Abstract
Dystonia is a common movement disorder seen by neurologists in clinic. Genetic forms of the disease are important to recognize clinically and also provide valuable information about possible pathogenic mechanisms within the wider disorder. In the past few years, with the advent of new sequencing technologies, there has been a step change in the pace of discovery in the field of dystonia genetics. In just over a year, four new genes have been shown to cause primary dystonia (CIZ1, ANO3, TUBB4A and GNAL), PRRT2 has been identified as the cause of paroxysmal kinesigenic dystonia and other genes, such as SLC30A10 and ATP1A3, have been linked to more complicated forms of dystonia or new phenotypes. In this review, we provide an overview of the current state of knowledge regarding genetic forms of dystonia—related to both new and well-known genes alike—and incorporating genetic, clinical and molecular information. We discuss the mechanistic insights provided by the study of the genetic causes of dystonia and provide a helpful clinical algorithm to aid clinicians in correctly predicting the genetic basis of various forms of dystonia.
Keywords: dystonia, genetics, molecular mechanisms, clinical phenotype
Introduction
New ‘next generation’ sequencing technologies have massively increased the speed of genetic discoveries in recent years and this has made itself felt in the field of dystonia research as in many others. In just over a year, four new genes have been shown to cause primary dystonia (CIZ1, ANO3, TUBB4A and GNAL) (Charlesworth et al., 2012; Fuchs et al., 2012; Hersheson et al., 2012; Lohmann et al., 2012; Xiao et al., 2012); PRRT2 has been identified as the cause of paroxysmal kinesigenic dystonia, and other genes (Wang et al., 2011), such as SLC30A10 and ATP1A3, have been linked to more complicated forms of dystonia or new phenotypes (Heinzen et al., 2012; Quadri et al., 2012; Rosewich et al., 2012; Tuschl et al., 2012). The identification of some of these genes strengthens the evidence for long-suspected molecular culprits in the pathophysiology of dystonia (e.g. dopaminergic transmission and transcription abnormalities), whereas others have highlighted less commonly implicated mechanisms that can lead to the disease (e.g. ion channel, microtubular or synaptic dysfunction). In this review, we provide a comprehensive overview of the major forms of dystonia for which the genetic cause is now known, with a particular emphasis on the primary, paroxysmal and major ‘dystonia-plus’ syndromes. Genetic, clinical and, where possible, molecular information are given and a helpful clinical algorithm is provided to aid clinicians in correctly predicting the genetic basis of various forms of dystonia.
The dystonias are a heterogenous group of hyperkinetic movement disorders, characterized by involuntary sustained muscle contractions affecting one or more sites of the body, which lead to twisting and repetitive movements or abnormal postures of the affected body part. It is the third most common movement disorder worldwide (Fanh et al., 1988; Geyer and Bressman, 2006; Defazio et al., 2007; Breakefield et al., 2008). Approximately 70 000 people are affected by dystonia in the UK alone, including some 8000 children and adolescents (Paudel et al., 2012). Affected individuals can suffer considerable physical and psychosocial distress, which has been demonstrated to have a significant impact on their quality of life (Skogseid et al., 2007; Soeder et al., 2009; Zoons et al., 2012). The pathophysiology of the disorder is poorly understood at present. In all probability, it is heterogeneous and arises from a dysfunction of the various central neural circuits that control and coordinate voluntary movements, such as those found in the basal ganglia, the cerebellum, the sensorimotor cortex, and the interactions between these three regions of the brain (Fahn, 1988; Quartarone et al., 2008; Argyelan et al., 2009; Teo et al., 2009).
Classification of dystonia
The classification of the dystonia is complex and not entirely satisfactory. Several approaches or systems operate in parallel. Clinically, the dystonias are usually classified according to one of four major variables: (i) age of onset (early onset versus adult onset); (ii) distribution of affected body parts (focal, multifocal, segmental or generalized); (iii) the underlying cause (primary, secondary or heredodegenerative); or (iv) special clinical features (paroxysmal, exercise-induced, task-specific or DOPA-responsive) (Albanese et al., 2011). The current European Federation of Neurological Societies recommended classification scheme is based on this approach (Table 1) (Albanese et al., 2011).
Table 1.
Classification of dystonia, based on the European Federation of Neurological Societies current scheme
| By aetiology | |
| 1. Primary dystonia | |
| 1.1 Primary pure dystonia | Torsion dystonia is the only clinical sign (apart from tremor) and there is no identifiable exogenous cause or other inherited or degenerative disease |
| 1.2 Primary plus dystonia | Torsion dystonia is a prominent sign but is associated with another movement disorder, for example myoclonus or parkinsonism. There is no evidence of neurodegeneration. |
| 1.3 Primary paroxysmal dystonia | Torsion dystonia occurs in brief episodes with normalcy in between. Three main forms are known depending on the triggering factor. |
| 2. Heredodegenerative dystonia | Dystonia is a feature, among other neurological signs, of a heredodegenerative disorder, such as Wilson's disease |
| 3. Secondary dystonia | Dystonia is a symptom of an identified neurological condition, such as a focal brain lesion, exposure to drugs or chemicals, e.g. dystonia because of a brain tumour, off-period dystonia in Parkinson's disease. |
| By age at onset | |
| 1. Early onset (<30 years of age) | Usually starts in a leg or arm and frequently progresses to involve other limbs and the trunk |
| 2. Late onset | Usually starts in the neck (including the larynx), the cranial muscles or one arm. Tends to remain localized with restricted progression to adjacent muscles |
| By distribution of affected body parts | |
| 1. Focal | Single body region (e.g. writer’s cramp, blepharospasm) |
| 2. Segmental | Contiguous body regions (e.g. cranial and cervical, cervical and upper limb) |
| 3. Multifocal | Non-contiguous body regions (e.g. upper and lower limb, cranial and upper limb) |
| 4. Generalized | Both legs and at least one other body region (usually one or both arms) |
| 5. Hemidystonia | Half of the body (usually secondary to a lesion in the contralateral basal ganglia) |
From a genetic point of view, hereditary dystonia can be classified either by the gene causing the condition, where it is known, or by reference to one of the ever expanding list of dystonia loci, of which there are currently 23 (Table 2). The system of DYT loci is particularly unsatisfactory. The system was designed to indicate genomic regions that had been linked to a specific hereditary disorder, but where the actual causative gene was not yet known (Kramer et al., 1990). DYT loci were assigned in chronological order based on the appearance of reports in the medical literature. In theory, once the underlying genetic cause was known, the locus was supposed to be withdrawn and the disorder merged into the entry for the cloned gene. However, in practice, this has not happened and both clinicians and researchers alike tend to use dystonia loci and genes names interchangeably, e.g. DYT1/TOR1A or DYT6/THAP1. With time, several other problems have arisen. The designation of some loci has never been replicated and is of questionable significance (e.g. DYT7 or DYT13), whereas others are known to be the result of incorrect assignations due to erroneous linkage (e.g. DYT9, DYT14 and DYT19) (Wider et al., 2008; Weber et al., 2011). Some DYT loci do not even designate any chromosomal location, but are based solely on the observation of a few families with a similar phenotype or mode of inheritance (e.g. DYT2) (Khan et al., 2003; Zlotogora, 2004). More importantly, not all pathogenic mutations causing dystonia have been assigned to a DYT locus (e.g. mutations in SPR, CIZ1 or GNAL), whereas some syndromes with prominent dystonic components have been assigned to loci belonging to other movement disorders (e.g. PARK13 and PARK14) or vice versa (DYT3 and DYT12).
Table 2.
The current DYT loci with brief description of associated phenotype, gene of linkage interval (where known), mode of inheritance and OMIM reference numbers
| Locus Symbol | Phenotype | Gene or linkage (if known) | Mode of inheritence | OMIM |
|---|---|---|---|---|
| DYT1 | Early-onset primary torsion dystonia | TOR1A | AD | 605204 |
| DYT2 | Early-onset primary dystonia with prominent cranio-cervical involvement | Not known | AR | 224500 |
| DYT3 | Adult onset dystonia-parkinsonism, prevalent in the Philippines. | TAF1 | X-linked | 31420 |
| DYT4 | Whispering dystonia (adult onset spasmodic dysphonia) with generalization and ‘hobby horse’ gait | TUBB4A | AD | 128101 |
| DYT5a | Progressive DOPA-responsive dystonia with diurnal variation | GCH1 | AD | 128230 |
| DYT5b | Akinetic rigid syndrome with DOPA-responsive dystonia or complex encephalopathy | TH | AR | 191290 |
| DYT6 | Adult-onset torsion dystonia with prominent cranio-cervical and laryngeal involvement | THAP1 | AD | 602629 |
| DYT7 | Adult-onset primary cervical dystonia | 18 p | AD | 602124 |
| DYT8 | Paroxysmal non-kinesigenic dyskinesia | MR-1 | AD | 118800 |
| DYT10 | Paroxysmal kinesigenic dyskinesia | PRRT2 | AD | 128200 |
| DYT11 | Myoclonic dystonia (often with alcohol responsiveness) | SGCE | AD | 159900 |
| DYT12 | Rapid onset dystonia parkinsonism and alternating hemiplegia of childhood | ATP1A3 | AD (often de novo) | 128235 |
| DYT13 | Early onset torsion dystonia in one Italian family | 1p36.32-p36.13 | AD | 607671 |
| DYT15 | Myoclonic dystonia with alcohol responsiveness in one Canadian kindred | 18p11 | AD | 607488 |
| DYT16 | Early-onset dystonia-parkinsonism | PRKRA | AR | 612067 |
| DYT17 | Primary focal dystonia with progression in one Lebanese family | 20p11.2-q13.12 | AR | 612406 |
| DYT18 | Paroxysmal exercise-induced dyskinesia ± epilepsy | SLC2A1 | AD | 612126 |
| DYT20 | Paroxysmal non-kinesiogenic dyskinesia 2, in one large Canadian family | 2q31 | AD | 611147 |
| DYT21 | Adult-onset mixed dystonia with generalization in one Swedish family | 2q14.3-q21.3 | AD | 614588 |
| DYT22 | Reserved, but not published | ? | ? | ? |
| DYT23 | Autosomal dominant, often tremulous cranio-cervical dystonia ±upper limb tremor | ANO3 | AD | 610110 |
DYT9, DYT14, DYT19 are not included in the table as they are now known to be synonymous with DYT18, DYT5a, and DYT19 respectively. AD = autosomal dominant; AR = autosomal recessive.
Investigation of dystonia
The diagnosis of dystonia is, fundamentally, clinical. It relies on the presence of repetitive or sustained abnormal postures (with or without tremor) and the recognition of specific features, such as a geste antagoniste or overflow and mirror movements. Geste antagoniste refers to a voluntary manoeuvre (such as touching the face or an affected body part) that temporarily reduces the severity of dystonic posture or movements. An overflow movement is an unintentional muscle contraction that accompanies, but is anatomically distinct, from the primary dystonic movement, i.e. posturing of a hand normally unaffected by dystonia when performing tasks with the affected hand. Conversely, mirror movements are dystonic postures of a body part normally affected by dystonia when performing a motor task with a body part that is not affected by dystonia.
In general, for primary dystonia, few, if any, tests are required. The main exception to this rule is early-onset (<30 years of age) dystonia of unknown aetiology, which should always prompt consideration of a diagnosis of DOPA-responisve dystonia or Wilson’s disease, as accurate identification of these diseases at an early stage will permit the introduction of potentially life-changing treatments. Therefore, many would advocate, at the very least, a metabolic analysis that includes measurement of serum copper and caeruloplasmin and, possibly, a trial of l-DOPA in this group. In practice, an MRI and genetic testing for TOR1A and THAP1 mutations are often also performed. Finally, given the recent identification of a new form of treatable dystonia caused by brain manganese deposition secondary to mutations in SLC30A10 (Quadri et al., 2012; Tuschl et al., 2012), serum manganese measurement should at least be considered.
The features listed in Table 3 are those that might raise suspicion that the dystonia is not primary and trigger further investigation. The purpose of such investigations is to identify a secondary cause for the dystonia or to further elucidate the cause of dystonia presenting as part of a heredodegenerative condition. In practice, a combination of blood tests, structural imaging and selected secondary investigations are usually required to secure the diagnosis and Table 4 gives an indication of some of the investigations that may be appropriate given the aetiology under consideration. As regards neuroimaging, MRI is generally the modality of choice, although secondary CT may be required to accurately distinguish calcium from iron deposition in the basal ganglia. A dopamine transporter (DaT) scan may be useful to distinguish DOPA-responsive dystonia or rapid-onset dystonia-parkinsonism (where it will be normal) from other causes of parkinsonism with secondary dystonia (Romero-Lopez et al., 2008; Zanotti-Fregonara et al., 2008).
Table 3.
Features suggestive of non-primary dystonia
| Abnormal birth or perinatal history | |
| Dysmorphia | |
| Delayed developmental milestones | |
| Seizures | |
| Hemidystonia | |
| Sudden onset or rapidly progressive dystonia | |
| Prominent oro-bulbar dystonia | |
| The presence of another movement disorder (except tremor) | |
| Neurological signs suggesting involvement of other neurological systems (pyramidal signs, cerebellar signs, neuropathy, cognitive decline) | |
| Signs suggesting disease outside of the nervous system (hepatomegaly, splenomegaly) |
Some features that should raise suspicion that dystonia is secondary or heredodegenerative and trigger further investigation. It should be noted that some of these features are found in some types of primary dystonia, but their presence should nonetheless trigger careful consideration of a secondary dystonia or heredodegenerative disorder.
Table 4.
