Abstract
Gait and balance disturbances typically emerge in advanced Parkinson’s disease with generally limited response to dopaminergic medication and subthalamic nucleus deep brain stimulation. Therefore, advanced programming with interleaved pulses was put forward to introduce concomittant nigral stimulation on caudal contacts of a subthalamic lead. Here, we hypothesized that the combined stimulation of subthalamic nucleus and substantia nigra pars reticulata improves axial symptoms compared with standard subthalamic nucleus stimulation. Twelve patients were enrolled in this 2 × 2 cross-over double-blind randomized controlled clinical trial and both the safety and efficacy of combined subthalamic nucleus and substantia nigra pars reticulata stimulation were evaluated compared with standard subthalamic nucleus stimulation. The primary outcome measure was the change of a broad-scaled cumulative axial Unified Parkinson’s Disease Rating Scale score (Scale II items 13–15, Scale III items 27–31) at ‘3-week follow-up’. Secondary outcome measures specifically addressed freezing of gait, balance, quality of life, non-motor symptoms and neuropsychiatric symptoms. For the primary outcome measure no statistically significant improvement was observed for combined subthalamic nucleus and substantia nigra pars reticulata stimulation at the ‘3-week follow-up’. The secondary endpoints, however, revealed that the combined stimulation of subthalamic nucleus and substantia nigra pars reticulata might specifically improve freezing of gait, whereas balance impairment remained unchanged. The combined stimulation of subthalamic nucleus and substantia nigra pars reticulata was safe, and of note, no clinically relevant neuropsychiatric adverse effect was observed. Patients treated with subthalamic nucleus and substantia nigra pars reticulata stimulation revealed no ‘global’ effect on axial motor domains. However, this study opens the perspective that concomittant stimulation of the substantia nigra pars reticulata possibly improves otherwise resistant freezing of gait and, therefore, highly warrants a subsequent phase III randomized controlled trial.
Keywords: Parkinson’s disease, DBS, gait, freezing, subthalamic nucleus
Introduction
Deep brain stimulation of the subthalamic nucleus (STN–DBS) in Parkinson’s disease is an established treatment for segmental motor symptoms and motor fluctuations (Deuschl et al., 2006; Kleiner-Fisman et al., 2006; Weaver et al., 2009) including early disease stages with beginning motor fluctuations (Schuepbach et al., 2013). However, debilitating axial motor symptoms are frequently observed during disease progression (Nutt et al., 2011) and contribute to a disproportional decline of the therapeutic response to standard dopaminergic treatment and to STN–DBS (Krack et al., 2003; St George et al., 2010; Castrioto et al., 2011). We postulate that these different therapeutic outcomes of segmental and axial motor domains may mirror differential functional sub-loops of pathological motor network processing. Whereas standard STN–DBS may primarily facilitate the thalamo-cortico-spinal motor control improving segmental symptoms (Salenius et al., 2002; Potter-Nerger et al., 2008; Kuriakose et al., 2010; Weiss et al., 2012a), gait disturbances in advanced disease stages may be associated with defective motor processing of mesencephalic locomotor pathways (Ferraye et al., 2010; Moro et al., 2010) including descending nigropontine projections to spinal motor neurons (Potter et al., 2008; Chastan et al., 2009; Tsang et al., 2010; Thevathasan et al., 2011b; Weiss et al., 2012a). An attractive approach to modulate nigropontine locomotor integration is to introduce co-stimulation of the substantia nigra pars reticulata (SNr) on a caudal electrode contact of a lead with rostral contacts located in the STN (Weiss et al., 2011a). Advanced programming with so-called ‘interleaved pulses’ allows independent stimulation of contacts with different amplitudes and pulse widths at a common frequency (Weiss et al., 2011a; Wojtecki et al., 2011; Kovacs et al., 2012) and therefore enables us to co-stimulate segregate functional motor loops at the level of the STN and SNr (Weiss et al., 2011a).
Materials and methods
This investigator-initiated phase II double-blind randomized controlled trial was registered at ClinicalTrials.gov (NCT01355835) and a detailed study protocol was published elsewhere (Weiss et al., 2011b). The trial was approved by the local Ethics committee in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Patients
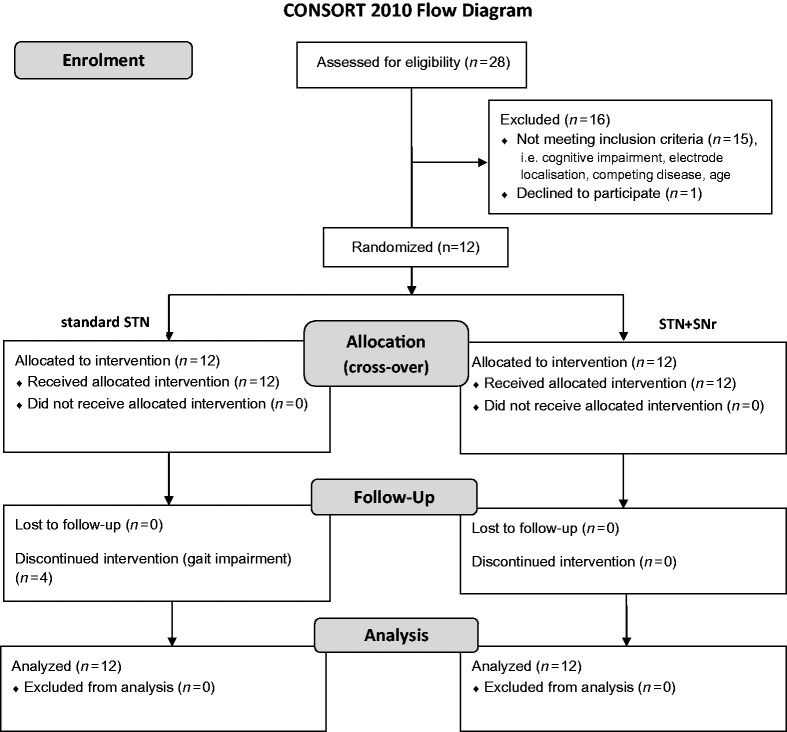
Patients with advanced Parkinson’s disease and gait and balance impairment resistant to optimized dopaminergic and STN–DBS treatment (Weiss et al., 2011b) were enrolled if they met the following inclusion criteria: age 18–80 years, disease duration >5 years, idiopathic Parkinson’s disease including genetic forms of typical Parkinson’s disease, therapy with STN–DBS and Activa® impulse generator (Medtronic), axial UPDRS ≥12 [sum score of Unified Parkinson’s disease Rating Scale (UPDRS) II, items 13–15 and UPDRS III, items 27–31], one of the two rostral electrode contacts located in the STN area and the lowermost electrode contacts located in the caudal STN–SNr border zone, dopaminergic medication unchanged for 4 weeks before study enrolment, and implantation of STN–DBS electrodes for at least 6 months. Exclusion criteria were cognitive impairment (Mini-Mental State Examination <25 points), participation in other clinical trials during the past 3 months or during study enrolment, acute suicidal tendency or psychosis, other chronic pathological conditions interfering with the study protocol or interpretability of the data, and pregnancy. Comprehensive data on patient screening and patient enrolment are given in the CONSORT flow diagram (Fig. 1). Patients were screened for mutations in the most frequent Parkinson’s disease associated genes. One patient (Patient PD11) was identified with a Parkin gene mutation (Supplementary material).
Figure 1.
CONSORT chart. [STN+SNr] = combined STN+SNr stimulation.
