
- 102.Zi X, Zhang J, Agarwal R, Pollak M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res. 2000;60(20):5617–20. [PubMed] [Google Scholar]
- 103.Lu W, Lin C, King TD, Chen H, Reynolds RC, Li Y. Silibinin inhibits Wnt/beta-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells. Cell Signal. 2012;24(12):2291–6. [DOI] [PMC free article] [PubMed]
- 104.Zhu W, Zhang JS, Young CY. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis. 2001;22(9):1399–403. doi: 10.1093/carcin/22.9.1399. [DOI] [PubMed] [Google Scholar]
- 105.Thelen P, Wuttke W, Jarry H, Grzmil M, Ringert RH. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. J Urol. 2004;171(5):1934–8. doi: 10.1097/01.ju.0000121329.37206.1b. [DOI] [PubMed] [Google Scholar]
- 106.Thelen P, Jarry H, Ringert RH, Wuttke W. Silibinin down-regulates prostate epithelium-derived Ets transcription factor in LNCaP prostate cancer cells. Planta Med. 2004;70(5):397–400. doi: 10.1055/s-2004-818965. [DOI] [PubMed] [Google Scholar]
- 107.Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomarkers Prev. 2003;12(9):933–9. [PubMed] [Google Scholar]
- 108.Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma PC-3 tumor xenograft. Carcinogenesis. 2007;28(12):2567–74. doi: 10.1093/carcin/bgm218. [DOI] [PubMed] [Google Scholar]
- 109.Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits constitutive and TNFalpha-induced activation of NF-kappaB and sensitizes human prostate carcinoma DU145 cells to TNFalpha-induced apoptosis. Oncogene. 2002;21(11):1759–67. doi: 10.1038/sj.onc.1205240. [DOI] [PubMed] [Google Scholar]
- 110.Wu K, Zeng J, Li L, Fan J, Zhang D, Xue Y, et al. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol Rep. 2010;23(6):1545–52. [PubMed]
- 111.Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25(7):1053–69. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 112.Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc Natl Acad Sci U S A. 1999;96(13):7490–5. [DOI] [PMC free article] [PubMed]
- 113.Tyagi A, Agarwal C, Agarwal R. Inhibition of retinoblastoma protein (Rb) phosphorylation at serine sites and an increase in Rb-E2F complex formation by silibinin in androgen-dependent human prostate carcinoma LNCaP cells: role in prostate cancer prevention. Mol Cancer Ther. 2002;1(7):525–32. [PubMed] [Google Scholar]
- 114.Bhatia N, Zhao J, Wolf DM, Agarwal R. Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin. Cancer Lett. 1999;147(1–2):77–84. doi: 10.1016/S0304-3835(99)00276-1. [DOI] [PubMed] [Google Scholar]
- 115.Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62(11):3063–9. [PubMed] [Google Scholar]
- 116.Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial–mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008;14(23):7773–80. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68(16):6822–30. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tyagi A, Agarwal C, Agarwal R. The cancer preventive flavonoid silibinin causes hypophosphorylation of Rb/p107 and Rb2/p130 via modulation of cell cycle regulators in human prostate carcinoma DU145 cells. Cell Cycle. 2002;1(2):137–42. [PubMed]
- 119.Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther. 2007;6(10):2696–707. doi: 10.1158/1535-7163.MCT-07-0104. [DOI] [PubMed] [Google Scholar]
- 120.Deep G, Oberlies NH, Kroll DJ, Agarwal R. Identifying the differential effects of silymarin constituents on cell growth and cell cycle regulatory molecules in human prostate cancer cells. Int J Cancer. 2008;123(1):41–50. doi: 10.1002/ijc.23485. [DOI] [PubMed] [Google Scholar]
- 121.Tyagi AK, Singh RP, Agarwal C, Chan DC, Agarwal R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin Cancer Res. 2002;8(11):3512–9. [PubMed] [Google Scholar]
- 122.Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, et al. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65(10):4448–57. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 123.Flaig TW, Su LJ, Harrison G, Agarwal R, Glode LM. Silibinin synergizes with mitoxantrone to inhibit cell growth and induce apoptosis in human prostate cancer cells. Int J Cancer. 2007;120(9):2028–33. doi: 10.1002/ijc.22465. [DOI] [PubMed] [Google Scholar]
- 124.Dhanalakshmi S, Agarwal P, Glode LM, Agarwal R. Silibinin sensitizes human prostate carcinoma DU145 cells to cisplatin- and carboplatin-induced growth inhibition and apoptotic death. Int J Cancer. 2003;106(5):699–705. doi: 10.1002/ijc.11299. [DOI] [PubMed] [Google Scholar]
- 125.Tyagi A, Bhatia N, Condon MS, Bosland MC, Agarwal C, Agarwal R. Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells. Prostate. 2002;53(3):211–7. doi: 10.1002/pros.10146. [DOI] [PubMed] [Google Scholar]
- 126.Deep G, Gangar SC, Agarwal C, Agarwal R. Role of E-cadherin in antimigratory and antiinvasive efficacy of silibinin in prostate cancer cells. Cancer Prev Res (Phila). 2011;4(8):1222–32. [DOI] [PMC free article] [PubMed]
- 127.Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D, Li L, et al. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol Sin. 2009;30(8):1162–8. doi: 10.1038/aps.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raina K, Serkova NJ, Agarwal R. Silibinin feeding alters the metabolic profile in TRAMP prostatic tumors: 1H-NMRS-based metabolomics study. Cancer Res. 2009;69(9):3731–5. doi: 10.1158/0008-5472.CAN-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jung HJ, Park JW, Lee JS, Lee SR, Jang BC, Suh SI, et al. Silibinin inhibits expression of HIF-1alpha through suppression of protein translation in prostate cancer cells. Biochem Biophys Res Commun. 2009;390(1):71–6. doi: 10.1016/j.bbrc.2009.09.068. [DOI] [PubMed] [Google Scholar]