Investigations used in the diagnosis of secondary and heredodegenerative dystonia with example indication
| Investigation | Example indications |
|---|---|
| Blood | |
| Acanthocytes | Neuroacanthocytosis, neuronal brain iron accumulation |
| Alpha fetoprotein | Ataxia telangiectasia |
| Creatinine kinase | Neuroacanthocytosis |
| Copper and caeruloplasmin | Wilson’s disease, neuronal brain iron accumulation |
| Lactate and pyruvate | Mitochondrial disorders |
| Serum manganese | Dystonia with brain manganese deposition due to SLC30A10 mutation |
| Serum ferritin | Neuroferritinopathy |
| White cell enzymes | Lysosomal storage disorders |
| Urine | |
| Urinary amino acids | Aminoacidaemias |
| 24 h urinary copper | Wilson’s disease |
| Neuroimaging | |
| MRI | Most secondary causes, looking for structural lesions, iron/calcium deposition, caudate atropy, white matter abnormalities, etc. |
| Dopamine transporter (DaT) scan | Parkinsonism |
| Other | |
| Nerve condition tests | Spinocerebellar ataxia, neuroacanthocytosis, metachromatic leukodystrophy |
| Trial of l-DOPA | Early onset dystonia (<30 years of age) of unknown aetiology |
| Autonomic function tests | Multiple system atrophy |
| Slit-lamp examination | Wilson’s disease |
| Liver biopsy | Wilson’s disease |
| Muscle biopsy | Mitochondrial disorders |
| Electro-retinography | Neuronal brain iron accumulation |
| Genetic tests | See Table 7 for the genetic causes of heredodegenerative dystonias |
This list is not exhaustive.
Genetic burden in dystonia and genetic testing
Current evidence suggests that there is a significant genetic contribution to many forms of dystonia. Monogenic inheritance is most often seen in early-onset cases, where a family history can often be elicited. However, the reduced penetrance of some monogenic forms of dystonia, such as those due to mutations in TOR1A and THAP1, means that many apparently sporadic cases may also fall into this category. Furthermore, it is likely that a number of genes responsible for familial dystonia remain to be discovered, such that negative genetic testing for all currently known dystonia genes does not imply that the disorder is not genetic.
Late-onset dystonia, which represents by far the greatest number of cases, also appears to have a strong genetic basis. Studies based on the clinical examination of first-degree relatives of patients with focal dystonia have reported a risk of developing the same or another form of dystonia in the range of 23 to 36% (Waddy et al., 1991; Schmidt et al., 2009). Epidemiological studies have suggested that, although often apparently sporadic, adult onset dystonia may sometimes be inherited in an autosomal dominant manner, but with a marked penetrance of 12 to 15% (Waddy et al., 1991; Stojanovic et al., 1995; Leube et al., 1997b). Unfortunately, this presents significant challenges for gene discovery and, at present, the genetic architecture of late onset dystonia remains largely unknown. It is possible that the use of endophenotypes, based on imaging or neurophysiological testing, to identify non-penetrant mutation carriers will help by permitting accurate linkage and segregation analyses in some of the larger kindreds (Defazio et al., 2007; Kimmich et al., 2011). However, multigenic inheritance, resulting from the combination of two or more genetic changes, each imparting a low to moderately increased risk of developing dystonia and acting in combination with environmental factors, may also underlie a significant proportion of the apparent heritability of late-onset dystonia. Large-scale genome-wide association studies will be helpful in dissecting out the genetic contribution in these cases.
At the present time, genetic testing is most profitably employed in familial or early-onset cases, where it is likely to have the highest yield. Despite the fact that the establishment of a molecular diagnosis rarely alters management radically, it can be helpful for patients to understand the cause of their dystonia and also bring a halt to unnecessary continued investigation, as well as allowing the physician to impart accurate information regarding the risk of recurrence in subsequent generations. A scheme for deciding which genes may be appropriate to test in which patients is provided in Fig. 1.
Figure 1.
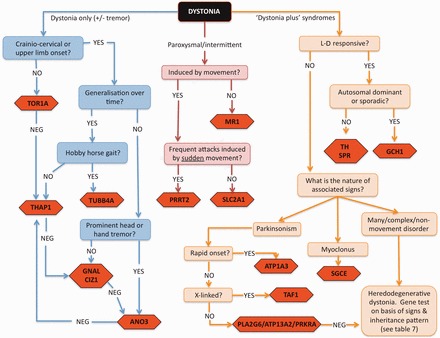
A workable strategy for identifying the likely genetic basis for some of the major forms of dystonia. Mutational screening for some genes is currently only available on a research basis.
Monogenic forms of dystonia
In the sections below, we present an overview of the major forms of dystonia exhibiting Mendelian inheritance for which an underlying genetic cause has been identified. Table 5 offers an overview of some of the key features of each of the various subtypes of primary dystonia where the causative gene is known.
Table 5.
Summary of the key clinical features of some of the major forms of primary dystonia by causative gene
| Gene | Typical age range for onset | Other notable non-dystonic signs | Typical distribution of dystonia (at onset) | Generalization or progression? | Clinical clues or other special features | |
|---|---|---|---|---|---|---|
| Primary pure | TOR1A (DYT1) | Childhood | – | Legs ≫ arms | Often Generalizes | Jewish ancestry; dystonia progressing to fixed deformity; laryngeal or cranial sparing |
| THAP1 (DYT6) | Adolescence to early adulthood | – | Laryngeal, cervical or brachial | Generalization common | Laryngeal or brachial onset with progression. | |
| CIZ1 | Adult | – | Cervical | No generalization | Only reported in pure focal cervical dystonia | |
| ANO3 (DYT23) | Adolescence to early adulthood | – | Craniocervical or brachial | No generalization | Prominent head, voice or arm tremor. | |
| TUBB4A (DYT4) | Adolescence to early adulthood | – | Laryngeal, craniocervical | Generalization observed | Ataxic, hobbyhorse gait; extrusional tongue dystonia; single family | |
| GNAL | Adolescence to mid-life | – | Craniocervical | Generalization observed | – | |
| Paraoxysmal | MR-1 (DYT8) | Childhood to adolescence | Choreatic dyskinesias; ballism | Limbs and face | No | Long (minutes to hours) attacks, triggered by alcohol, caffeine and emotional stress. |
| SLC2A1 (DYT18) | Childhood to adolescence | Epilepsy (see clinical clues also) | Legs | Progression to static dystonia can occur | Exercise or hunger induced. May be associated with other complex neurology: ataxia, spasticity etc, or haemolytic anaemia. | |
| PRRT2 (DYT10) | Childhood to adolescence | Choreatic dyskinesias; childhood epilepsy. | Limbs (generally the limb affected is the limb that is moving) | No | Frequent, brief (seconds to minutes) attacks triggered by movement. Family history of childhood epilepsy or hemiplegic migraine | |
| Dystonia-plus | GCH1 (DYT5a) | Childhood | Parkinsonism in some | Legs | Progressive, but often becomes static later. | Diurnal variation; dramatic response to l-DOPA; may mimic a spastic paraparesis. |
| TH (DYT5b) | Infancy to childhood | Progressive hypokinetic rigidity; complex encephalopathy in some; ‘lethargy-irritability’ crises. | Generalized but often with axial hypotonia. | Progressive without treatment | l-DOPA response related to phenotype: response tends to be less good in those with complex encephalopathy. | |
| SPR | Infancy to childhood | Many: motor/speech delay, axial hypotonia, parkinsonism, dysarthria, autonomic symptoms. Oculogyric crises are common. | Generalized but often with axial hypotonia. | Progressive without treatment | l-DOPA response related to phenotype: response tends to be less good in those with complex encephalopathy. 5-HTP may be a useful adjunct | |
| SGCE (DYT11) | Childhood | Myoclonus. Neuropsychiatric manifestations | Cervical | Dystonia usually remains segmental | Myoclonus usually more prominent than dystonia. Often alcohol responsive | |
| ATP1A3† (DYT12) | Adolescence to adulthood | Parkinsonism | Rostocaudal gradient | Rapid onset then stabilizes | Onset in context of febrile illness or other stressor. No response to l-DOPA | |
| PRKRA (DYT16) | Childhood | Variable parkinsonism | Any body part | Generalization | No response to l-DOPA |
Features described relate to typical cases and it must be borne in mind that there is often wide phenotypic variation between cases. † = clinical description restricted to rapid-onset dystonia-parkinsonism only (for alternating hemiplegia of childhood, see main text).
The primary pure dystonias
Primary pure dystonia is usually defined as a syndrome in which dystonia is the only clinical feature (except for tremor of the arms or head and neck) without any evidence of neurodegeneration or any obvious secondary cause (e.g. trauma, autoimmune, post-infectious etc.).
TOR1A mutations (DYT1/Oppenheim’s disease)
In 1911, Oppenheim proposed the term ‘dystonia musculorum deformans’ to describe a syndrome in children with twisting or jerking movements, muscular spasm, and gait abnormalities that often progressed to a fixed postural deformity. The hereditary nature of the condition was subsequently recognized and, in 1990, the disease was linked to chromosome 9q32-34 and designated with the first dystonia locus (DYT1) (Kramer et al., 1990). It was quickly realized that this locus was associated with a significant proportion of all childhood-onset dystonia, particularly in Ashkenazi Jews (Ozelius et al., 1992; Kramer et al., 1994). Seven years later the gene responsible for the condition was identified and named TOR1A (Ozelius et al., 1997). The TOR1A gene comprises five exons and is widely expressed. In almost all cases of DTY1-related dystonia, the underlying genetic mutation is an inframe GAG deletion located in exon 5 of the gene, resulting in the loss of a single glutamic acid in the final protein product (torsin A). This mutation has been shown to be responsible for ∼80% of all primary, early-onset dystonia in Ashkenazi Jewish populations and up to 50% of primary, early-onset dystonia in non-Jewish populations (Bressman, 2006; Jamora et al., 2006). Only two other missense variants (p.A288G and p.F205I) and one frameshift, 4-base pair deletion (c.934_937delAGAG) in this gene have since been reported to cause dystonia, though their pathogenicity is by no means certain (Kabakci et al., 2004; Zirn et al., 2008; Calakos et al., 2010).
The penetrance of the GAG deletion is notably low, with current estimates somewhere within the region of 30–40% (Kramer et al., 1994; Bressman, 2006). Moreover, even when the condition does manifest, the spectrum of severity of the symptoms is wide, ranging from mild focal dystonia to severe and disabling generalized dystonia. The exact mechanism underlying this variability remains unclear, but is presumed that other genetic or environmental modifiers must exist that influence the penetrance and presentation of the disease. To date, only one coding polymorphism in exon 4 of TOR1A (rs1801968), which encodes either aspartic acid (D) or histidine (H), has convincingly been shown to modify the risk of developing symptoms of dystonia. It appears that carrying the H allele in trans (that is, on the opposite allele from that harbouring the GAG deletion) may reduce the risk of developing symptoms of dystonia by 10-fold, to as little as 3% (Risch et al., 2007; Kamm et al., 2008a). Interestingly, in cellular models, the overexpression of TOR1A containing the H allele was sufficient by itself to induce formation of inclusions of torsin A in a manner similar to expression of GAG-deleted TOR1A, though at a much lower level (Kock et al., 2006). It is thought that this may be the result of a disruption of protein interactions induced by the presence of the exposed histidine residue. However, expression of GAG-deleted TOR1A containing the H allele in cis actually reduced the formation of inclusions, implying that these two changes act to cancel each other out to some degree (Kock et al., 2006). It has even been suggested that the GAG-deletion may need to be carried in conjunction with a D allele in cis to be penetrant (Risch et al., 2007), though this has not yet been proven. Yet, although the effect of the 216H coding polymorphism appears to be robust (in trans, at least), its relatively low population frequency (∼12% in Europeans and less in other populations) means it cannot fully explain the reduced penetrance or variable expressivity seen in this condition; other factors clearly remain to be identified.
In terms of non-genetic factors that might affect the penetrance of TOR1A mutations, one small study has assessed exposure to perinatal adversity, childhood infections, general anaesthesia and trauma in manifesting and non-manifesting carriers as well as non-carriers from a series of 28 families with DYT1-related dystonia by means of a retrospective questionnaire (Martino et al., 2012). Only self-reported perinatal adversity, in particular complications of vaginal delivery, showed a statistically significant (P = 0.02) positive correlation with manifestation of the disease.
Clinically, DYT1-related dystonia typically presents in childhood with dystonic posturing of the foot or leg, though it may begin in any part of the body, and evolves to generalized dystonia with fixed deformities. As mentioned above, however, late-onset or much milder forms of the disorder are recognized (Jamora et al., 2006). There is generally a family history consistent with autosomal dominant inheritance, albeit with reduced penetrance, but apparently sporadic early and late onset cases are also seen (Hjermind et al., 2002).
The TOR1A gene encodes a protein torsin A, which is a member of the AAA+ family of ATPases and is believed to be involved in maintenance of both structural integrity and/or normal function of protein processing and trafficking. Torsin A is expressed throughout the CNS in humans, but is found at particularly high levels in the dopaminergic neurons of substantia nigra pars compacta, locus coeruleus, Purkinje cells, cerebellar dentate nucleus, basis pontis, thalamus, hippocampal formation, oculomotor nuclei and frontal cortex (Shashidharan et al., 2000; Konakova et al., 2001; Rostasy et al., 2003). The exact mechanisms by which mutant torsin A causes dystonia are still unclear, but there is accumulating data to suggest that torsin A is important for cellular compartments and pathways, including the cytoskeleton, the nuclear envelope, the secretory pathway and the synaptic vesicle machinery (Goodchild and Dauer, 2005; Goodchild et al., 2005; Hewett et al., 2006, 2007; Henriksen et al., 2009; Kakazu et al., 2012).
THAP1 mutations (DYT6)
After the discovery of TOR1A and the subsequent uptake of genetic testing for mutations in this gene, it became clear that a second, distinct form of pure primary dystonia existed. Affected individuals, who were negative for TOR1A mutations, tended to have an older age at onset—in their adolescence or adulthood—and presented predominantly with cranio-cervical or focal dystonia affecting the upper limbs. In 1997, the DYT6 locus was assigned to chromosome 8p21-q22 by linkage analysis in two Mennonite families, but it was not until 2009 that the gene responsible for the condition was finally identified as THAP1 (thanatos associated protein domain containing, apoptosis associated protein 1) (Almasy et al., 1997; Fuchs et al., 2009). Missense, nonsense and frameshift mutations, spread throughout most of the coding portion of the gene, have all been associated with disease. Mutations in this gene have been detected in genetically diverse populations throughout the world and, unlike TOR1A, do not seem to be especially prevalent in any one particular population. Inheritance is autosomal dominant with a reduced penetrance. To date, penetrance has only been measured in Amish–Mennonite families, where it appears to be ∼60%, but this may not be true of all populations or mutations (Saunders-Pullman et al., 2007). Most mutations are found in the heterozygous state, but homozygotes are described (Fuchs et al., 2009; Houlden et al., 2010).