Study design
This study is a randomized double-blinded 2 × 2 cross-over single centre clinical trial. After trial commencement, there were no changes to methods or outcome assessments. We tested the hypothesis that combined STN+SNr stimulation is superior to improve axial motor symptoms compared with active subthalamic standard therapy after 3-week active treatment.
Patients underwent a detailed ‘baseline’ assessment after overnight withdrawal of dopaminergic medication (OFF medication, OFF stimulation). In the same session, ‘immediate testings’ of standard STN stimulation versus combined STN+SNr stimulation treatment were performed. These three treatment conditions [i.e. (Baseline) in OFF medication OFF stimulation; standard STN stimulation in OFF medication; and combined STN+SNr stimulation in OFF medication] were introduced 30 min before the clinical ratings in randomized order. This session performed in an OFF medication state was considered to assess short-term efficacy and to ensure that parameters on subthalamic contacts were optimally adjusted. At the end of the immediate testing patients entered the ‘3-week follow-up’ stage with both standard STN stimulation and combined STN+SNr stimulation active treatment in randomized order, prepared by the Institute for Clinical Epidemiology and Applied Biometry, Tübingen, Germany using a computer generated randomization. Endpoint assessments were obtained at the end of the ‘3-week follow-up’ period and included both clinical and anamnestic measures as detailed below. In this cross-over trial we did not consider a second baseline assessment after the first ‘3-week follow-up’ period (before entering the second ‘3-week’ period). Based on current literature it is highly improbable, that carry-over effects from either combined STN+SNr stimulation or standard STN stimulation treatment might outlast a ‘3-week follow-up' given: (i) the immediate recurrence of motor symptoms when the stimulator is switched OFF; and (ii) clearly discriminable motor effects of subthalamic and nigral stimulation were demonstrated within short time intervals (Chastan et al., 2009; Weiss et al., 2011a). This was recently confirmed by an independent study that described fast clinical wash-out after turning off the DBS that was most pronounced in advanced disease stages (Cooper et al., 2013). Therefore, and for ethical reasons, we did not implement a second baseline assessment that would have necessitated another l-DOPA and stimulation withdrawal after the first ‘3-week follow-up’.
Because both standard STN stimulation and combined STN+SNr stimulation similarly controlled for segmental symptoms and did not induce acute adverse events or other sensations using the study parameters applied, there was no indication that the patients were able to distinguish between the two stimulation programs. However, patients and the endpoint assessor may have noticed when stimulators were switched OFF in the baseline condition due to the recurrence of segmental Parkinson’s disease symptoms. For ethical reasons patients were informed that the two programs of standard STN and combined STN+SNr stimulation were designed to study the differential therapeutic efficacy on gait measures.
The initial titration of subthalamic and nigral stimulation parameters was performed by the principal investigator (D.W.) who also stored the allocation code and held it closed until all endpoint assessments and final statistical analyses were performed. In order to keep both patients and endpoint assessors blinded to the treatment condition, parameters were changed several times between standard STN and combined STN+SNr stimulation before maintaining the intended program. Double-blind clinical endpoint assessments were performed by a specialized expert neurologist trained in Parkinson’s disease and DBS treatment (T.W.). Patients were able to discontinue study treatment in accordance with the accepted ethical standard.
Electrode localization
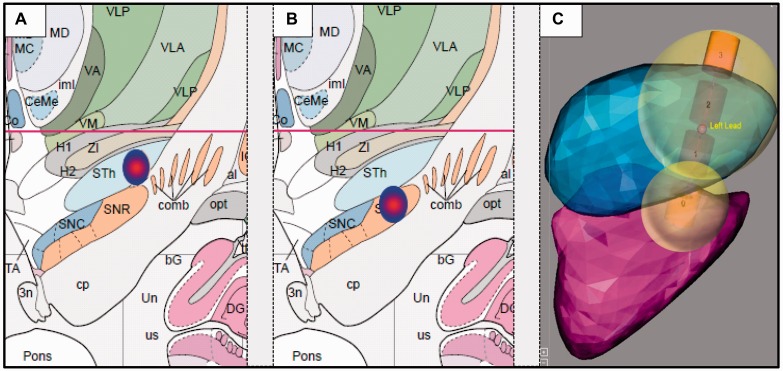
Localization of the active STN and SNr contacts were determined by coregistration analyses of preoperative 3D T1-weighted MPRAGE and postoperative 3D T1-weighted FLASH sequences. Coregistration analyses were performed with Matlab 7.0 and the open-source toolbox SPM5 and indicated electrode localization of active contacts in the dorsolateral portions of STN and SNr, respectively (Fig. 2).
Figure 2.
Localization of active electrode contacts of (A) dorsolateral STN and (B) dorsolateral SNr. Coordinates relative to the midcommisural point (MCP) were: left STN −11.4 ± 0.8, −0.9 ± 2.0, −3.0 ± 1.7; right STN 13.5 ± 1.1, −0.5 ± 1.7, −2.2 ± 1.5; left SNr −10.0 ± 0.9, −3.4 ± 2.1, −6.4 ± 1.8; right SNr 12.1 ± 1.3, −3.3 ± 1.7, −5.8 ± 1.5 (x, y, z; x = medio-lateral, y = anterio-posterior, z = rostro-caudal). Electrode coordinates (mean ± standard deviation in x- and y-direction) are visualized in coronal view on the Atlas of the Human Brain with permission (Mai et al., 2007). (C) An additional illustrative image of electrode localization including a simulation on volume of tissue activated was kindly provided by Medtronic based on work by Yelnik et al. (2007) (atlas) and D’Haese et al. (2012) (atlas and algorithms).
Therapy and stimulation parameters
At the time of study enrolment, all patients were implanted with Activa® pulse generators (enabling advanced interleaved programming). Some individual patients with longer follow-up periods of DBS therapy (up to 79 months) initially received Kinetra® pulse generators; however these were changed to Activa® pulse generators after battery depletion during regular clinical follow-up. Activa® pulse generators were available at our study site from 2009.
The stimulation parameters applied during the study phase were established according to our stringent predefined study protocol (Weiss et al., 2011b) to achieve the best individual parameters for active subthalamic contacts in patients with emerging gait disturbances. This protocol provides a standardized procedure including the concept of ‘better side reduction’ (Fasano et al., 2011) in order to ensure best individual STN stimulation parameters before entering the study. Here, in general the rostral contacts 2 (second upper left STN of the quadripolar electrode) and 10 (second upper right STN) were chosen, that also prevented current spreading from the subthalamic active contacts to SNr. This programming was performed before patients entered the study.
Importantly, when entering the study protocol, we again ascertained that segmental motor symptoms were optimally controlled from standard STN stimulation. Therefore, the medication OFF session was considered to verify optimal stimulation parameters as gold standard (i.e. for tremor, bradykinesia and rigidity) before entering the ‘immediate testing'. Nigral stimulation was standardized on a common pulse width of 60 µs and all subthalamic and nigral contacts were stimulated at a common frequency (125 Hz). Detailed information on the stimulation parameters is provided (Supplementary Tables 1 and 2).
Throughout the study the stimulation parameters of the active subthalamic contacts as well as medication were held constant, including ‘immediate testing' and both ‘3-week follow-up’ assessments. Of note, owing to the delayed onset of dyskinesia after introduction of combined STN+SNr stimulation, medication in one patient and nigral stimulation parameters in two patients had to be adjusted during the ‘3-week follow-up’' according to the intention-to-treat principle (detailed below).