The clinical spectrum of THAP1 mutations is wide. Presentation with oromandibular, cranio-cervical or laryngeal dystonia is common, but presentations with focal dystonia of the limbs, segmental or generalized dystonia are all described in the literature. In a recent analysis by Xiromerisiou et al. (2012) of 100 patients reported to carry THAP1 mutations, the mean age at onset of dystonia was 24 years, 60% of patients were females and the distribution was generalized in 37%, segmental in 30%, multifocal in 6% and focal in 27%.
The THAP1 protein is an atypical zinc finger protein characterized by an N-terminal THAP domain, a proline-rich region and a C-terminal nuclear localization domain (Roussigne et al., 2003). The THAP domain is conserved in both vertebrates and invertebrates (Clouaire et al., 2005). It has DNA binding properties and is thought to be involved in the regulation of transcription, either on its own or in concert with other proteins. Thus, one possible mechanism by which mutations in THAP1 might cause dystonia is by dysregulated transcription of key genes. THAP1 protein is also known to interact with the prostate apoptosis response protein 4 (PAR-4), which is a transcription regulator involved in apoptosis (Roussigne et al., 2003). Under conditions of cellular stress or toxicity, this protein is rapidly upregulated and it has been linked to neurodegeneration in a number of disease models (Guo et al., 1998; Duan et al., 1999; Pedersen et al., 2000). One intriguing finding has been that THAP1 may regulate transcription of TOR1A by binding to one of two sites in its promoter region (Gavarini et al., 2010; Kaiser et al., 2010). Mutations in THAP1 have been shown to disrupt binding to the TOR1A promoter and decrease TOR1A-driven luciferase expression, thus suggesting that under some conditions TOR1A is negatively regulated by THAP1 protein and that mutations in THAP1 may lead to abnormally high levels of torsin A (Kaiser et al., 2010). Although these data come from experimental cell lines and thus are of uncertain physiological significance, this does at least raise the possibility that the disease mechanisms in DYT1 and DYT6 dystonia may be linked. However, if this is the case, the question arises as to how to link this mechanistically with the prevailing idea that the GAG-deletion in TOR1A leads to a loss of function of the protein (Goodchild et al., 2005). One possibility is that TOR1A expression must be maintained within a set range in relation to its binding partners and that dysfunction may result from either increased expression or loss of function (Bragg et al., 2011). Indeed, transgenic mice overexpressing wild-type TOR1A have been shown to display evidence of neurohistological, neurochemical and behavioural abnormalities, which may provide some support for this hypothesis (Grundmann et al., 2007).
ANO3 mutations (DYT23)
Recently, our laboratory employed a combination of exome sequencing and linkage analysis in moderately-sized, three generation Caucasian kindred with autosomal dominant cranio-cervical dystonia to identify a mutation in ANO3 (p.Arg494Trp) that segregated with the disease in the family (Charlesworth et al., 2012). Subsequent screening of this gene in 188 probands with cervical dystonia revealed five further novel variants that were not found in any databases of sequence variation, scattered throughout the gene, including one variant in its 5’ UTR. Segregation analysis was possible for two of these additional variants (p.Trp490Cys and p.Ser685Gly), which were seen to segregate with disease. The expression pattern of the gene was supportive of its role in dystonia, with expression being several folds higher in the striatum than anywhere else (Charlesworth et al., 2012).
Clinically, patients with mutations in this gene exhibited focal or segmental dystonia, variably affecting the cranio-cervical, laryngeal or brachial regions. There was often dystonic tremor with a jerky quality affecting the head, voice or upper limbs. The age at onset ranged from the very early childhood to 40 years old. Interestingly, despite prolonged follow-up and a gradual increase in severity, the dystonia was never seen to generalize in any affected individual, remaining confined to the head, neck and upper limbs. Some individuals manifested upper limb tremor alone and had been misclassified as essential tremor.
ANO3 encodes a protein called anoctamin 3, about which little is yet known. It belongs to a family of genes (ANO1–10) that appear to be closely related in sequence and topology, but with distinct expression patterns. Several of these genes have already been linked to various diseases, suggesting they play an important role within their tissue types (Duran and Hartzell, 2011). ANO1 and ANO2, the best studied members of the family, encode proteins that function as Ca2+-activated chloride channels and it is possible anoctamin 3 may function in the same manner (Caputo et al., 2008). Certainly, hydropathy analysis suggests a similar topology, with eight hydrophobic helices that are likely to be transmembrane domains and cytosolic N- and C-termini, but more recent work has suggested that anoctamin 3 may in fact be targeted to the endoplasmic reticulum, rather than the cell surface, like anoctamins 1 and 2 (Duran et al., 2012). Moreover, patient fibroblasts habouring the p.Trp490Cys mutation showed evidence of a potential defect in endoplasmic reticulum related Ca2+ handling. It is believed that Ca2+-activated chloride channels have a role to play in the modulation of neuronal excitability (Hartzell et al., 2005; Huang et al., 2012) and, in view of high expression of ANO3 in the striatum, it is possible that mutations in this gene lead to abnormal excitability of striatal neurons, which manifests itself clinically in unwanted dystonic movements, though further functional work will be required to test this hypothesis.
GNAL mutations
At the same time, mutations in another gene, GNAL, were identified as further cause of familial primary pure dystonia (Fuchs et al., 2012). Using whole exome sequencing and linkage in two unrelated families, two mutations in GNAL were initially identified, a nonsense mutation (p.Ser293*) resulting in a premature stop codon in one family and a missense mutation (p.Val137Met) in the other. Screening of 39 additional families identified six further novel mutations, with segregation confirmed in all families for which DNA from additional affected individuals was available.
Clinically, of the 28 individuals with dystonia resulting from a mutation in GNAL, 82% had onset in the region of the neck and 93% had cervical involvement when examined for the study. However, progression to other sites had occurred in at least half of those affected and generalized dystonia was seen in ∼10% of cases. Thus, the clinical phenotype in GNAL-related dystonia appears to be not dissimilar to that caused by mutations in THAP1. Though the authors noted that onset in GNAL mutations was never brachial, this may just be a chance finding given the small number of cases identified so far, rather than a true distinguishing factor.
GNAL encodes Gαolf, the alpha subunit of triheteromeric G protein Golf, which is involved in dopamine (D1) signalling. Experimental evidence exists to show that Gαolf is mostly responsible for the coupling of D1 receptors to adenylyl cyclase in striatal neurons and that Gαolf is required for D1-mediated behaviour and biochemical effects (Herve et al., 1993; Zhuang et al., 2000; Corvol et al., 2001). Because D1 dopamine receptors have a known role in mediating locomotor activity, the link between GNAL and dystonia is biologically plausible. The gene is located on the short arm of chromosome 18 in a region that has been linked not only to DYT7 dystonia (though the original DYT7 family do not appear to carry a mutation in GNAL), but also to bipolar disorder, schizophrenia and attention deficit hyperactivity disorder (Leube et al., 1997a; Berrettini, 2000; Segurado et al., 2003; Laurin et al., 2008; Winter et al., 2012). Interestingly, homozygous knockout mice for this gene are hyposmic and display hyperactive behaviours (Belluscio et al., 1998).
CIZ1 mutations
In early 2012, Xiao et al. (2012) reported that they had used a combination of linkage analysis and whole exome sequencing to identify a mutation in CIZ1 (p.Ser264Gly) as the likely causal variant in a large Caucasian kindred with primary cervical dystonia inherited as an autosomal dominant trait. Those affected in the family exhibited focal cervical dystonia, occasionally with mild tremor, having its onset in early adulthood to late midlife (18–66 years of age). However, affectation status was not always clear cut: five family members were said to have ‘definite’ cervical dystonia, whereas five family members were said to have ‘possible’ cervical dystonia, making linkage analysis difficult (Uitti and Maraganore, 1993).
CIZ1 encodes CDKN1A interacting zinc finger protein 1, a p21Cip1/Waf1-interacting zinc finger protein expressed in the brain and involved in DNA synthesis and cell-cycle control. Functional work suggests that the p.Ser264Gly mutation may alter splicing of the gene and normal subnuclear localization of CIZ1 protein (Xiao et al., 2012). Screening in a cohort of patients with adult-onset dystonia identified two additional missense mutations in three individuals (p.Pro47Ser and p.Arg672Met), all with focal cervical dystonia developing in mid-to-late life.
Some researchers feel it may be too early to place full confidence in the association of mutations in CIZ1 and focal cervical dystonia. Firstly, databases of normal sequence variation reveal that the CIZ1 gene contains a large number of missense variants in supposedly healthy individuals (a third of which are predicted to be pathogenic) and, in the study by Xiao et al. (2012) an equal number of novel missense variants were found in controls as were found in patients. Secondly, and more importantly, segregation was not demonstrated in a second independent family for any of the purported mutations. Thirdly, there is some question regarding the quality of the exome data used in this study: coverage was only >20 reads for ∼60% of the exome and the number of false-positive variants appears unusually high (Klein et al., 2012). Screening of this gene in further cohorts of cervical dystonia cases from various populations will be required to decide if these reservations are warranted or not.
TUBB4A mutations (DYT 4)
DYT4 was first described in 1985 by forensic psychiatrist Neville Parker in a large family with third decade onset of autosomal dominantly inherited ‘whispering dysphonia’ and generalized dystonia (Parker, 1985). Over 25 affected individuals have been reported, typically presenting with a laryngeal dysphonia progressing to a generalized dystonia and a peculiar ‘hobby horse’ gait (Wilcox et al., 2011). Alcohol responsiveness was not uncommon, leading to severe alcohol abuse in some DYT4 patients (Wilcox et al., 2011). The family is descended from an affected male who was born in 1801 in the small rural coastal town of Heacham in Norfolk and, to date, no other kindred has been described worldwide with a similar phenotype.
Using a combination of linkage analysis and exome sequencing, a mutation in the gene TUBB4A (p.Arg2Gly) has recently been identified as causal in the DYT4 kindred by two groups independently (Hersheson et al., 2012; Lohmann et al., 2012). TUBB4A encodes β-tubulin 4 a, a constituent of microtubules, and the mutation results in an arginine to glycine amino acid substitution in the key, highly conserved autoregulatory MREI (methionine–arginine–glutamic acid–isoleucine) domain of the protein. The gene is expressed throughout the brain, but at the highest levels in the cerebellum, which has been linked to the pathogenesis of dystonia (Hersheson et al., 2012). The MREI tetrapeptide sequence at the start of the N-terminal domain is known to be necessary for the autoregulation of the β-tubulin messenger RNA transcript and separate in vitro studies using site-directed mutagenesis have previously demonstrated that the p.Arg2Gly mutation abrogates this autoregulatory ability (Yen et al., 1988a, b). One further possibly pathogenic variant (p.Ala271Thr) was detected during the screening of a cohort of 394 unrelated dystonia patients: the individual concerned exhibited spasmodic dysphonia with oromandibular dystonia and dyskinesia with an age at onset of 60. Her mother had been similarly affected, but was now deceased so that segregation analysis was not possible, meaning the pathogenicity of the variant is uncertain (Lohmann et al., 2012).
The paroxysmal dystonias
The paroxysmal dystonias are characterized, in theory at least, by episodes of dystonia or other dyskinesia separated periods of neurological normality. In practice, there may be other associated neurological features besides the movement disorder and, especially in the case of SLC2A1-related disease, both the movement disorder and the other associated neurological symptoms may become relatively fixed with time.
MR-1 mutations (DYT8)
Symptoms of paroxysmal non-kinesigenic dyskinesia (PNKD) typically begin in childhood or adolescence and include dystonic and choreatic dyskinesias or ballistic movements, lasting from minutes to hours. There are often premonitory symptoms of an impending attack (paraesthesia or tension in the affected area) and sleep can prevent or abort attacks. The frequency of attacks varies between individuals, ranging from daily attacks to only a few in a lifetime. Typically, they are precipitated by alcohol, caffeine or stress, but less often by exercise, fatigue or cold (Bruno et al., 2007). Neurological examination is normal between attacks. Treatment is with carbamazepine or benezodiazepines, particularly clonazepam.
The condition results from mutations in the myofribillogenesis regulator gene (MR-1, also known as PNKD) and is inherited as an autosomal dominant trait. Only three mutations have been described to date, all clustered in the N-terminus of the protein: p.Ala7Val, p.Ala9Val and p.Ala33Pro (Lee et al., 2004; Rainier et al., 2004; Chen et al., 2005; Djarmati et al., 2005; Hempelmann et al., 2006; Ghezzi et al., 2009). Despite this, haplotype analysis has failed to reveal a common founder, suggesting that these represent independent mutational events (Chen et al., 2005). It is currently believed that this region of the protein may represent a mitochondrial targeting sequence (Ghezzi et al., 2009). Mutations may act by disrupting protein processing in vivo, a hypothesis that is supported by evidence from transgenic mice (Shen et al., 2011). The function of the MR-1 protein is not fully understood, but it shows close homology to glyoxalase hydroxyacylglutathione hydrolase, which is known to detoxify methylglyoxal, a compound found in coffee and alcohol and a by-product of glycolysis, thus providing a link to the standard triggers (Lee et al., 2004).
PRRT2 mutations (DYT10)
Mutations in PRRT2 cause paroxysmal kinesigenic dyskinesia (PKD), which has an estimated prevalence 1 in 150 000 individuals (Bruno et al., 2004). Affected individuals have short (seconds to minutes) and frequent (up to 100 times per day) attacks of dystonic or choreiform movements, precipitated by sudden movements or startle. As with paroxysmal non-kinesigenic dyskinesia, there is often warning of an impending attack—a so-called ‘aura’—consisting of numbness or paraesthesia in the affected body part. The attacks usually begin in childhood and are highly responsive to anticonvulsant therapy, such as carbamazepine (Silveira-Moriyama et al., 2013). Reports of what was, in reterospect, probably this disorder have appeared in the literature from as early as 1892 and the condition was designated dystonia 10 in 1998 (Smith and Heersema, 1941; Muller et al., 1998). However, the discovery of the gene underlying the condition, PRRT2, would wait until exome sequencing approaches could be applied to the problem (Chen et al., 2011; Wang et al., 2011).
Most mutations are truncating and by far the most common of these is the c.649dupC mutation, but missense variants (possibly with reduced penetrance) have also been described (Cao et al., 2012; Friedman et al., 2012a; Hedera et al., 2012; Steinlein et al., 2012). The PRRT2 protein is highly expressed in the CNS and is probably localized to the synapse (Lee et al., 2012). Using yeast 2-hybrid screening, it has been shown to interact with synaptosomal protein 25 (SNAP25), suggesting a role in the fusion of the synaptic vesicles to the plasma membrane (Stelzl et al., 2005). Both PRRT2 and SNAP25 are highly expressed in the basal ganglia and disrupted neurotransmitter release has been suggested as a possible pathogenic mechanism in PRRT2 mutations. Truncating mutations produce a protein lacking the transmembrane domain, and are thought to result in altered subcellular localization (Chen et al., 2011). Missense mutations may cause loss of function or act in a dominant-negative manner (Wang et al., 2011; Li et al., 2012).
PRRT2-related disease is notable for its varied presentation, differing not merely between individuals carrying different mutations, but also between individuals carrying the same mutation and even between affected members within the same family (Silveira-Moriyama et al., 2013). As well as the classical paroxysmal kinesigenic dyskinesia phenotype described above, infantile convulsions with choreoathetosis (ICCA) with or without paroxysmal kinesigenic dyskinesia, benign familial infantile seizures, episodic ataxia, hemiplegic migraine and even benign paroxysmal torticollis of infancy all appear to be possible manifestations of mutations in this gene (Dale et al., 2012; Gardiner et al., 2012; Heron et al., 2012; Lee et al., 2012). The common thread would seem to be the paroxysmal nature of all PRRT2-related disorders.
Finally, it would appear that the family used to define DYT19 also carry the common c.649dup mutation in PRRT2, suggesting that the initial linkage was incorrect (Gardiner et al., 2012).
SLC2A1 mutations (DYT18)
Dominantly inherited mutations in the SLC2A1 gene are now known to be the cause of paroxysmal exercise-induced dyskinesia (PED) (Suls et al., 2008; Weber et al., 2008). In fact, SLC2A1 mutations cause a wide spectrum of disease, resulting from a deficiency of the encoded glucose transporter type 1, which is still commonly referred to as GLUT1 despite the change in the recommended gene name. GLUT1 is the principal glucose transporter in the brain and it has generally been assumed that neuronal dysfunction arises from energy failure. Under this assumption, the prevalence of movement disorders was thought to reflect the heightened sensitivity of the basal ganglia to energy deficits (Perez-Duenas et al., 2009). However, recent studies in a mouse model of GLUT1-deficiency, which recaptures many of the features of the disease, demonstrated the preservation of aerobic metabolism, such that mechanisms other than impaired aerobic glycolysis must be responsible for the manifestations of the disorder (Marin-Valencia et al., 2012). Possible culprits include reduced transfer of lactate generated through anaerobic glycolysis from the astrocyte into the neuron and reduced anaerobic ATP formation in the astrocyte having a negative impact on glutamate-glutamine recycling, which, if proven, would place the emphasis on glial dysfunction in the pathogenesis of GLUT1-deficiency (Magistretti and Pellerin, 1996; Marin-Valencia et al., 2012).
At its most severe, GLUT1 deficiency can present with early onset psychomotor delay, drug resistant epilepsy, acquired microcephaly and other signs of widespread neurological dysfunction, such as spasticity, ataxia, tremor, dystonia, choreoathetosis and ballism, which may all be paroxysmal, static or static with paroxysmal worsening. From this extreme, there exists an entire spectrum of decreasing disease severity, ranging through developmental delay and movement disorders without epilepsy, right down to the mildest cases, in which only minimal phenotypic abnormalities are detectable (Brockmann, 2009; Leen et al., 2010). There can be considerable heterogeneity of clinical presentation even within the same family (Leen et al., 2010).
Paroxysmal exercise-induced dyskinesia is, therefore, probably best regarded as just one possible manifestation of the complex and variable disorder that is GLUT1-deficiency syndrome. It is characterized by attacks of combined chorea, athetosis, and dystonia precipitated by exercise—particularly brisk walking or running—that usually begin in childhood (Suls et al., 2008; Weber et al., 2008). The legs and feet are by far the most commonly affected body parts during attacks, which generally last from a few minutes to an hour (Bruno et al., 2004). Unlike paroxysmal kinesigenic dyskinesia or paroxysmal non-kinesigenic dyskinesia, affected individuals do not report aura-like symptoms before an attack. The symptoms tend to improve with intravenously administered glucose and with permanent ketogenic diet, though they can become static with time (Weber et al., 2008). Because paroxysmal exercise-induced dyskinesia is simply a manifestation of GLUT1 deficiency, it may occur alone in minimally affected patients or may be accompanied by various other manifestations of the disease, such as epilepsy, ataxia, haemolytic anaemia or spastic paraplegia. No doubt this wide variation in clinical presentation contributed to the erroneous assignation of DYT9 (paroxysmal choreoathetosis with spasticity) and DYT18 (PED with or without epilepsy) as two separate conditions; it is now known that the causative gene in both cases was, in fact, SLC2A1 (Weber et al., 2011).
Given the variable expressivity of SLC2A1 mutations, researchers have sought correlations between genotype and phenotype. It has been suggested that multiple exon deletion is associated with the early-onset, more severe form of the disorder, whereas missense mutations are associated with less severe cognitive deficits and a lower rate of movement disorders (Leen et al., 2010).
The dystonia-plus syndromes
The dystonia-plus syndromes represent a heterogeneous group of diseases, where dystonia is accompanied by other neurological features but is not part of a complex neurodegenerative disorder.
SGCE mutations (DYT11)
Mutations in SGCE cause myoclonus-dystonia, the commonest of the ‘dystonia-plus’ syndromes with a prevalence of about 2 per million in Europe (Asmus et al., 2007). The condition is inherited in an autosomal dominant fashion but with a significant role played by imprinting (see below). It usually begins in childhood, with a mean age of onset of ∼6 years, and is characterized by brief lightning-like myoclonic jerks, most often affecting the neck, trunk and upper limbs, in combination with focal or segmental dystonia in around two-thirds of patients (Asmus and Gasser, 2004). Myoclonus usually dominants the clinical picture and can be reliably provoked by complex motor activities, such as writing or copying a picture. There is often a dramatic improvement after alcohol, but this feature is not specific to the condition and is not present in all cases (Nardocci, 2011).
The SGCE gene encodes the 438 amino acid protein ε-sarcoglycan, which contains a single transmembrane domain. Despite its ubiquitous expression pattern and close homology to α-sarcoglycan (68%), mutations in SGCE do not lead to any detectable deficits in peripheral nerve or muscle function. Over 50 different mutations have been reported as causing myoclonus-dystonia, most of them located within the portion of the gene encoding the extracellular domain of the protein (Kinugawa et al., 2009). It is thought that mutations lead to mislocalization of the protein from the plasma membrane to the endoplasmic reticulum and to the promotion of its degradation by the proteasome (Esapa et al., 2007). Interestingly, in humans, transcription occurs almost exclusively from the paternal allele, whereas transcription from the maternal allele is silenced by promoter methylation (Grabowski et al., 2003). Because of this fact, 95% of those inheriting an SGCE mutation from their mother will not manifest any symptoms of the disease.
In patients exhibiting alcohol responsiveness, GABAergic drugs such as clonazepam or gamma-hydroxybutyrate often produce a pronounced but temporary improvement in the condition. However, their short duration of action can lead to overuse and addiction and this danger is compounded by apparently higher rates of psychiatric comorbidity—particularly obsessive–compulsive disorder, anxiety and alcohol dependency—in SGCE mutation carriers (Hess et al., 2007; Peall et al., 2013). The myoclonus seen in this condition is subcortical and does not respond to agents used to treat cortical myoclonus, such as valproate, levetiractem or piracetam (Roze et al., 2008). Levodopa responsiveness (of both the myoclonic and dystonic elements of the condition) has been reported in two cases and some have suggested a trial of levodopa is warranted (Luciano et al., 2009).
ATP1A3 mutations (DYT12)
Mutations in ATP1A3 are primarily associated with rapid onset dystonia-parkinsonism. Familial cases show autosomal dominant inheritance with reduced penetrance (∼90%), but de novo mutations are common so that the absence of a family history should not preclude consideration of the condition (de Carvalho Aguiar et al., 2004). Most mutations are missense, affecting highly conserved transmembrane or N-terminus domains. The mechanism by which such mutations actually cause rapid onset dystonia parkinsonism is not fully understood. ATP1A3 encodes the catalytic unit of a sodium pump that uses ATP hydrolysis to exchange Na+ and K+ across the cell membrane, thus maintaining the ionic gradients important for electrical excitability, neurotransmitter transport, volume regulation and other key cellular functions. Studies using cells transfected with mutant ATP1A3 have suggested mutations may reduce affinity for Na+ and that the resultant dysfunction in ion transport may impair cell viability, but the relative importance of these mechanisms to the actual pathophysiology of rapid onset dystonia-parkinsonism remains uncertain (Rodacker et al., 2006; Blanco-Arias et al., 2009).
Clinically, the condition is characterized by the onset, over minutes to days, of dystonia and parkinsonism, usually in the context of specific physical or psychological stressors, particularly febrile illness. After this period of rapid development of symptoms, the condition usually stabilizes and continued progression has only been reported once (Brashear et al., 2007). There is often a rostrocaudal gradient of symptoms. Despite clinical parkinsonism, dopamine transporter PET and single-photon emission computed tomography studies reveal normal dopamine uptake (Brashear et al., 1999; Zanotti-Fregonara et al., 2008) and, unfortunately, patients with rapid-onset dystonia parkinsonism do not respond to levodopa or pallidal deep brain stimulation (Deutschlander et al., 2005; Kamm et al., 2008b).
Interestingly, de novo heterozygous mutations in ATP1A3 also appear to underlie a rare neuropaediatric condition termed alternating hemiplegia of childhood (AHC) (Heinzen et al., 2012; Rosewich et al., 2012). Alternating hemiplegia of childhood is characterized by the onset before the age of 18 months of paroxysmal neurological events, such as hemiplegia alternating in laterality, quadriplegia, dystonic spells, oculomotor abnormalities and autonomic dysfunction, all of which abate during sleep. Non-paroxysmal manifestations develop after a few months or years of the disease, comprising developmental delay, intellectual disability of variable degree, ataxia, dysarthria, choreoathetosis, and, in some patients, pyramidal tract signs. In one study, a mutation in ATP1A3 was demonstrated in all 24 patients with alternating hemiplegia of childhood (Rosewich et al., 2012). A second study found ATP1A3 mutations is just under three-quarters of their cases (Heinzen et al., 2012). One mutation, p.Asp801Asn, was found 30–40% of all cases, suggesting it is a mutational hot spot.
PRKRA mutations (DYT16)
Mutations in the gene PRKRA appear to be a rare cause of autosomal recessive dystonia-parkinsonism. Camargos et al. (2008) identified two apparently unrelated consanguineous Brazilian families with a total of six individuals exhibiting young-onset, generalized dystonia or dystonia-parkinsonism. Autozygosity mapping revealed a shared segment of homozygosity segregating with the disease and subsequent mutational screening of the region identified a homozygous missense mutation (p.Pro222Lys) in all six individuals (Camargos et al., 2008). PRKRA encodes protein kinase, interferon-inducible double-stranded RNA-dependent activator, which, in response to extracellular stress activates the latent protein kinase PKR, a protein involved in signal transduction, cell differentiation, cell proliferation, antiviral response and apoptosis (Patel et al., 2000). The Pro222Lys mutation alters a conserved amino acid between the second and third RNA-binding motifs, but the manner by which it causes disease is remains elusive. As with patients carrying mutations in ATP1A3, response to standard medical therapy was minimal or absent.
DOPA-responsive dystonia
Technically, the DOPA-responsive dystonias fall within the category of the ‘dystonia-plus’ syndromes, but they have been separated out in this discussion because they are bound together aetiologically by their connection to the endogenous pathway for the synthesis of dopamine, which accounts for their shared clinic characteristic of an improvement in symptoms in response to treatment with oral l-DOPA. To date, three genes have been convincingly shown to cause DOPA-responsive dystonia: GCH1 (GTP cyclohydrolase 1), TH (tyrosine hydroxylase) and SPR (sepiapterin reductase) (Ichinose et al., 1994; Brautigam et al., 1998; Bonafe et al., 2001). All three genes encode enzymes required for the biosynthesis of dopamine (Fig. 2).
Figure 2.
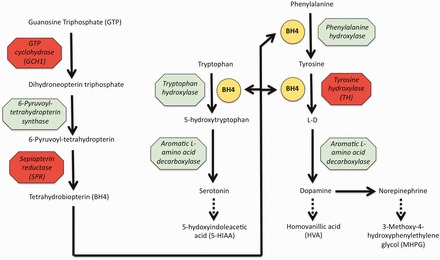
Schematic illustration of the pathways for the synthesis of catecholamines and serotonin. Breakdown products are indicated by broken arrows. Enzymes in which defects cause DOPA-responsive dystonia are shown in red with the gene symbol in brackets. Tetrahydrobiopterin is a key cofactor in some enzymatic reactions.