Outcome measures
In this phase II study we primarily aimed to investigate a broad spectrum of axial motor symptoms. Therefore, a broad-scaled primary endpoint was defined as ‘axial score’ built from eight items of the anamnestic UPDRS II (items 13–15: falling unrelated to freezing, freezing when walking, walking) and the clinical UPRDS III (items 27–31: arising from chair, posture, gait, postural stability, body bradykinesia and hypokinesia), all 5-point rated. For the statistical evaluation the five rating points are represented by the numbers 0 to 4, which represent increasing levels of impairment on different axial motor domains including freezing of gait, independence of gait, balance and posture. This ‘axial score’ was summed of the ratings across the eight items (range 0–32). Secondary clinical endpoint assessments tested axial motor function (UPDRS III, items 27–31), balance (Berg Balance Scale; Berg et al., 1992), gait [timed walking test from Core Assessment Program for Surgical Interventional Therapies in Parkinson's Disease (CAPSIT-PD)], and freezing of gait (Freezing of Gait Assessment Course) (Ziegler et al., 2010). The Freezing of Gait Assessment Course reliably detects freezing of gait given its episodic nature and dependence on environmental factors and includes elements like ‘walking through a narrow door’, ‘turning in tight space’ and ‘dual tasking’ that are well-known to provoke freezing of gait. These clinical ratings were obtained at baseline, upon ‘immediate testing’ and at ‘3-week follow-up’ in all treatment conditions. Further anamnestic measures were assessed at baseline and at ‘3-week follow-up’ on: (i) gait impairment related to freezing (Giladi Freezing of Gait Questionnaire) (Giladi et al., 2009); (ii) quality of life (PDQ-39); (iii) neuropsychiatric symptoms (Beck’s Depression Index, Barrett Impulsiveness Scale); and (iv) non-motor symptoms (Non-motor Symptoms Scale) (Storch et al., 2010). Note that the anamnestic scores and the primary endpoint (including anamnestic items) were not considered for ‘immediate testing' as treatment conditions were separated by only 30 min.
Statistical analysis
The primary endpoint for the confirmatory statistical analysis was the difference in the ‘axial score’ between standard STN and combined STN+SNr stimulation at ‘3-week follow-up’. A sample size of 10 patients was estimated to be sufficient to detect a difference of 4 points on the primary outcome measure with 80% power, assuming a standard deviation of 4.0 (effect size: 1.0; NQuery Advisor 7.0). Assuming normal distribution a two-sided paired t-test with α = 0.05 on the null hypothesis of equality of the two therapies was applied. The normality assumption on the primary endpoint was confirmed using the Shapiro-Wilk test. To adjust for a maximum of two dropouts n = 12 patients were enrolled.
Assuming normal distributions, the statistical analysis of the primary and all secondary outcomes includes a control for period effects. Therefore, unpaired t-tests were used to compare the sum of the scores in the two periods, i.e. the group of six patients who were randomized to standard STN stimulation followed by combined STN+SNr stimulation versus the group of six patients who were randomized to combined STN+SNr stimulation followed by standard STN stimulation (Wellek and Blettner, 2012). In the second step we analyzed the difference between the scores for combined STN+SNr stimulation and for standard STN stimulation. First we confirmed the normal distribution and if no evidence against the normality assumption was found (P > 0.05 on the Shapiro-Wilk tests) we compared the differences with paired t-tests. In case of evidence against the normality assumption we used sign tests.
The primary endpoint was statistically analyzed with the paired t-test. The outcome on the primary endpoint was decided on a two-sided significance level of 0.05. All secondary endpoints were analyzed with an exploratory intention and no confirmatory interpretation was drawn. As in this situation the ‘use of multiple test procedures will not solve the problem of making valid statistical inference for hypotheses that were generated by the data’ (Bender and Lange, 2001) findings from the exploratory analyses are subject to testing in confirmatory follow-up trials and accordingly not corrected for multiple comparisons here.
Moreover, given some clinical heterogeneity of our cohort concerning disease duration and time from DBS implantation (as typically observed along the variable endophenotypic spectrum of idiopathic Parkinson’s disease) we additionally performed non-parametric testing on the primary endpoint and further on secondary endpoints without normal distribution (Non-motor Symptoms Scale, CAPSIT-PD, Berg Balance Scale) using a sign test. All measurements are presented with mean ± standard deviation for parametric tests and median (range) for non-parametric tests. The results presented were two-sided P-values without adjustments.
Results
Of 28 patients assessed for eligibility in our study centre 12 patients with advanced Parkinson’s disease (nine male, age 65.0 ± 8.9 years) were enrolled between January 2011 and June 2012 at the Department for Neurodegenerative Diseases (Table 1). Reasons that precluded study participation were cognitive impairment (Mini-Mental State Examination < 25; n = 5), caudal electrode contact located outside STN–SNr border zone (n = 4), other disease that interfered with gait (n = 5), age > 80 years (n = 1), retracted consent (n = 1) (Fig. 1). The study cohort had age at Parkinson’s disease onset of 47.0 ± 8.3 years, disease duration 17.6 ± 5.2 years, and time since DBS implantation 31.3 ± 24.4 (range: 6–79) months. The mean Mini-Mental State Examination score was 28.7 ± 1.3 (no patient <25). Four patients wished to discontinue standard STN stimulation treatment prematurely (Patients PD3, PD7, PD10 and PD11) owing to more pronounced gait impairment (Patients PD10 and PD11), immobility (Patients PD3 and PD7) or falls (Patient PD7). Three of these patients had been treated with combined STN+SNr stimulation first. A detailed overview on immediate (Table 2) and ‘3-week follow-up’ results (Table 3) is given.
Table 1.
Patient characteristics
| ID | Age, years | Gender | Age at onset, years | Disease duration, years | Time with DBS, months | LED, mg | Axial score at enrolment |
|---|---|---|---|---|---|---|---|
| PD1 | 63 | F | 42 | 21 | 18 | 490 | 20 |
| PD2 | 72 | M | 58 | 14 | 20 | 890 | 20 |
| PD3 | 74 | F | 48 | 26 | 61 | 275 | 15 |
| PD4 | 68 | M | 51 | 16 | 8 | 934 | 14 |
| PD5 | 61 | M | 44 | 16 | 53 | 150 | 14 |
| PD6 | 71 | F | 53 | 17 | 30 | 575 | 17 |
| PD7 | 71 | M | 57 | 13 | 6 | 807 | 23 |
| PD8 | 61 | M | 37 | 23 | 51 | 785 | 18 |
| PD9 | 61 | M | 47 | 14 | 7 | 1098 | 12 |
| PD10 | 67 | M | 41 | 26 | 79 | 440 | 14 |
| PD11 | 41 | M | 31 | 10 | 10 | 350 | 14 |
| PD12 | 70 | M | 55 | 15 | 33 | 1000 | 12 |
F = female, M = male; LED = l-DOPA equivalent dosage.
Table 2.