GCH1 mutations (DYT5a/Segawa’s disease)
Mutations in this gene account for ∼60–80% of autosomal dominant DOPA-responsive dystonia (Furukawa, 2004). Penetrance is incomplete and lower for males (∼40–50%) than females (∼80%) (Wider et al., 2008). GCH1 encodes the enzyme GTP cyclohydrolase 1, which catalyzes the first step in the reaction of tetrahydrobiopterin neo-synthesis from GTP. Tetrahydrobiopterin is an essential cofactor for tyrosine hydroxylase, the rate-limiting enzyme for catecholamine synthesis, as well as for tryptophan hydroxylase and phenylalanine hydroxylase, which are involved in the production of serotonin. GCH1 mutations act in a dominant negative manner, with measurable enzyme activity usually being <20% of normal, resulting in a relative deficiency of dopamine and serotonin (Ichinose and Nagatsu, 1997; Hwu et al., 2000). Diagnosis can be confirmed on the basis of reduced CSF levels of total biopterin (of which tetrahydrobiopterin is the main component) and neopterin (a product of the reaction involving GCH1) (Table 6) or an abnormal phenylalanine loading test (Bandmann et al., 2003; Furukawa, 2003).
Table 6.
CSF neurotransmitter metabolite profiles in DOPA-responisve dystonia secondary to GCH1, TH and SPR mutations
| CSF metabolite | GCH1 | TH | SPR |
|---|---|---|---|
| Biopterin | Decreased | Normal | Normal to slightly raised |
| Neopterin | Decreased | Normal | Increased |
| Homovanillic acid | Normal to mildly decreased | Decreased | Very low |
| 5-HIAA | Normal to mildly decreased | Normal | Very low |
| MHPG | Normal to mildy decreased | Decreased | Decreased |
5-HIAA = 5-hydroxyindoleactetic acid; MHPG = 3-methoxy-4-hydroxy-phenylethylene glycol.
Clinical presentation is typically with lower limb dystonia and gait disturbance in the first decade of life, which occasionally leads to misdiagnosis as cerebral palsy or spastic paraparesis (Nygaard et al., 1990). Diurnal fluctuation in the severity of the dystonia is common, though this may abate with age. With time there is usually gradual generalization, and parkinsonism and dystonic tremor are possible complications of the condition (Segawa et al., 2003; Cobb et al., 2009). In a subgroup of patients, the dystonia is mild, progresses only very slowly and may require no treatment (Trender-Gerhard et al., 2009). Infrequently, patients may present later in life with a dystonic tremor or akinetic-rigid parkinsonism (Tassin et al., 2000; Trender-Gerhard et al., 2009). There is some evidence that subtle neuropsychiatric features may be associated with mutations in this gene. In one recent study of 18 patients, major depressive and sleep disorders occurred in about half of those over 20 years of age, whereas obsessive–compulsive disorder was found in 25% of cases (Van Hove et al., 2006). In terms of treatment, the response even to low doses of l-DOPA is generally excellent (70–100% improvement in clinical symptoms), sustained and is not generally associated with the late-onset dyskinesias that often accompany prolonged use of l-DOPA in other conditions, such as Parkinson’s disease (Clot et al., 2009).
It should be noted that a previously-proposed novel form of DOPA-responsive dystonia, DYT14, is now known to be synonymous with DYT5a. Misclassification of one patient from the ‘DYT14’ family had led to incorrect locus assignment on the basis of erroneous linkage. After removal of this patient, the linkage peak now included the GCH1 gene, which was found to habour a causative deletion (Wider et al., 2008).
Finally, autosomal recessive mutations in GCH1 have also been reported, resulting in no detectable enzyme activity in the liver. As might be expected the phenotype is generally distinct and much more severe, with complex neurological dysfunction, including developmental delay, spasticity, seizures and physiological hyperphenylalaninaemia, which is generally picked up on routine screening of newborn infants (Ichinose et al., 1995). However, atypical presentations have been described with DOPA-responsive dystonia (Hwu et al., 1999), with a neonatal DOPA-responsive extrapyramidal syndrome (Nardocci et al., 2003) or without hyperphenylaninaemia (Horvath et al., 2008; Opladen et al., 2011).
TH mutations (DYT5b)
Rarely, DOPA-responsive dystonia can also be inherited as an autosomal recessive disorder associated with mutations in the gene encoding tyrosine hydroxylase itself (Ludecke et al., 1996). It is sometimes referred to as DYT5b. Only ∼40 cases have been reported worldwide but with over 30 different pathogenic mutations, scattered throughout the length of the gene, including its promoter regions (Willemsen et al., 2010).
As shown in Fig. 2, tyrosine hydroxylase is involved in the conversion of phenylalanine to dopamine, but it is also the rate-limiting enzyme in the synthesis of other catecholamines, such as norepinephrine and epinephrine. The enzyme thus plays a key role in normal functioning of both dopaminergic and noradrenergic neuronal populations and, indeed, complete loss of tyrosine hydroxylase activity appears to be lethal in knock-out mice (Zhou et al., 1995). Patients with biallelic mutations in this gene tend to present with a more severe disease than is seen in those carrying heterozygous GCH1 mutations, which presumably reflects the more profound deficiency of dopamine and norephinephrine. Two major phenotypes can be discerned: (i) a progressive hypokinetic-rigid syndrome with dystonia beginning in the first year of life and significant improvement in response to l-DOPA; and (ii) a more complex encephalopathy, associated with variable degrees of hypokinesia, bradykinesia, hypotonia, dystonia, myoclonus, ptosis and eye movement abnormalities that has its onset in the first few months of life, is often poorly responsive to l-DOPA and carries a far worse long term prognosis (Clot et al., 2009; Willemsen et al., 2010). In the latter subtype, autonomic functions are often disturbed leading to so-called ‘lethargy-irritability crises’, consisting of excessive drooling, sweating, body temperature instability and marked periods of ‘pyrexia of unknown origin’ (Willemsen et al., 2010).
In keeping with the underlying biochemical defects, CSF levels of homovanillic acid and 3-methoxy-4-hydroxyphenylethylene glycol (MHPG), the breakdown products of dopamine and norepinephrine, respectively, are found to be low. CSF levels of biopterin and 5-hydroxyindoleacetic acid are normal, however, reflecting the fact that the synthesis of tetrahydrobiopterin and serotonin is unaffected by mutations in the tyrosine hydroxylase gene (Table 6 and Fig. 2).
SPR mutations
Mutations in the gene SPR result in a second form of autosomal recessive DOPA-responsive dystonia. Deficiency of the encoded enzyme, sepiapterin reductase, results in neurologic deterioration due to severe catecholamine and serotonin deficiencies in the CNS, caused by the defect in tetrahydrobiopterin synthesis. As well as being a cofactor for tyrosine hydroxylase, tetrahydrobiopterin is also a required cofactor for phenylalanine hydroxylase, but patients with sepiapterin reductase deficiency do not exhibit overt hyperphenylalaninaemia as is seen in other autosomal recessive disorders of tetrahydrobiopterin synthesis as alternative enzymes are able to replace sepiapterin reductase in peripheral tissues and can thus compensate to some degree. In the brain, however, the low activity of dihydrofolate reductase means that an alternate pathway for tetrahydrobiopterin synthesis cannot complete and an intermediate, dihydrobiopterin, accumulates instead (Blau et al., 2001). The accumulation of dihydrobiopterin is probably of pathological significance since it is a competitive inhibitor of both tyrosine and tryptophan hydroxylase, thus further compounding the deficits in catecholamine and serotonin production caused by the subnormal tetrahydrobiopterin concentrations. Furthemore, by displacing prebound tetrahydrobiopterin, it has been suggested that dihydrobiopterin may uncouple nitric oxide synthase and cause the release of potentially apoptotic levels of superoxide and peroxynitrite, resulting in neuronal cell death (Blau et al., 2001; Jones et al., 2001).
The complex biochemical abnormalities described above are reflected in the CSF metabolite measurements, which show severe decreases in homovanillic acid and 5-hydroxyindoleacetic acid in the context of a normal neopterin and a normal or slightly raised total biopterin (Table 6). The neurological phenotype of SPR deficiency is quite variable, with milder cases being particularly susceptible to misdiagnosis as cerebral palsy (Friedman et al., 2012b). Most affected individuals will display motor and speech delay, axial hypotonia, dystonia, weakness and oculogyric crises with diurnal variation and sleep benefit, whereas dysarthria, parkinsonian features, hyperreflexia, behavioural abnormalities, mental retardation, and autonomic signs are not uncommon accompaniments (Bonafe et al., 2001; Friedman et al., 2012b). Many patients will show considerable benefit from treatment with low-dose l-DOPA, with most improvement being observed in motor and sleep symptoms. Significant cognitive problems may remain, however, and some patients have shown further benefit from the addition of 5-hydroxytryptophan, a serotonin precursor, leading to the suggestion that it should be trialled in all patients without complete symptom resolution on l-DOPA alone (Friedman et al., 2012b).
Mechanistic insights from the monogenic primary dystonias
As with other disorders, it is believed that an understanding of the genetic architecture of dystonia offers that best starting point for the complex task of unravelling the molecular pathophysiology of the disorder. Identification of genes involved in rarer, Mendelian forms of dystonia, along with a knowledge of their cellular function, allows us to identify key molecular pathways, which, when compromised, might contribute to the onset of dystonia. Since primary dystonia seems to result from a chronic cellular dysfunction, rather than a degenerative process, there are greater grounds for the belief that it may eventually be possible to ameliorate the disease by correcting the underlying dysfunction in these pathways. With this in mind, what do the functions of the causative genes identified so far tell us about the pathophysiology of primary dystonia?
One inescapable observation, evident from even a cursory look at the genes mentioned in the preceding sections, is the apparent range of cellular pathways implicated in the pathogenesis of dystonia. This suggests that the phenotypic heterogeneity of the dystonias is matched by a similar heterogeneity of pathological mechanism and that dysfunction in distinct pathways or cellular components is capable of resulting in the common clinical manifestations of the disease. Nonetheless, several themes can be discerned on closer examination. Firstly, and perhaps unsurprisingly, dopamine signalling problems are clearly an important pathogenic mechanism in dystonia. All three of the genes involved in DOPA-responsive dystonia (GCH1, TH and SPR) are directly involved in the dopamine synthesis pathway (Fig. 2); GNAL encodes the alpha subunit of G protein that directly couples to D1 receptors; and knock-in mouse models of the GAG deletion in TOR1A have been shown to exhibit neurochemical and structural changes in the dopamine pathways of the brain (Song et al., 2012). Regulation of gene expression and cell cycle dynamics also appear to be important: the protein products of THAP1 and TAF3 are both transcription factors, whereas that of CIZ1 is a DNA replication factor that acts to transform nuclei poised to begin DNA synthesis into nuclei actively synthesizing DNA (Coverley et al., 2005). With the recent identification of TUBB4A as the cause of whispering dysphonia, the function of several genes now point to cell structure as playing an important role in dystonia. TUBB4A encodes tubulin, the major component of the cytoskeleton; torsin A appears to be important for the structure of the nuclear envelope where it may form a bridge complex between it and the cytoskeleton (Atai et al., 2012) and also interacts with vimentin, a type III intermediate filament important for motility, chemotaxis, adhesion, intracellular signalling and neurite outgrowth (Hewett et al., 2006); and SGCE, a gene linked to myoclonus-dystonia, encodes a member of the dystrophin-glycoprotein complex that connects the cytoskeleton to the extracellular matrix in muscle and may play a role in synaptic organization in the CNS (Anastasi et al., 2012). Synaptic dysfunction is also highlighted by the recent identification of PRRT2, the protein product of which interacts with the SNARE protein, SNAP25 (Lee et al., 2012), in addition to evidence suggesting torsin A may regulate vesicular traffic and, by extension, neurotransmitter turnover (Warner et al., 2010). Finally, the identification of mutations in ANO3, which encodes a Ca2+-gated chloride channel predominantly expressed in the striatum, as a cause of craniocervical dystonia suggests that altered neuronal excitability due to ion channel pathology could also result in dystonia and raises the possibility that medication aimed at correcting the kinetics of the channel might be an effective treatment in a subset of patients. ATP1A3, the cause of rapid onset dystonia-parkinsonism and alternating hemiplegia of childhood, encodes the catalytic subunit of a Na+/K+ pump that is expressed in neurons and cardiac cells and that is involved in maintaining ionic gradients (de Carvalho Aguiar et al., 2004).
The heredodegenerative dystonias
This category comprises a large number of complex neurological disorders of which dystonia can sometimes be a significant feature. However, it is important to recognize that dystonia may not be present in all cases or, more often still, may not be the dominant neurological sign within the clinical picture. Many of the conditions have a known genetic cause, but a full exposition of their clinical features and molecular basis is beyond the scope of this review. Instead, they are summarized in Table 7 by mode of inheritance.
Table 7.