Results from ‘immediate testing’
| Baseline | ‘Immediate Testing’ |
||||
|---|---|---|---|---|---|
| OFF medication OFF stimulation | Standard STN stimulation | Combined STN+SNr stimulation | P-value | ||
| Secondary endpoints | |||||
| Axial UPDRS III (items 27–31) | 11.17 ± 3.56 | 9.25 ± 4.67 | 8.17 ± 4.09 | 0.041a | |
| Segmental UPDRS III (items 20–26) | 38.0 ± 5.10 | 29.17 ± 6.62 | 27.58 ± 7.96 | 0.1347a | |
| FOG-AC | 22.17 ± 11.74 | 16.25 ± 12.78 | 8.67 ± 10.92 | 0.0056a | |
| CAPSIT [steps] | 18.5 (13–82)§ | 14.5 (8–51.5)§ | 14.5 (8.5–36)§ | 0.5488b | |
| CAPSIT [time] | 12 (6.5–105)§ | 7.5 (5.5–67.5)§ | 8.5 (5–28)§ | 0.7539b | |
| CAPSIT [freezing] | 0.5 (0–3)§ | 0.5 (0–3)§ | 0 (0–0.5)§ | >0.99b | |
| Berg Balance Scale | 41.5 (11–56)§ | 47 (15–56)§ | 50 (9–56)§ | 0.7266b | |
FOG-AC = Freezing of Gait Assessment Course.
at-Test.
bSign Test.
§Median (Min–Max).
Table 3.
Results from the ‘3-week follow-up’
| Baseline | ‘3-week follow-up’ |
|||
|---|---|---|---|---|
| OFF medication OFF stimulation | Standard STN stimulation | Combined STN+SNr stimulation | P-value | |
| Primary endpoint (axial UPDRS II + III) | 17.25 ± 4.31 | 14.25 ± 5.75 | 13.42 ± 6.47 | 0.470a, 0.5078b |
| Secondary endpoints | ||||
| Segmental UPDRS III (items 20–26) | 38.0 ± 5.10 | 28.75 ± 6.03 | 29.75 ± 5.53 | 0.5180a |
| Axial UPDRS III (items 27–31) | 11.17 ± 3.56 | 8.08 ± 4.01 | 8.08 ± 4.38 | >0.99a |
| FOG-AC | 22.17 ± 11.74 | 14.42 ± 13.19 | 8.33 ± 10.91 | 0.0468a |
| CAPSIT [steps] | 18.5 (13–82)§ | 14.25 (8–115)§ | 13 (8.5–28.5)§ | 0.2266b |
| CAPSIT [time] | 12 (6.5–105)§ | 7.5 (4.5–71)§ | 7 (5–22.5)§ | 0.3438b |
| CAPSIT [freezing] | 0.5 (0–3)§ | 0.25 (0–3.5)§ | 0 (0–0.5)§ | 0.0625b |
| Berg Balance Scale | 41.5 (11–56)§ | 51.5 (19–56)§ | 51.5 (17–56)§ | >0.99b |
| FOG-Q | 14.67 ± 4.70 | 16.17 ± 3.83 | 14.50 ± 4.89 | 0.1013a |
| PDQ-39 | ||||
| Mobility | 53.96 ± 23.78 | 54.32 ± 27.23 | 49.38 ± 25.30 | 0.2925a |
| Activities of daily living | 42.01 ± 20.45 | 45.08 ± 23.04 | 45.14 ± 22.46 | 0.4825a |
| Emotional well-being | 26.74 ± 15.02 | 25.38 ± 21.45 | 23.96 ± 17.87 | 0.5697a |
| Stigma | 21.88 ± 27.24 | 22.73 ± 25.35 | 20.31 ± 21.01 | 0.4592a |
| Social support | 18.06 ± 23.26 | 18.94 ± 23.89 | 11.81 ± 10.93 | 0.2767a |
| Cognition | 31.25 ± 24.28 | 23.30 ± 22.89 | 24.48 ± 21.89 | 0.4933a |
| Communication | 40.97 ± 18.62 | 31.82 ± 21.99 | 36.81 ± 22.88 | 0.6250a |
| Bodily discomfort | 35.42 ± 21.06 | 34.85 ± 22.61 | 36.81 ± 16.84 | 0.7623a |
| BDI | 8.67 ± 3.37 | 7.91 ± 3.94 | 9.25 ± 5.55 | 0.3497a |
| NMSS | ||||
| Cardiovascular | 1 (0–9)§ | 0 (0–6)§ | 0 (0–9)§ | 0.3750b |
| Sleep | 9 (0–20)§ | 8 (0–24)§ | 11.5 (0–28)§ | 0.1797b |
| Mood | 5.5 (2–18)§ | 8 (0–28)§ | 7 (0–49)§ | 0.7539b |
| Cognition | 0 (0–12)§ | 0 (0–4)§ | 0 (0–13)§ | >0.99b |
| Concentration | 6 (0–27)§ | 4 (0–24)§ | 5 (0–32)§ | 0.2891b |
| Gastrointestinal | 8 (0–25)§ | 8 (0–20)§ | 6.5 (0–20)§ | 0.7266b |
| Micturition | 7 (0–30)§ | 8 (0–28)§ | 8.5 (0–18)§ | >0.99b |
| Sexual function | 4 (0–18)§ | 0 (0–12)§ | 1 (0–12)§ | >0.99b |
| Sundries | 7 (0–24)§ | 4 (0–26)§ | 9 (0–18)§ | 0.7266b |
| Barrett Impulsiveness Scale | 62.6 ± 5.91 | 63.55 ± 4.3 | 61.67 ± 5.18 | 0.2894a |
| UPDRS IV | 5.75 ± 1.96 | 6.27 ± 2.45 | 5.17 ± 3.04 | 0.2335a |
FOG-AC = Freezing of Gait Assessment Course; FOG-Q = Freezing of Gait Questionnaire; CAPSIT = timed walking test from the Core Assessment Program; PDQ-39 = Parkinson’s disease questionnaire (Quality of life, 39 items); BDI = Beck’s Depression Scale Index; NMSS = Non-motor Symptoms Scale; UPDRS = Unified Parkinson’s Disease Rating Scale. Two-sided P-values are given.
at-Test.
bSign Test, § Median (Min–Max).
Primary outcome parameter on axial motor impairment
At baseline (medication OFF, stimulation off) patients demonstrated severe impairment on the axial score as primary endpoint (17.25 ± 4.31). At ‘3-week follow-up’, no statistically significant difference was found on the axial score between conditions [combined STN+SNr stimulation: 13.42 ± 6.47; standard STN stimulation: 14.25 ± 5.75; effect = 0.83 ± 3.86; 95% confidence interval (CI)−1.62–3.82; P = 0.470; Fig. 3]. An additional non-parametric testing with the sign test revealed similar results. Four patients wished to discontinue standard STN stimulation treatment prematurely (Patient PD3: 3 h, Patient PD7: 19 days, Patient PD10: 2 days, Patient PD11: 9 days) but completed the entire combined STN+SNr stimulation follow-up. In these patients, the individual axial UPDRS scores improved in the combined STN+SNr stimulation condition compared with standard STN stimulation (Patient PD3: 16 versus 19; Patient PD7: 25 versus 27; Patient PD10: 9 versus 13; Patient PD11: 4 versus 9) with endpoint assessments performed according to the intention-to-treat principle. Three were randomized to combined STN+SNr stimulation first (Patients PD3, PD10 and PD11).
Figure 3.
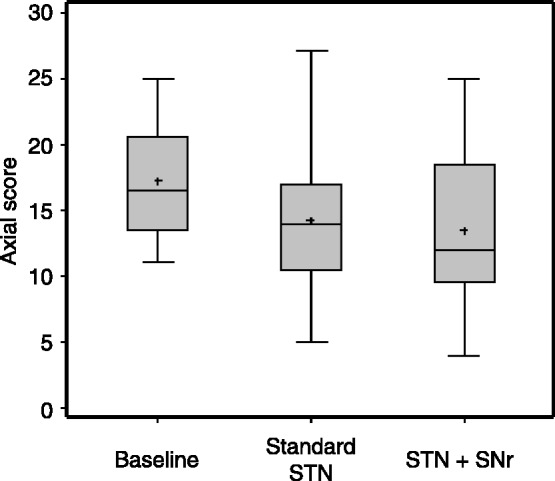
Primary endpoint at ‘3-week follow-up’. Results are given as box plots. x-axis: therapeutic condition; y-axis: axial score. [STN+SNr] = combined STN+SNr stimulation.
Secondary outcome measures: differentiation of distinct axial motor domains
For all secondary endpoints, no significant period effects were detected. In the ‘immediate testing' and at ‘3-week follow-up’' the segmental UPDRS III (items 20–26) was improved with both standard STN stimulation and combined STN+SNr stimulation compared with medication OFF stimulation OFF (baseline), as expected (Tables 2 and 3). At baseline, patients presented with severe axial motor symptoms according to the axial UPDRSIII (items 27–31) (11.17 ± 3.56). Greater improvement was observed in the ‘immediate testing’ with combined STN+SNr stimulation compared with standard STN stimulation on only active subthalamic contacts (8.17 ± 4.09 versus 9.25 ± 4.67; P = 0.041), however, no difference was found at the ‘3-week follow-up’ (8.08 ± 4.38 versus 8.08 ± 4.01; P > 0.99). Similarly, patients presented with severe freezing of gait at baseline according to the Freezing of Gait Assessment Course (22.17 ± 11.74). This improved more with combined STN+SNr stimulation compared with standard STN stimulation in the ‘immediate testing’ (8.67 ± 10.92 versus 16.25 ± 12.78; P = 0.006) and at the ‘3-week follow-up’ (8.33 ± 10.91 versus 14.42 ± 13.19; P = 0.047). Of note, freezing of gait presented with similar severity both at ‘immediate testing’ and at ‘3-week follow-up’ in both treatment conditions, although at ‘3-week follow-up’ patients were ON their regular dopaminergic medication unlike ‘immediate testing’ (Fig. 4). In the CAPSIT-PD timed walking test no relevant differences were observed between combined STN+SNr stimulation and standard STN stimulation in the number of steps and time. Freezing episodes occurred more frequently with standard STN stimulation compared with combined STN+SNr stimulation at ‘3-week follow-up’ (P = 0.063; Supplementary material) but not at ‘immediate testing’. In the Giladi Freezing of Gait Questionnaire, freezing of gait improved with combined STN+SNr stimulation compared with standard STN stimulation, although not significantly (14.50 ± 4.89 versus 16.17 ± 3.83; P = 0.1). No differences were observed in the Berg Balance Scale.
Figure 4.
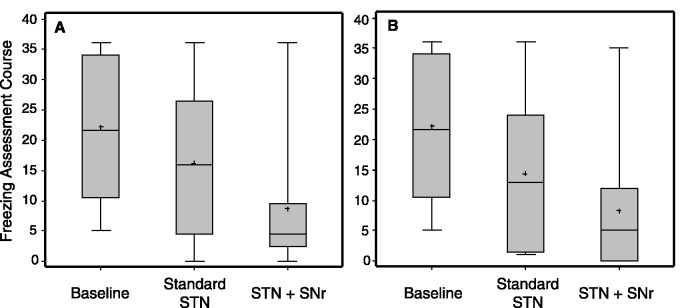
Secondary endpoint: results at (A) ‘immediate testing’ and at (B) ‘3-week follow-up’ are given for the Freezing of Gait Assessment Course. Results are given as box plots. x-axis: therapeutic condition; y-axis: score of the Freezing of Gait Assessment Course. [STN+SNr] = combined STN+SNr stimulation.
Ten of 12 patients wished to continue combined STN+SNr stimulation treatment at the end of the study.
Quality of life and non-motor issues
The PDQ-39 summary index was unchanged in both treatment arms. At baseline, patients presented with highest impairment of quality of life in the ‘mobility’ domain, as expected (53.96 ± 23.78). A slightly greater improvement was observed with combined STN+SNr stimulation compared with standard STN stimulation on ‘mobility’ (49.38 ± 25.30 versus 54.32 ± 27.23; not statistically significant) and ‘social support’ (11.81 ± 10.93 versus 18.94 ± 23.89; not statistically significant). No differences were identified in the distinct non-motor symptom domains.
Adverse events
In both treatment arms no serious adverse events were observed. Four patients wished to discontinue standard STN stimulation treatment prematurely. During combined STN+SNr stimulation active treatment, no acute side effects were observed, however, four adverse events were reported during the ‘3-week follow-up’. Two patients (Patients PD2 and PD9) reported delayed onset of dyskinesias within the first few days after introduction of combined STN+SNr stimulation, which completely resolved after therapy adjustment: in Patient PD2, stimulation amplitudes were lowered on the caudal contacts (−0.4V, both electrodes). Patient PD9 had already self-administered a reduction of the daily l-DOPA dosage by 125 mg when the patient informed the study site; this had already ameliorated the dyskinesias. The patient was rescheduled and as slight dyskinesias persisted, the SNr amplitudes were lowered by −0.1 V on both sides. After therapy adjustment both patients were followed for the complete ‘3-week follow-up’ period according to the intention-to-treat principle. One patient (Patient PD8) reported at the ‘3-week follow-up’ visit that a few intermittent episodes of double vision during combined STN+SNr stimulation treatment, each lasting for a few seconds, had occurred. Patient PD7 reported increased immobility and recurrent falls during the last week of follow-up under combined STN+SNr stimulation, whereas patient and caregiver consistently reported initial improvement of freezing of gait during the first 2 weeks.
Safety measures
No suicidality was reported. No change was found on the Beck’s Depression Scale Index on group level; Patient PD1 presented with increased Beck’s Depression Scale Index scores during combined STN+SNr stimulation compared to standard STN stimulation (18 versus 7). Patient PD7 reported visual ‘benign hallucinations with insight retained’ (UPDRS I, item 2) during combined STN+SNr stimulation consistent with a former personal history of hallucinations as documented in the preoperative records. UPDRS I, item 2 was unchanged at the group level between therapeutic conditions. No patient presented with psychosis. The comparison of standard STN stimulation and combined STN+SNr stimulation showed no differences between treatments on the Barrett Impulsivity Scale, on segmental motor symptoms (UPDRS III, items 20–26) and on motor fluctuations (UPDRS IV).