The major forms of heredodegenerative dystonia where a genetic cause has been identified, arranged by mode of inheritance
| Disease | Gene | Major features besides dystonia |
|---|---|---|
| Autosomal dominant | ||
| Dentatorubral-pallidoluysian atrophy | ATN1 | Ataxia, chorea and cognitive decline. |
| Huntington’s disease | IT-15 | Chorea, depression and cognitive decline. |
| Huntington’s disease-like 2 | JPH3 | Chorea, parkinsonism, ataxia and cognitive decline |
| Neuroferritinopathy | FTL | Chorea and parkinsonism. |
| SCA 2 | ATXN2 | Ataxia, ocular movement disorders, spasticity and parkinsonism |
| SCA 3 | ATXN3 | Ataxia, spasticity, parkinsonism and ocular movement disorders. |
| SCA 7 | ATXN7 | Ataxia, pigmentary macular degeneration, brisk reflexes |
| SCA 17 | TBP | Chorea, parkinsonism, ataxia and cognitive decline |
| Autosomal recessive | ||
| Ataxia-telangectasia | ATM | Ataxia, oculomotor apraxia, telangiectasia, susceptibility to malignancy. |
| Choreoacanthocytosis | VPS13A | Chorea, orofacial dyskinesias, cognitive decline, axonal neuropathy |
| Ceroid-lipofuscinosis | Multiple genes | Visual failure, cerebral atrophy and seizures |
| Dystonia with brain manganese deposition | SLC30A10 | Hepatic cirrhosis, polycythemia and hypermanganesaemia. |
| Fucosidosis | FUCA1 | Mental retardation, growth retardation, dysostosis multiplex, angiokeratoma |
| FBX07-associated neurodegeneration | FBX07 | Parkinsonism |
| Glutaric acidaemia type 1 | GCDH | Macrocepahaly, encephalopathic crises, axial hypotonia, parkinsonism. |
| Hereditary dopamine deficiency syndrome | SLC6A3 | Pyramidal tract signs, eye movement disoreder, hyper and hypokinetic movement disorders |
| Kufor-Rakeb syndrome | ATP13A2 | Parkinsonism |
| Infantile striatonigral degeneration | NUP62 | Choreoathetosis, spasticity, nystagmus, developmental regression |
| Metachromatic leukodystrophy | ARSA | Mental retardation, spasticity and bulbar palsy |
| Mitochondrial membrane protein-associated neurodegeneration (MPAN) | C19orf12 | Cognitive decline, prominent neuropsychiatric abnormalities, motor neuronopathy |
| Neimann Pick type C and D | NPC1/NPC2 | Ataxia, ocular motor abnormalities (vertical gaze palsy), seizures |
| Neurodegeneration with brain iron accumulation type 1 | PANK2 | Parkinsonism, behavioural changes, pigmentary retinopathy in 50% |
| Neurodegeneration with brain iron accumulation type 2 | PLA2G6 | Parkinsonism, pyramidal signs, cognitive decline, cerebellar ataxia |
| Parkin-related parkinsonism | PRKN | Parkinsonism |
| Tay-Sach’s disease | HEXA | Psychomotor regression, dementia, blindness. |
| Wilson’s disease | ATP7B | Tremor, parkinsonism |
| X-Linked | ||
| Dystonia-deafness syndrome | TIMM8A | Progressive hearing loss, spasticity, cortical blindness |
| Lesch-Nyhan syndrome | HPRT | Choreoathetosis, ballismus, cognitive and attentional deficits, self-injurious behaviours |
| Lubag disease (DYT3) | TAF1 | Parkinsonism |
| Pelizaeus-Merzbacher disease | PLP1 | Pyramidal dysfunction, cerebellar ataxia, head tremor |
| Rett syndrome | MECP2 | Mental retardation, motor regression, autistic behaviours, seizures |
| Static encephalopathy of childhood with neurodegeneration in adulthood | WDR45 | De novo inheritance pattern, global developmental delay, parkinsonism and dementia |
| Mitochondrial | ||
| Leber’s optic neuropathy | ND1, ND4, ND6 | Bilateral or sequential visual failure |
| Leigh syndrome | Multiple genes | Optic atrophy, ophthalmoplegia, ataxia, spasticity and developmental delay/regression. May also be autosomale recessive inheritance. |
| Myoclonic Epilepsy with Ragged Red Fibres (MERRF) | Mainly tRNA(lys) but others reported | Epilepsy, short stature, hearing loss |
Major clinical features besides dystonia are indicated.
Notably, DYT3-related dystonia belongs to this category of disease and is perhaps worth a more detailed consideration. It is primarily found in Filipino males, due to a founder mutation, and is inherited in an X-linked recessive fashion. It is due to a 2.6 kb retrotransposon insertion in intron 32 of the TAF1 (TATA box-binding protein-associated factor 1) gene (Makino et al., 2007). The insertion appears to reduce neuron-specific expression of TAF1 and the dopamine receptor D2 gene in the caudate nucleus (Makino et al., 2007; Muller, 2009). Symptoms start as focal dystonia and progress to multifocal/generalized dystonia, sometimes with parkinsonism. Neuronal degeneration on post-mortem analysis has been described in association with mutations in this gene (Waters et al., 1993; Evidente et al., 2002).
The genetics of sporadic dystonia
To date, no genome-wide association study has been reported for sporadic dystonia. Previous experience in allied diseases, such as Parkinson’s disease or Alzheimer’s disease, suggests that to be successful such a study would need to be a large-scale, international effort that included thousands of case and control subjects. Such research is, of course, expensive and would require a considerable funding commitment. A second problem that a potential association study would face is defining its case cohort. Sporadic dystonia is an umbrella term, comprising multiple phenotypic subtypes that may well have distinct genetic architectures. In this respect, it is not unlike epilepsy, a condition for which large association studies have been somewhat disappointing (Kasperaviciute et al., 2010). Difficult decisions will have to be made regarding whether to group all sporadic dystonia together and risk ‘diluting’ individual signals associated with particular subtypes or whether to spilt them and risk compromising the power of the studies to detect an association.
In the case of sporadic primary dystonia, only candidate gene association studies have been published. Six of these studies have examined common single nucleotide polymorphisms (SNPs) in and around the TOR1A gene. Two showed strong associations between individual SNPs and susceptibility for primary dystonia in populations from Iceland, southern Germany and Austria, and the United States (Clarimon et al., 2005; Kamm et al., 2006; Sharma et al., 2010). However, others have shown only modest associations in Indian, Italian and northern German populations (Naiya et al., 2006; Clarimon et al., 2007; Bruggemann et al., 2009) and yet other similar studies have been unable to replicate these associations (Sibbing et al., 2003; Hague et al., 2006). In contrast to TOR1A, no strong associations have been reported with polymorphisms in the THAP1 gene (Xiao et al., 2011; Kaffe et al., 2012). One recent study employed a haplotype tagging strategy to cover the majority of common variability in TOR1A, TAF1, GCH1, THAP1, MR-1, SGCE, ATP1A3 and PRKRA (Newman et al., 2012). No association survived correction for multiple testing in this study, though three variants in GCH1 did show significant association before correction and would merit follow-up in a larger case-control cohort.
Conclusions
In recent years, advances in sequencing technology have accelerated the pace of gene discovery in neurology and the field of dystonia has been no exception, with several new genes appearing on the scene. Indeed, there is every reason to believe that the pace of discovery will continue to accelerate as sequencing costs fall and exome sequencing gives way to affordable whole-genome sequencing, allowing examination for the first time of non-coding and regulatory regions of DNA and their potential contributions to disease.
By pointing researchers in the direction of cellular pathways that are dysfunctional in familial dystonia, the discovery of new causative genes is the first step in unravelling the complex molecular pathophysiology that underlies not just familial but also, one would hope, sporadic forms of dystonia. Current data suggest that dystonia can result from dysfunction is a wide variety of cellular pathways, which probably reflects and in part explains the wide phenotypic variety seen in the disorder. Further functional work, particularly on those new genes that have only recently been published and still require independent confirmation, may help us identify a core set of pathogenic mechanisms in dystonia that might form the target for novel treatments. In this respect, induced pluripotent stem cell-derived neurons would offer the advantage of a more accurate model system, as well as an initial means of assessing the effect of any proposed treatments. Finally, it remains important to examine the genetics of sporadic dystonia independently by means of a large scale, genome-wide association study but, in order for this to be successful, international co-operation and a considerable funding commitment will be required.
Acknowledgements
The authors would like to thank our funders for enabling us to complete this work.
Funding
This work was supported by the Medical Research council and the Wellcome Trust [grant number WT089698/Z/09/Z] and the Bachmann-Strauss Dystonia and Parkinson Foundation.
References
- Albanese A, Asmus F, Bhatia KP, Elia AE, Elibol B, Filippini G, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- Almasy L, Bressman SB, Raymond D, Kramer PL, Greene PE, Heiman GA, et al. Idiopathic torsion dystonia linked to chromosome 8 in two Mennonite families. Ann Neurol. 1997;42:670–3. doi: 10.1002/ana.410420421. [DOI] [PubMed] [Google Scholar]
- Anastasi G, Tomasello F, Di Mauro D, Cutroneo G, Favaloro A, Conti A, et al. Expression of sarcoglycans in the human cerebral cortex: an immunohistochemical and molecular study. Cells Tissues Organs. 2012;196:470–80. doi: 10.1159/000336842. [DOI] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–7. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmus F, Devlin A, Munz M, Zimprich A, Gasser T, Chinnery PF. Clinical differentiation of genetically proven benign hereditary chorea and myoclonus-dystonia. Mov Disord. 2007;22:2104–9. doi: 10.1002/mds.21692. [DOI] [PubMed] [Google Scholar]
- Asmus F, Gasser T. Inherited myoclonus-dystonia. Adv Neurol. 2004;94:113–19. [PubMed] [Google Scholar]
- Atai NA, Ryan SD, Kothary R, Breakefield XO, Nery FC. Untethering the nuclear envelope and cytoskeleton: biologically distinct dystonias arising from a common cellular dysfunction. Int J Cell Biol. 2012;2012:634214. doi: 10.1155/2012/634214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandmann O, Goertz M, Zschocke J, Deuschl G, Jost W, Hefter H, et al. The phenylalanine loading test in the differential diagnosis of dystonia. Neurology. 2003;60:700–2. doi: 10.1212/01.wnl.0000048205.18445.98. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Berrettini WH. Susceptibility loci for bipolar disorder: overlap with inherited vulnerability to schizophrenia. Biol Psychiatry. 2000;47:245–51. doi: 10.1016/s0006-3223(99)00226-7. [DOI] [PubMed] [Google Scholar]
- Blanco-Arias P, Einholm AP, Mamsa H, Concheiro C, Gutierrez-de-Teran H, Romero J, et al. A C-terminal mutation of ATP1A3 underscores the crucial role of sodium affinity in the pathophysiology of rapid-onset dystonia-parkinsonism. Hum Mol Genet. 2009;18:2370–7. doi: 10.1093/hmg/ddp170. [DOI] [PubMed] [Google Scholar]
- Blau N, Bonafe L, Thony B. Tetrahydrobiopterin deficiencies without hyperphenylalaninemia: diagnosis and genetics of dopa-responsive dystonia and sepiapterin reductase deficiency. Mol Genet Metab. 2001;74:172–85. doi: 10.1006/mgme.2001.3213. [DOI] [PubMed] [Google Scholar]
- Bonafe L, Thony B, Penzien JM, Czarnecki B, Blau N. Mutations in the sepiapterin reductase gene cause a novel tetrahydrobiopterin-dependent monoamine-neurotransmitter deficiency without hyperphenylalaninemia. Am J Hum Genet. 2001;69:269–77. doi: 10.1086/321970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg DC, Armata IA, Nery FC, Breakefield XO, Sharma N. Molecular pathways in dystonia. Neurobiol Dis. 2011;42:136–47. doi: 10.1016/j.nbd.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear A, Dobyns WB, de Carvalho Aguiar P, Borg M, Frijns CJ, Gollamudi S, et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain. 2007;130(Pt 3):828–35. doi: 10.1093/brain/awl340. [DOI] [PubMed] [Google Scholar]
- Brashear A, Mulholland GK, Zheng QH, Farlow MR, Siemers ER, Hutchins GD. PET imaging of the pre-synaptic dopamine uptake sites in rapid-onset dystonia-parkinsonism (RDP) Mov Disord. 1999;14:132–7. doi: 10.1002/1531-8257(199901)14:1<132::aid-mds1022>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Brautigam C, Wevers RA, Jansen RJ, Smeitink JA, de Rijk-van Andel JF, Gabreels FJ, et al. Biochemical hallmarks of tyrosine hydroxylase deficiency. Clin Chem. 1998;44:1897–904. [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–34. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Bressman S. Genetics of dystonia. J Neural Transm Suppl. 2006;70:489–95. doi: 10.1007/978-3-211-45295-0_73. [DOI] [PubMed] [Google Scholar]
- Brockmann K. The expanding phenotype of GLUT1-deficiency syndrome. Brain Dev. 2009;31:545–52. doi: 10.1016/j.braindev.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Bruggemann N, Kock N, Lohmann K, Konig IR, Rakovic A, Hagenah J, et al. The D216H variant in the DYT1 gene: a susceptibility factor for dystonia in familial cases? Neurology. 2009;72:1441–3. doi: 10.1212/WNL.0b013e3181a1861e. [DOI] [PubMed] [Google Scholar]