Discussion
In this randomized controlled phase II trial, intractable gait impairment as one of the major unmet needs in the treatment of advanced Parkinson’s disease was treated with interleaved pulses of STN and SNr for the first time. This trial particularly addressed the therapeutic response of a broad spectrum of axial motor symptoms and secondarily disentangled the efficacy on distinct axial subdomains. The broad-scaled primary endpoint revealed no significant improvement of axial motor functioning with combined STN+SNr stimulation compared with stimulation on only active subthalamic contacts. Similarly, as a secondary endpoint analysis, there was only a slight improvement of the clinical axial motor items (UPDRS III, items ‘27-31’) in the ‘immediate testing’ from combined STN+SNr stimulation compared with standard STN stimulation that did not present at the ‘3-week follow-up’. More specifically, we observed an improvement of freezing of gait on combined STN+SNr stimulation in the Freezing of Gait Assessment Course as secondary exploratory endpoint analysis, whereas postural control according to the Berg Balance Scale remained unchanged. This was in line with a (not statistically significant) five-point improvement in the mobility domain of the PDQ-39 with combined STN+SNr stimulation compared with standard STN stimulation. Although, no final conclusion can be drawn owing to the small sample size and exploratory nature of the secondary endpoints in this phase II trial, a difference of 3.2 points on the PDQ-39 ‘mobility’ subdomain was identified as meaningful to improve the patients’ subjective clinical impression in large Parkinson’s disease cohorts (Peto et al., 2001) and this may be verified in a larger follow-up trial. Of note, 4 of 12 patients discontinued standard STN stimulation treatment, three of them after switching from combined STN+SNr stimulation to the standard STN stimulation condition. Consistently, in all of these patients, the individual primary endpoint scores were superior with combined STN+SNr stimulation and 10 of 12 patients preferred to continue combined STN+SNr stimulation after completion of the study. Group-level data of the primary endpoint and anamnestic secondary outcome measures have to be interpreted with caution given the premature drop-outs in only the standard stimulation on only active subthalamic contacts treatment arm.
Generally, it should be noted that the variability within the endophenotypic spectrum of idiopathic Parkinson’s disease was also reflected by this study cohort and cannot be excluded. Even with genetic classifications, e.g. a spread in ‘age at disease onset’ and a variable disease progression was reported in LRRK2 mutation carriers (Schiesling et al., 2008). The clinical heterogeneity includes variable disease progression after STN–DBS with emerging axial symptoms resistant to standard therapy. However, regarding axial symptoms and cognitive decline, genetic biomarkers like the most common genetic susceptibility factor for Parkinson’s disease, i.e. heterozygous mutations in the glucocerebrosidase (GBA) gene, might help to predict the individual profile of disease progression more accurately in the future (Weiss et al., 2012b; Winder-Rhodes et al., 2013). In this context, another strength of this study was to identify patients with Parkinson’s disease with predominant freezing of gait as a future subgroup of interest for neuromodulation trials on the level of SNr. This is also important for related neurostimulation strategies on axial motor symptoms, as a heterogeneous spectrum of treatment response was observed in previous trials on pedunculopontine stimulation modulating balance or freezing of gait to a variable degree (Ferraye et al., 2010; Hamani et al., 2011). Therefore, the detailed phenotypic classification of patients with Parkinson’s disease according to the clinical criteria identified here may help to reduce the heterogeneity of study cohorts in future trials on gait impairment.
Concomittant stimulation of the SNr was safe and well-tolerated. Mild side-effects were delayed by a few days and were resolved completely. The motor response of segmental symptoms remained unchanged and, similarly, motor fluctuations remained well controlled. Therefore, as a major advantage of the concomittant nigral stimulation, the best individual subthalamic stimulation parameters can be maintained for reprogramming. Most importantly, SNr stimulation was safe on non-motor issues and major neuropsychiatric domains including depressive symptoms, impulsivity, suicidality and psychotic symptoms. Previously, acute depressive (Bejjani et al., 1999; Blomstedt et al., 2008) or hypomanic clinical states (Ulla et al., 2011) were described in few selected cases with high-frequency stimulation on SNr contacts, and similarly, mood changes were described to emerge basically on ‘ventral subthalamic’ contacts in the COMPARE trial (Okun et al., 2009). However, the incidence of neuropsychiatric interference from SNr stimulation in unselected DBS cohorts remains undetermined. Recognizing these previous findings, we carefully monitored for neuropsychiatric symptoms and found that nigral stimulation may be applied safely. Nevertheless, patients with subthalamic and nigral stimulation should be followed with caution for neuropsychiatric symptoms. Larger cohorts and longer follow-up ranges are needed to draw a final conclusion.
To interpret the results of this and related studies on freezing of gait in advanced Parkinson’s disease (Moreau et al., 2008, 2012; Chastan et al., 2009) and to strategize future directions of DBS for axial motor symptoms other aspects have to be considered: STN–DBS reprogramming (e.g. by modulating parameters to lower frequencies) was limited by recurrence of segmental symptoms (Moreau et al., 2008; Ricchi et al., 2012). This also applies when considering stimulation on a single SNr contact, which did not sufficiently control segmental motor symptoms (Chastan et al., 2009) and, therefore, was not considered in this trial. Similarly, one might argue that the intermediate ventral subthalamic contact might have been more efficacious; however, several previous findings argue against this: the progressive amplitude increase on a dorsolateral subthalamic contact is likely to activate the ventral portion of the subthalamic nucleus, although, this was associated with a disproportional decline of gait impairment including freezing of gait (Moreau et al., 2008). Consistently, no differential therapeutic response of gait or balance impairments was found with dorsal versus ventral subthalamic nucleus stimulation (McNeely et al., 2011). The present study characterized a novel target of interest for future neuromodulation trials. The dorsolateral part of the SNr (Fig. 2) with mainly GABAergic and cholinergic projection neurons mediated our findings. Whereas, at the level of the pedunculopontine nucleus stimulation at lower frequencies below 35 Hz (Stefani et al., 2007; Ferraye et al., 2010; Moro et al., 2010; Thevathasan et al., 2011a) and at 70 Hz in unilateral stimulation (Moro et al., 2010) was put forward for gait therapy, stimulation on high frequencies might be superior on the level of SNr in the light of previous converging experimental evidence: SNr demonstrated pathological overactivity in Parkinson’s disease (Breit et al., 2006) and high frequency stimulation may suppress SNr activity (Lafreniere-Roula et al., 2010). Similarly, pharmacological inhibition of SNr activity presented with ‘prokinetic’ effects and elicited dyskinesias (Dybdal et al., 2013) as similarly observed in two of our patients. Consistently, high-frequency SNr stimulation at 130 Hz (unlike 50 Hz) improved forelimb akinesia in a rat model of Parkinson’s disease (Sutton et al., 2013). GABAergic inhibitory output from the SNr to the pedunculopontine nucleus was demonstrated in animal research including experiments in rats (Childs and Gale, 1983; Grofova and Zhou, 1998), cat (Noda and Oka, 1986; Nakamura et al., 1989), and non-human primates (Carpenter et al., 1981). Given the efferent monosynaptic GABAergic transmission from SNr to the pedunculopontine nucleus (Nandi et al., 2008), high-frequency stimulation at the level of SNr might attenuate an overinhibitory drive.
A large body of clinical trials provides compelling evidence that axial impairment emerges along disease progression after primarily effective STN–DBS (Krack et al., 2003; St George et al., 2010; Castrioto et al., 2011; Nutt et al., 2011), however evidence-based data on how to treat these resistant symptoms is still limited. It has to be kept in mind that clinical trials generally select for cognitively competent Parkinson’s disease patients given that axial and cognitive impairments may demonstrate with coincidence. Whether the potential benefit from nigral stimulation applies to a larger proportion of patients with advanced Parkinson’s disease remains to be determined and this consideration may include patients with predominant preoperative freezing of gait that are often precluded from DBS treatments. Genetic predictors and endophenotypes might be defined to indicate optimal target selection (Weiss et al., 2012b).
This phase II trial opens the perspective that concomittant SNr stimulation might improve intractable freezing of gait. Field steering applications like interleaved programming can be utilized as a reprogramming option if patients develop resistant freezing of gait along disease progression. A larger randomized controlled phase III clinical trial to assess the efficacy of concomittant nigral stimulation on ‘freezing of gait’ and ‘quality of life’ is highly warranted.
Supplementary Material
Acknowledgements
We are grateful to Ruth Bösel and Dagmar Henke (Department of Biometry) for support in data management and statistical analyses. We wish to thank John Hammargren (Medtronic Inc., Minnesota, USA) for reconstruction of an exemplary image of volume of tissue activated by interleaved STN+SNr stimulation. We greatly acknowledge Dr. Robert Chen (MBBChir, MSc, FRCPC) for proof reading and the valuable comments on the manuscript (Toronto Western Hospital, Edmond J. Safra Program in Parkinson’s Disease, University Health Network, and the Division of Neurology, University of Toronto, Canada).
Glossary
Abbreviations
- STN–DBS
subthalamic nucleus deep brain stimulation
- SNr
substantia nigra pars reticulata
- UPDRS
Unified Parkinson’s Disease Rating Scale
Funding
The study was supported by a Research Grant (AKF 259-0-0) of the Eberhard Karls University Tübingen and by Medtronic Europe Sarl. Daniel Weiss is supported by a research grant of the German Research Council (DFG) WE5375/1-1 and was supported by a Research Grant of the Medical Faculty of the University of Tübingen (AKF 259-0-0). Daniel Weiss received speaker’s honoraria and a travel grant from Medtronic, Abott Pharmaceutical, UCB, and the Movement Disorder Society. Margarete Walach received a travel grant from GlaxoSmithKline. Christoph Meisner received reimbursement from the Department of Neurodegenerative Diseases as provided by Medtronic study support. Melanie Fritz received a travel grant from Ipsen Pharmaceuticals.
Christian Plewnia received resarch grants from the German Research Council (DFG; PL 525/1–1), the University of Tübingen (AKF #238-0-0) and the Werner Reichardt Centre for Integrative Neuroscience (CIN, PP2011_11). He received speaker’s honoraria by Inomed Medizintechnik GmbH. Sorin Breit was supported by a Research Grant of the Medical Faculty of the University of Tübingen (AKF 246-0-1). Benjamin Bender received a travel grant by Bayer Vital and was supported by research grants of the German Research Council (DFG) BE4609/1-1 and the Wilhelm-Schuler-Stiftung. Alireza Gharabaghi is supported by grants from the German Research Council [DFG GH 94/2-1, DFG EC 307], Federal Ministry for Education and Research [BFNT 01GQ0761, BMBF 16SV3783, BMBF 03160064B, BMBF V4UKF014, and European Union [ERC 2276329]. Alireza Gharabaghi received speaker’s honoraria and travel grants from Medtronic. Tobias Wächter: received speaker‘s honoraria and travel reimbursement for scientific meetings from Medtronic, Solvay, Abbott Pharma, Cephalon, Merz Pharmaceuticals, Ipsen Pharma and Schwarz Pharma. He has also received financial support for research from and conducted commissioned research for Medtronic, Abbott Pharma, Merz Pharmaceuticals, Ipsen Pharma and Pharm-Allergan and worked on advisory boards for Ipsen Pharma and Merz Pharmaceuticals. Rejko Krüger serves as Editor of European Journal of Clinical Investigation, Journal of Neural Transmission and Associate Editor of BMC Neurology; has received research grants of the German Research Council (DFG; KR2219/2-3 and KR2119/8-1), the Michael J Fox Foundation, the Fritz Thyssen foundation (10.11.2.153) and the Federal Ministry for Education and Research [BMBF, NGFNplus; 01GS08134], as well as speaker’s honoraria and/or travel grants from UCB Pharma, Cephalon, Abott Pharmaceutical, Takeda Pharmaceuticals and Medtronic.
Supplementary material
Supplementary material is available at Brain online.
References
- Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476–80. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol. 2001;54:343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83:S7–11. [PubMed] [Google Scholar]
- Blomstedt P, Hariz MI, Lees A, Silberstein P, Limousin P, Yelnik J, et al. Acute severe depression induced by intraoperative stimulation of the substantia nigra: a case report. Parkinsonism Relat Disord. 2008;14:253–6. doi: 10.1016/j.parkreldis.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Breit S, Lessmann L, Unterbrink D, Popa RC, Gasser T, Schulz JB. Lesion of the pedunculopontine nucleus reverses hyperactivity of the subthalamic nucleus and substantia nigra pars reticulata in a 6-hydroxydopamine rat model. Eur J Neurosci. 2006;24:2275–82. doi: 10.1111/j.1460-9568.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Carleton SC, Keller JT, Conte P. Connection of the subthalamic nucleus in the monkey. Brain Res. 1981;224:1–29. doi: 10.1016/0006-8993(81)91113-6. [DOI] [PubMed] [Google Scholar]
- Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. 2011;68:1550–6. doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- Chastan N, Westby GW, Yelnik J, Bardinet E, Do MC, Agid Y, et al. Effects of nigral stimulation on locomotion and postural stability in patients with Parkinson's disease. Brain. 2009;132:172–84. doi: 10.1093/brain/awn294. [DOI] [PubMed] [Google Scholar]
- Childs JA, Gale K. Neurochemical evidence for nigrotegmental GABAergic projection. Brain Res. 1983;258:109–114. doi: 10.1016/0006-8993(83)91233-7. [DOI] [PubMed] [Google Scholar]
- Cooper SE, McIntyre CC, Fernandez HH, Vitek JL. Association of deep brain stimulation washout effects with Parkinson disease duration. JAMA Neurol. 2013;70:95–9. doi: 10.1001/jamaneurol.2013.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Dybdal D, Forcelli PA, Dubach M, Oppedisano M, Holmes A, Malkova L, et al. Topography of dyskinesias and torticollis evoked by inhibition of substantia nigra pars reticulata. Mov Disord. 2013;28:460–8. doi: 10.1002/mds.25215. [DOI] [PubMed] [Google Scholar]
- Fasano A, Herzog J, Seifert E, Stolze H, Falk D, Reese R, et al. Modulation of gait coordination by subthalamic stimulation improves freezing of gait. Mov Disord. 2011;26:844–51. doi: 10.1002/mds.23583. [DOI] [PubMed] [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. 2010;133:205–14. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov Disord. 2009;24:655–61. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- Grofova I, Zhou M. Nigral innervation of cholinergic and glutamatergic cells in the rat mesopontine tegmentum: light and electron microscopic anterograde tracing and immunohistochemical studies. J Comp Neurol. 1998;395:359–79. [PubMed] [Google Scholar]
- Hamani C, Moro E, Lozano AM. The pedunculopontine nucleus as a target for deep brain stimulation. J Neural Transm. 2011;118:1461–8. doi: 10.1007/s00702-010-0547-8. [DOI] [PubMed] [Google Scholar]
- Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(Suppl 14):S290–304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- Kovacs N, Janszky J, Nagy F, Balas I. Changing to interleaving stimulation might improve dystonia in cases not responding to pallidal stimulation. Mov Disord. 2012;27:163–5. doi: 10.1002/mds.23962. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Kuriakose R, Saha U, Castillo G, Udupa K, Ni Z, Gunraj C, et al. The nature and time course of cortical activation following subthalamic stimulation in Parkinson's disease. Cereb Cortex. 2010;20:1926–36. doi: 10.1093/cercor/bhp269. [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula M, Kim E, Hutchison WD, Lozano AM, Hodaie M, Dostrovsky JO. High-frequency microstimulation in human globus pallidus and substantia nigra. Exp Brain Res. 2010;205:251–61. doi: 10.1007/s00221-010-2362-8. [DOI] [PubMed] [Google Scholar]