- Bruno MK, Hallett M, Gwinn-Hardy K, Sorensen B, Considine E, Tucker S, et al. Clinical evaluation of idiopathic paroxysmal kinesigenic dyskinesia: new diagnostic criteria. Neurology. 2004;63:2280–7. doi: 10.1212/01.wnl.0000147298.05983.50. [DOI] [PubMed] [Google Scholar]
- Bruno MK, Lee HY, Auburger GW, Friedman A, Nielsen JE, Lang AE, et al. Genotype-phenotype correlation of paroxysmal nonkinesigenic dyskinesia. Neurology. 2007;68:1782–9. doi: 10.1212/01.wnl.0000262029.91552.e0. [DOI] [PubMed] [Google Scholar]
- Calakos N, Patel VD, Gottron M, Wang G, Tran-Viet KN, Brewington D, et al. Functional evidence implicating a novel TOR1A mutation in idiopathic, late-onset focal dystonia. J Med Genet. 2010;47:646–50. doi: 10.1136/jmg.2009.072082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargos S, Scholz S, Simon-Sanchez J, Paisan-Ruiz C, Lewis P, Hernandez D, et al. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7:207–15. doi: 10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- Cao L, Huang XJ, Zheng L, Xiao Q, Wang XJ, Chen SD. Identification of a novel PRRT2 mutation in patients with paroxysmal kinesigenic dyskinesias and c.649dupC as a mutation hot-spot. Parkinsonism Relat Disord. 2012;18:704–6. doi: 10.1016/j.parkreldis.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–4. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Charlesworth G, Plagnol V, Holmstrom KM, Bras J, Sheerin UM, Preza E, et al. Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. Am J Hum Genet. 2012;91:1041–50. doi: 10.1016/j.ajhg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DH, Matsushita M, Rainier S, Meaney B, Tisch L, Feleke A, et al. Presence of alanine-to-valine substitutions in myofibrillogenesis regulator 1 in paroxysmal nonkinesigenic dyskinesia: confirmation in 2 kindreds. Arch Neurol. 2005;62:597–600. doi: 10.1001/archneur.62.4.597. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Lin Y, Xiong ZQ, Wei W, Ni W, Tan GH, et al. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;43:1252–5. doi: 10.1038/ng.1008. [DOI] [PubMed] [Google Scholar]
- Clarimon J, Asgeirsson H, Singleton A, Jakobsson F, Hjaltason H, Hardy J, et al. Torsin a haplotype predisposes to idiopathic dystonia. Ann Neurol. 2005;57:765–7. doi: 10.1002/ana.20485. [DOI] [PubMed] [Google Scholar]
- Clarimon J, Brancati F, Peckham E, Valente EM, Dallapiccola B, Abruzzese G, et al. Assessing the role of DRD5 and DYT1 in two different case-control series with primary blepharospasm. Mov Disord. 2007;22:162–6. doi: 10.1002/mds.21182. [DOI] [PubMed] [Google Scholar]
- Clot F, Grabli D, Cazeneuve C, Roze E, Castelnau P, Chabrol B, et al. Exhaustive analysis of BH4 and dopamine biosynthesis genes in patients with Dopa-responsive dystonia. Brain. 2009;132(Pt 7):1753–63. doi: 10.1093/brain/awp084. [DOI] [PubMed] [Google Scholar]
- Clouaire T, Roussigne M, Ecochard V, Mathe C, Amalric F, Girard JP. The THAP domain of THAP1 is a large C2CH module with zinc-dependent sequence-specific DNA-binding activity. Proc Natl Acad Sci USA. 2005;102:6907–12. doi: 10.1073/pnas.0406882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SA, Wider C, Ross OA, Mata IF, Adler CH, Rajput A, et al. GCH1 in early-onset Parkinson's disease. Mov Disord. 2009;24:2070–5. doi: 10.1002/mds.22729. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Studler JM, Schonn JS, Girault JA, Herve D. Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76:1585–8. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- Coverley D, Marr J, Ainscough J. Ciz1 promotes mammalian DNA replication. J Cell Sci. 2005;118(Pt 1):101–12. doi: 10.1242/jcs.01599. [DOI] [PubMed] [Google Scholar]
- Dale RC, Gardiner A, Antony J, Houlden H. Familial PRRT2 mutation with heterogeneous paroxysmal disorders including paroxysmal torticollis and hemiplegic migraine. Dev Med Child Neurol. 2012;54:958–60. doi: 10.1111/j.1469-8749.2012.04394.x. [DOI] [PubMed] [Google Scholar]
- de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, et al. Mutations in the Na+/K+-ATPase Œ±3 Gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–75. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130(Pt 5):1183–93. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- Deutschlander A, Asmus F, Gasser T, Steude U, Botzel K. Sporadic rapid-onset dystonia-parkinsonism syndrome: failure of bilateral pallidal stimulation. Mov Disord. 2005;20:254–7. doi: 10.1002/mds.20296. [DOI] [PubMed] [Google Scholar]
- Djarmati A, Svetel M, Momcilovic D, Kostic V, Klein C. Significance of recurrent mutations in the myofibrillogenesis regulator 1 gene. Arch Neurol. 2005;62:1641. doi: 10.1001/archneur.62.10.1641-a. [DOI] [PubMed] [Google Scholar]
- Duan W, Zhang Z, Gash DM, Mattson MP. Participation of prostate apoptosis response-4 in degeneration of dopaminergic neurons in models of Parkinson's disease. Ann Neurol. 1999;46:587–97. [PubMed] [Google Scholar]
- Duran C, Hartzell HC. Physiological roles and diseases of Tmem16/Anoctamin proteins: are they all chloride channels? Acta Pharmacol Sin. 2011;32:685–92. doi: 10.1038/aps.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran C, Qu Z, Osunkoya AO, Cui Y, Hartzell HC. ANOs 3-7 in the anoctamin/Tmem16 Cl- channel family are intracellular proteins. Am J Physiol Cell Physiol. 2012;302:C482–93. doi: 10.1152/ajpcell.00140.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esapa CT, Waite A, Locke M, Benson MA, Kraus M, McIlhinney RA, et al. SGCE missense mutations that cause myoclonus-dystonia syndrome impair epsilon-sarcoglycan trafficking to the plasma membrane: modulation by ubiquitination and torsinA. Hum Mol Genet. 2007;16:327–42. doi: 10.1093/hmg/ddl472. [DOI] [PubMed] [Google Scholar]
- Evidente VG, Advincula J, Esteban R, Pasco P, Alfon JA, Natividad FF, et al. Phenomenology of “Lubag” or X-linked dystonia-parkinsonism. Mov Disord. 2002;17:1271–7. doi: 10.1002/mds.10271. [DOI] [PubMed] [Google Scholar]
- Fahn S. Concept and classification of dystonia. Adv Neurol. 1988;50:1–8. [PubMed] [Google Scholar]
- Fanh S, Marsden CD, Caine DB. Dystonia 2. Proceedings of the Second International Symposium on Torsion Dystonia. Harriman, New York, 1986. Adv Neurol. 1988;50:1–705. [PubMed] [Google Scholar]
- Friedman J, Olvera J, Silhavy JL, Gabriel SB, Gleeson JG. Mild paroxysmal kinesigenic dyskinesia caused by PRRT2 missense mutation with reduced penetrance. Neurology. 2012a;79:946–8. doi: 10.1212/WNL.0b013e318266fabf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Roze E, Abdenur JE, Chang R, Gasperini S, Saletti V, et al. Sepiapterin reductase deficiency: a treatable mimic of cerebral palsy. Ann Neurol. 2012b;71:520–30. doi: 10.1002/ana.22685. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Gavarini S, Saunders-Pullman R, Raymond D, Ehrlich ME, Bressman SB, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–8. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Saunders-Pullman R, Masuho I, Luciano MS, Raymond D, Factor S, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2012;45:88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y. Genetics and biochemistry of dopa-responsive dystonia: significance of striatal tyrosine hydroxylase protein loss. Adv Neurol. 2003;91:401–10. [PubMed] [Google Scholar]
- Furukawa Y. Update on dopa-responsive dystonia: locus heterogeneity and biochemical features. Adv Neurol. 2004;94:127–38. [PubMed] [Google Scholar]
- Gardiner AR, Bhatia KP, Stamelou M, Dale RC, Kurian MA, Schneider SA, et al. PRRT2 gene mutations: From paroxysmal dyskinesia to episodic ataxia and hemiplegic migraine. Neurology. 2012;79:2115–21. doi: 10.1212/WNL.0b013e3182752c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavarini S, Cayrol C, Fuchs T, Lyons N, Ehrlich ME, Girard JP, et al. Direct interaction between causative genes of DYT1 and DYT6 primary dystonia. Ann Neurol. 2010;68:549–53. doi: 10.1002/ana.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer HL, Bressman SB. The diagnosis of dystonia. Lancet Neurol. 2006;5:780–90. doi: 10.1016/S1474-4422(06)70547-6. [DOI] [PubMed] [Google Scholar]
- Ghezzi D, Viscomi C, Ferlini A, Gualandi F, Mereghetti P, DeGrandis D, et al. Paroxysmal non-kinesigenic dyskinesia is caused by mutations of the MR-1 mitochondrial targeting sequence. Hum Mol Genet. 2009;18:1058–64. doi: 10.1093/hmg/ddn441. [DOI] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. The AAA+ protein torsinA interacts with a conserved domain present in LAP1 and a novel ER protein. J Cell Biol. 2005;168:855–62. doi: 10.1083/jcb.200411026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron. 2005;48:923–32. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Zimprich A, Lorenz-Depiereux B, Kalscheuer V, Asmus F, Gasser T, et al. The epsilon-sarcoglycan gene (SGCE), mutated in myoclonus-dystonia syndrome, is maternally imprinted. Eur J Hum Genet. 2003;11:138–44. doi: 10.1038/sj.ejhg.5200938. [DOI] [PubMed] [Google Scholar]
- Grundmann K, Reischmann B, Vanhoutte G, Hubener J, Teismann P, Hauser TK, et al. Overexpression of human wildtype torsinA and human DeltaGAG torsinA in a transgenic mouse model causes phenotypic abnormalities. Neurobiol Dis. 2007;27:190–206. doi: 10.1016/j.nbd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Guo Q, Fu W, Xie J, Luo H, Sells SF, Geddes JW, et al. Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer disease. Nat Med. 1998;4:957–62. doi: 10.1038/nm0898-957. [DOI] [PubMed] [Google Scholar]
- Hague S, Klaffke S, Clarimon J, Hemmer B, Singleton A, Kupsch A, et al. Lack of association with TorsinA haplotype in German patients with sporadic dystonia. Neurology. 2006;66:951–2. doi: 10.1212/01.wnl.0000203344.43342.18. [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–58. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- Hedera P, Xiao J, Puschmann A, Momcilovic D, Wu SW, Ledoux MS. Novel PRRT2 mutation in an African-American family with paroxysmal kinesigenic dyskinesia. BMC Neurol. 2012;12:93. doi: 10.1186/1471-2377-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen EL, Swoboda KJ, Hitomi Y, Gurrieri F, Nicole S, de Vries B, et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet. 2012;44:1030–4. doi: 10.1038/ng.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempelmann A, Kumar S, Muralitharan S, Sander T. Myofibrillogenesis regulator 1 gene (MR-1) mutation in an Omani family with paroxysmal nonkinesigenic dyskinesia. Neurosci Lett. 2006;402:118–20. doi: 10.1016/j.neulet.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Henriksen C, Madsen LB, Bendixen C, Larsen K. Characterization of the porcine TOR1A gene: The first step towards generation of a pig model for dystonia. Gene. 2009;430:105–15. doi: 10.1016/j.gene.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Heron SE, Grinton BE, Kivity S, Afawi Z, Zuberi SM, Hughes JN, et al. PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am J Hum Genet. 2012;90:152–60. doi: 10.1016/j.ajhg.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersheson J, Mencacci NE, Davis M, MacDoanld HN, Trabzuni D, Ryten M, et al. Mutations in the autoregulatory domain of β-tubulin 4a cause hereditary dystonia. Ann Neurol. 2013 doi: 10.1002/ana.23832. doi: 10.1002/ana.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, et al. G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13:2237–48. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CW, Raymond D, Aguiar Pde C, Frucht S, Shriberg J, Heiman GA, et al. Myoclonus-dystonia, obsessive-compulsive disorder, and alcohol dependence in SGCE mutation carriers. Neurology. 2007;68:522–4. doi: 10.1212/01.wnl.0000253188.76092.06. [DOI] [PubMed] [Google Scholar]
- Hewett JW, Tannous B, Niland BP, Nery FC, Zeng J, Li Y, et al. Mutant torsinA interferes with protein processing through the secretory pathway in DYT1 dystonia cells. Proc Natl Acad Sci USA. 2007;104:7271–6. doi: 10.1073/pnas.0701185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett JW, Zeng J, Niland BP, Bragg DC, Breakefield XO. Dystonia-causing mutant torsinA inhibits cell adhesion and neurite extension through interference with cytoskeletal dynamics. Neurobiol Dis. 2006;22:98–111. doi: 10.1016/j.nbd.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hjermind LE, Werdelin LM, Sorensen SA. Inherited and de novo mutations in sporadic cases of DYT1-dystonia. Eur J Hum Genet. 2002;10:213–6. doi: 10.1038/sj.ejhg.5200782. [DOI] [PubMed] [Google Scholar]
- Horvath GA, Stockler-Ipsiroglu SG, Salvarinova-Zivkovic R, Lillquist YP, Connolly M, Hyland K, et al. Autosomal recessive GTP cyclohydrolase I deficiency without hyperphenylalaninemia: evidence of a phenotypic continuum between dominant and recessive forms. Mol Genet Metab. 2008;94:127–31. doi: 10.1016/j.ymgme.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Houlden H, Schneider SA, Paudel R, Melchers A, Schwingenschuh P, Edwards M, et al. THAP1 mutations (DYT6) are an additional cause of early-onset dystonia. Neurology. 2010;74:846–50. doi: 10.1212/WNL.0b013e3181d5276d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Xiao S, Huang F, Harfe BD, Jan YN, Jan LY. Calcium-activated chloride channels (CaCCs) regulate action potential and synaptic response in hippocampal neurons. Neuron. 2012;74:179–92. doi: 10.1016/j.neuron.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu WL, Chiou YW, Lai SY, Lee YM. Dopa-responsive dystonia is induced by a dominant-negative mechanism. Ann Neurol. 2000;48:609–13. [PubMed] [Google Scholar]