- McNeely ME, Hershey T, Campbell MC, Tabbal SD, Karimi M, Hartlein JM, et al. Effects of deep brain stimulation of dorsal versus ventral subthalamic nucleus regions on gait and balance in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2011;82:1250–5. doi: 10.1136/jnnp.2010.232900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C, Defebvre L, Destee A, Bleuse S, Clement F, Blatt JL, et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology. 2008;71:80–4. doi: 10.1212/01.wnl.0000303972.16279.46. [DOI] [PubMed] [Google Scholar]
- Moreau C, Delval A, Defebvre L, Dujardin K, Duhamel A, Petyt G, et al. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson's disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol. 2012;11:589–96. doi: 10.1016/S1474-4422(12)70106-0. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. 2010;133:215–24. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Tokuno H, Moriizumi T, Kitao Y, Kudo M. Monosynaptic nigral inputs to the pedunculopontine tegmental nucleus neurons which send their axons to the medial reticular formation in the medulla oblongata. An electron microscopic study in the cat. Neurosci Lett. 1989;103:145–50. doi: 10.1016/0304-3940(89)90566-1. [DOI] [PubMed] [Google Scholar]
- Nandi D, Jenkinson N, Stein J, Aziz T. The pedunculopontine nucleus in Parkinson's disease: primate studies. Br J Neurosurg. 2008;22(Suppl 1):S4–8. doi: 10.1080/02688690802448350. [DOI] [PubMed] [Google Scholar]
- Noda T, Oka H. Distribution and morphology of tegmental neurons receiving nigral inhibitory inputs in the cat: an intracellular HRP study. J Comp Neurol. 1986;244:254–66. doi: 10.1002/cne.902440211. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–44. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65:586–95. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ-39 Parkinson's disease questionnaire. Age Ageing. 2001;30:299–302. doi: 10.1093/ageing/30.4.299. [DOI] [PubMed] [Google Scholar]
- Potter M, Herzog J, Siebner HR, Kopper F, Steigerwald F, Deuschl G, et al. Subthalamic nucleus stimulation modulates audiospinal reactions in Parkinson disease. Neurology. 2008;70:1445–51. doi: 10.1212/01.wnl.0000310422.49977.ea. [DOI] [PubMed] [Google Scholar]
- Potter-Nerger M, Ilic TV, Siebner HR, Deuschl G, Volkmann J. Subthalamic nucleus stimulation restores corticospinal facilitation in Parkinson's disease. Mov Disord. 2008;23:2210–5. doi: 10.1002/mds.22284. [DOI] [PubMed] [Google Scholar]
- Ricchi V, Zibetti M, Angrisano S, Merola A, Arduino N, Artusi CA, et al. Transient effects of 80 Hz stimulation on gait in STN DBS treated PD patients: a 15 months follow-up study. Brain Stimul. 2012;5:388–92. doi: 10.1016/j.brs.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Salenius S, Avikainen S, Kaakkola S, Hari R, Brown P. Defective cortical drive to muscle in Parkinson's disease and its improvement with levodopa. Brain. 2002;125:491–500. doi: 10.1093/brain/awf042. [DOI] [PubMed] [Google Scholar]
- Schiesling C, Kieper N, Seidel K, Kruger R. Review: Familial Parkinson's disease—genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol Appl Neurobiol. 2008;34:255–71. doi: 10.1111/j.1365-2990.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013;368:610–22. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- St George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010;75:1292–9. doi: 10.1212/WNL.0b013e3181f61329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007;130:1596–607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Storch A, Odin P, Trender-Gerhard I, Fuchs G, Reifschneider G, Ray Chaudhuri K, et al. Non-motor Symptoms Questionnaire and Scale for Parkinson's disease. Cross-cultural adaptation into the German language [in German] Nervenarzt. 2010;81:980–5. doi: 10.1007/s00115-010-3010-z. [DOI] [PubMed] [Google Scholar]
- Sutton AC, Yu W, Calos ME, Smith AB, Ramirez-Zamora A, Molho ES, et al. Deep brain stimulation of the substantia nigra pars reticulata improves forelimb akinesia in the hemiparkinsonian rat. J Neurophysiol. 2013;109:363–74. doi: 10.1152/jn.00311.2012. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, et al. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery. 2011a;69:1248–53; discussion 1254. doi: 10.1227/NEU.0b013e31822b6f71. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Bogdanovic M, Coyne TJ, et al. A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain. 2011b;134:2085–95. doi: 10.1093/brain/awr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang EW, Hamani C, Moro E, Mazzella F, Poon YY, Lozano AM, et al. Involvement of the human pedunculopontine nucleus region in voluntary movements. Neurology. 2010;75:950–9. doi: 10.1212/WNL.0b013e3181f25b35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulla M, Thobois S, Llorca PM, Derost P, Lemaire JJ, Chereau-Boudet I, et al. Contact dependent reproducible hypomania induced by deep brain stimulation in Parkinson's disease: clinical, anatomical and functional imaging study. J Neurol Neurosurg Psychiatry. 2011;82:607–14. doi: 10.1136/jnnp.2009.199323. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Breit S, Hoppe J, Hauser AK, Freudenstein D, Kruger R, et al. Subthalamic nucleus stimulation restores the efferent cortical drive to muscle in parallel to functional motor improvement. Eur J Neurosci. 2012a;35:896–908. doi: 10.1111/j.1460-9568.2012.08014.x. [DOI] [PubMed] [Google Scholar]
- Weiss D, Breit S, Wachter T, Plewnia C, Gharabaghi A, Kruger R. Combined stimulation of the substantia nigra pars reticulata and the subthalamic nucleus is effective in hypokinetic gait disturbance in Parkinson's disease. J Neurol. 2011a;258:1183–5. doi: 10.1007/s00415-011-5906-3. [DOI] [PubMed] [Google Scholar]
- Weiss D, Brockmann K, Srulijes K, Meisner C, Klotz R, Reinbold S, et al. Long-term follow-up of subthalamic nucleus stimulation in glucocerebrosidase-associated Parkinson's disease. J Neurol. 2012b;259:1970–2. doi: 10.1007/s00415-012-6469-7. [DOI] [PubMed] [Google Scholar]
- Weiss D, Wachter T, Meisner C, Fritz M, Gharabaghi A, Plewnia C, et al. Combined STN/SNr-DBS for the treatment of refractory gait disturbances in Parkinson's disease: study protocol for a randomized controlled trial. Trials. 2011b;12:222. doi: 10.1186/1745-6215-12-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2012;109:276–81. doi: 10.3238/arztebl.2012.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, et al. Glucocerebrosidase mutations influence the natural history of Parkinson's disease in a community-based incident cohort. Brain. 2013;136:392–9. doi: 10.1093/brain/aws318. [DOI] [PubMed] [Google Scholar]
- Wojtecki L, Vesper J, Schnitzler A. Interleaving programming of subthalamic deep brain stimulation to reduce side effects with good motor outcome in a patient with Parkinson's disease. Parkinsonism Relat Disord. 2011;17:293–4. doi: 10.1016/j.parkreldis.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Ziegler K, Schroeteler F, Ceballos-Baumann AO, Fietzek UM. A new rating instrument to assess festination and freezing gait in Parkinsonian patients. Mov Disord. 2010;25:1012–8. doi: 10.1002/mds.22993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.