- Hwu WL, Wang PJ, Hsiao KJ, Wang TR, Chiou YW, Lee YM. Dopa-responsive dystonia induced by a recessive GTP cyclohydrolase I mutation. Hum Genet. 1999;105:226–30. doi: 10.1007/s004390051093. [DOI] [PubMed] [Google Scholar]
- Ichinose H, Nagatsu T. Molecular genetics of hereditary dystonia–mutations in the GTP cyclohydrolase I gene. Brain Res Bull. 1997;43:35–8. doi: 10.1016/s0361-9230(96)00353-x. [DOI] [PubMed] [Google Scholar]
- Ichinose H, Ohye T, Matsuda Y, Hori T, Blau N, Burlina A, et al. Characterization of mouse and human GTP cyclohydrolase I genes. Mutations in patients with GTP cyclohydrolase I deficiency. J Biol Chem. 1995;270:10062–71. doi: 10.1074/jbc.270.17.10062. [DOI] [PubMed] [Google Scholar]
- Ichinose H, Ohye T, Takahashi E, Seki N, Hori T, Segawa M, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet. 1994;8:236–42. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- Jamora RD, Tan EK, Liu CP, Kathirvel P, Burgunder JM, Tan LC. DYT1 mutations amongst adult primary dystonia patients in Singapore with review of literature comparing East and West. J Neurol Sci. 2006;247:35–7. doi: 10.1016/j.jns.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Jones CL, Vasquez-Vivar J, Kalyanaraman B, Griscavage-Ennis M, Gross SS. Tetrahydropterins but not dihydropterins attenuate the reduction of superoxide from eNOS. Pteridines. 2001;12:52–3. [Google Scholar]
- Kabakci K, Hedrich K, Leung JC, Mitterer M, Vieregge P, Lencer R, et al. Mutations in DYT1: extension of the phenotypic and mutational spectrum. Neurology. 2004;62:395–400. doi: 10.1212/01.wnl.0000113024.84178.f7. [DOI] [PubMed] [Google Scholar]
- Kaffe M, Gross N, Castrop F, Dresel C, Gieger C, Lichtner P, et al. Mutational screening of THAP1 in a German population with primary dystonia. Parkinsonism Relat Disord. 2012;18:104–6. doi: 10.1016/j.parkreldis.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Kaiser FJ, Osmanoric A, Rakovic A, Erogullari A, Uflacker N, Braunholz D, et al. The dystonia gene DYT1 is repressed by the transcription factor THAP1 (DYT6) Ann Neurol. 2010;68:554–9. doi: 10.1002/ana.22157. [DOI] [PubMed] [Google Scholar]
- Kakazu Y, Koh JY, Ho KW, Gonzalez-Alegre P, Harata NC. Synaptic vesicle recycling is enhanced by torsinA that harbors the DYT1 dystonia mutation. Synapse. 2012;66:453–64. doi: 10.1002/syn.21534. [DOI] [PubMed] [Google Scholar]
- Kamm C, Asmus F, Mueller J, Mayer P, Sharma M, Muller UJ, et al. Strong genetic evidence for association of TOR1A/TOR1B with idiopathic dystonia. Neurology. 2006;67:1857–9. doi: 10.1212/01.wnl.0000244423.63406.17. [DOI] [PubMed] [Google Scholar]
- Kamm C, Fischer H, Garavaglia B, Kullmann S, Sharma M, Schrader C, et al. Susceptibility to DYT1 dystonia in European patients is modified by the D216H polymorphism. Neurology. 2008a;70:2261–2. doi: 10.1212/01.wnl.0000313838.05734.8a. [DOI] [PubMed] [Google Scholar]
- Kamm C, Fogel W, Wachter T, Schweitzer K, Berg D, Kruger R, et al. Novel ATP1A3 mutation in a sporadic RDP patient with minimal benefit from deep brain stimulation. Neurology. 2008b;70(Pt 2):1501–3. doi: 10.1212/01.wnl.0000310431.41036.e0. [DOI] [PubMed] [Google Scholar]
- Kasperaviciute D, Catarino CB, Heinzen EL, Depondt C, Cavalleri GL, Caboclo LO, et al. Common genetic variation and susceptibility to partial epilepsies: a genome-wide association study. Brain. 2010;133(Pt 7):2136–47. doi: 10.1093/brain/awq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NL, Wood NW, Bhatia KP. Autosomal recessive, DYT2-like primary torsion dystonia: a new family. Neurology. 2003;61:1801–3. doi: 10.1212/01.wnl.0000099076.17187.9a. [DOI] [PubMed] [Google Scholar]
- Kimmich O, Bradley D, Whelan R, Mulrooney N, Reilly RB, Hutchinson S, et al. Sporadic adult onset primary torsion dystonia is a genetic disorder by the temporal discrimination test. Brain. 2011;134(Pt 9):2656–63. doi: 10.1093/brain/awr194. [DOI] [PubMed] [Google Scholar]
- Kinugawa K, Vidailhet M, Clot F, Apartis E, Grabli D, Roze E. Myoclonus-dystonia: an update. Mov Disord. 2009;24:479–89. doi: 10.1002/mds.22425. [DOI] [PubMed] [Google Scholar]
- Klein C, König IR, Lohmann K. Exome sequencing for gene discovery: time to set standard criteria. Ann Neurol. 2012;72:627–8. doi: 10.1002/ana.23658. [DOI] [PubMed] [Google Scholar]
- Kock N, Naismith TV, Boston HE, Ozelius LJ, Corey DP, Breakefield XO, et al. Effects of genetic variations in the dystonia protein torsinA: identification of polymorphism at residue 216 as protein modifier. Hum Mol Genet. 2006;15:1355–64. doi: 10.1093/hmg/ddl055. [DOI] [PubMed] [Google Scholar]
- Konakova M, Huynh DP, Yong W, Pulst SM. Cellular distribution of torsin A and torsin B in normal human brain. Arch Neurol. 2001;58:921–7. doi: 10.1001/archneur.58.6.921. [DOI] [PubMed] [Google Scholar]
- Kramer PL, de Leon D, Ozelius L, Risch N, Bressman SB, Brin MF, et al. Dystonia gene in Ashkenazi Jewish population is located on chromosome 9q32-34. Ann Neurol. 1990;27:114–20. doi: 10.1002/ana.410270203. [DOI] [PubMed] [Google Scholar]
- Kramer PL, Heiman GA, Gasser T, Ozelius LJ, de Leon D, Brin MF, et al. The DYT1 gene on 9q34 is responsible for most cases of early limb-onset idiopathic torsion dystonia in non-Jews. Am J Hum Genet. 1994;55:468–75. [PMC free article] [PubMed] [Google Scholar]
- Laurin N, Ickowicz A, Pathare T, Malone M, Tannock R, Schachar R, et al. Investigation of the G protein subunit Galphaolf gene (GNAL) in attention deficit/hyperactivity disorder. J Psychiatr Res. 2008;42:117–24. doi: 10.1016/j.jpsychires.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Huang Y, Bruneau N, Roll P, Roberson ED, Hermann M, et al. Mutations in the novel protein PRRT2 cause paroxysmal kinesigenic dyskinesia with infantile convulsions. Cell Rep. 2012;1:2–12. doi: 10.1016/j.celrep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Xu Y, Huang Y, Ahn AH, Auburger GW, Pandolfo M, et al. The gene for paroxysmal non-kinesigenic dyskinesia encodes an enzyme in a stress response pathway. Hum Mol Genet. 2004;13:3161–70. doi: 10.1093/hmg/ddh330. [DOI] [PubMed] [Google Scholar]
- Leen WG, Klepper J, Verbeek MM, Leferink M, Hofste T, van Engelen BG, et al. Glucose transporter-1 deficiency syndrome: the expanding clinical and genetic spectrum of a treatable disorder. Brain. 2010;133(Pt 3):655–70. doi: 10.1093/brain/awp336. [DOI] [PubMed] [Google Scholar]
- Leube B, Hendgen T, Kessler KR, Knapp M, Benecke R, Auburger G. Evidence for DYT7 being a common cause of cervical dystonia (torticollis) in Central Europe. Am J Med Genet. 1997a;74:529–32. doi: 10.1002/(sici)1096-8628(19970919)74:5<529::aid-ajmg15>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Leube B, Kessler KR, Goecke T, Auburger G, Benecke R. Frequency of familial inheritance among 488 index patients with idiopathic focal dystonia and clinical variability in a large family. Mov Disord. 1997b;12:1000–6. doi: 10.1002/mds.870120625. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu X, Wang X, Sun W, Feng B, Du T, et al. Targeted genomic sequencing identifies PRRT2 mutations as a cause of paroxysmal kinesigenic choreoathetosis. J Med Genet. 2012;49:76–8. doi: 10.1136/jmedgenet-2011-100635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann K, Wilcox RA, Winkler S, Ramirez A, Rakovic A, Park JS, et al. Whispering dysphonia (DYT4 dystonia) is caused by a mutation in the TUBB4 gene. Ann Neurol. 2013 doi: 10.1002/ana.23829. doi: 10.1002/ana.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano MS, Ozelius L, Sims K, Raymond D, Liu L, Saunders-Pullman R. Responsiveness to levodopa in epsilon-sarcoglycan deletions. Mov Disord. 2009;24:425–8. doi: 10.1002/mds.22375. [DOI] [PubMed] [Google Scholar]
- Ludecke B, Knappskog PM, Clayton PT, Surtees RA, Clelland JD, Heales SJ, et al. Recessively inherited L-DOPA-responsive parkinsonism in infancy caused by a point mutation (L205P) in the tyrosine hydroxylase gene. Hum Mol Genet. 1996;5:1023–8. doi: 10.1093/hmg/5.7.1023. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism. Relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci. 1996;777:380–7. doi: 10.1111/j.1749-6632.1996.tb34449.x. [DOI] [PubMed] [Google Scholar]
- Makino S, Kaji R, Ando S, Tomizawa M, Yasuno K, Goto S, et al. Reduced neuron-specific expression of the TAF1 gene is associated with X-linked dystonia-parkinsonism. Am J Hum Genet. 2007;80:393–406. doi: 10.1086/512129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Valencia I, Good LB, Ma Q, Duarte J, Bottiglieri T, Sinton CM, et al. Glut1 deficiency (G1D): epilepsy and metabolic dysfunction in a mouse model of the most common human phenotype. Neurobiol Dis. 2012;48:92–101. doi: 10.1016/j.nbd.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino D, Gajos A, Gallo V, Cif L, Coubes P, Tinazzi M, et al. Extragenetic factors and clinical penetrance of DYT1 dystonia: an exploratory study. J Neurol. 2012;260:1081–6. doi: 10.1007/s00415-012-6765-2. [DOI] [PubMed] [Google Scholar]
- Muller U. The monogenic primary dystonias. Brain. 2009;132(Pt 8):2005–25. doi: 10.1093/brain/awp172. [DOI] [PubMed] [Google Scholar]
- Muller U, Steinberger D, Nemeth AH. Clinical and molecular genetics of primary dystonias. Neurogenetics. 1998;1:165–77. doi: 10.1007/s100480050025. [DOI] [PubMed] [Google Scholar]
- Naiya T, Biswas A, Neogi R, Datta S, Misra AK, Das SK, et al. Clinical characterization and evaluation of DYT1 gene in Indian primary dystonia patients. Acta Neurol Scand. 2006;114:210–5. doi: 10.1111/j.1600-0404.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- Nardocci N. Myoclonus-dystonia syndrome. Handb Clin Neurol. 2011;100:563–75. doi: 10.1016/B978-0-444-52014-2.00041-0. [DOI] [PubMed] [Google Scholar]
- Nardocci N, Zorzi G, Blau N, Fernandez Alvarez E, Sesta M, Angelini L, et al. Neonatal dopa-responsive extrapyramidal syndrome in twins with recessive GTPCH deficiency. Neurology. 2003;60:335–7. doi: 10.1212/01.wnl.0000044049.99690.ad. [DOI] [PubMed] [Google Scholar]
- Newman JR, Sutherland GT, Boyle RS, Limberg N, Blum S, O'Sullivan JD, et al. Common polymorphisms in dystonia-linked genes and susceptibility to the sporadic primary dystonias. Parkinsonism Relat Disord. 2012;18:351–7. doi: 10.1016/j.parkreldis.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Nygaard TG, Trugman JM, de Yebenes JG, Fahn S. Dopa-responsive dystonia: the spectrum of clinical manifestations in a large North American family. Neurology. 1990;40:66–9. doi: 10.1212/wnl.40.1.66. [DOI] [PubMed] [Google Scholar]
- Opladen T, Hoffmann G, Horster F, Hinz AB, Neidhardt K, Klein C, et al. Clinical and biochemical characterization of patients with early infantile onset of autosomal recessive GTP cyclohydrolase I deficiency without hyperphenylalaninemia. Mov Disord. 2011;26:157–61. doi: 10.1002/mds.23329. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–8. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Ozelius LJ, Kramer PL, de Leon D, Risch N, Bressman SB, Schuback DE, et al. Strong allelic association between the torsion dystonia gene (DYT1) andloci on chromosome 9q34 in Ashkenazi Jews. Am J Hum Genet. 1992;50:619–28. [PMC free article] [PubMed] [Google Scholar]
- Parker N. Hereditary whispering dysphonia. J Neurol Neurosurg Psychiatry. 1985;48:218–24. doi: 10.1136/jnnp.48.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel CV, Handy I, Goldsmith T, Patel RC. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J Biol Chem. 2000;275:37993–8. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- Paudel R, Hardy J, Revesz T, Holton JL, Houlden H. Review: Genetics and neuropathology of primary pure dystonia. Neuropathol Appl Neurobiol. 2012;38:520–34. doi: 10.1111/j.1365-2990.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- Peall KJ, Smith DJ, Kurian MA, Wardle M, Waite AJ, Hedderly T, et al. SGCE mutations cause psychiatric disorders: clinical and genetic characterization. Brain. 2013;136(Pt 1):294–303. doi: 10.1093/brain/aws308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WA, Luo H, Kruman I, Kasarskis E, Mattson MP. The prostate apoptosis response-4 protein participates in motor neuron degeneration in amyotrophic lateral sclerosis. FASEB J. 2000;14:913–24. doi: 10.1096/fasebj.14.7.913. [DOI] [PubMed] [Google Scholar]
- Perez-Duenas B, Prior C, Ma Q, Fernandez-Alvarez E, Setoain X, Artuch R, et al. Childhood chorea with cerebral hypotrophy: a treatable GLUT1 energy failure syndrome. Arch Neurol. 2009;66:1410–4. doi: 10.1001/archneurol.2009.236. [DOI] [PubMed] [Google Scholar]
- Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet. 2012;90:467–77. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant'angelo A, Rizzo V, Bagnato S, Terranova C, et al. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–90. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- Rainier S, Thomas D, Tokarz D, Ming L, Bui M, Plein E, et al. Myofibrillogenesis regulator 1 gene mutations cause paroxysmal dystonic choreoathetosis. Arch Neurol. 2004;61:1025–9. doi: 10.1001/archneur.61.7.1025. [DOI] [PubMed] [Google Scholar]
- Risch NJ, Bressman SB, Senthil G, Ozelius LJ. Intragenic Cis and Trans modification of genetic susceptibility in DYT1 torsion dystonia. Am J Hum Genet. 2007;80:1188–93. doi: 10.1086/518427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodacker V, Toustrup-Jensen M, Vilsen B. Mutations Phe785Leu and Thr618Met in Na+, K+-ATPase, associated with familial rapid-onset dystonia parkinsonism, interfere with Na+ interaction by distinct mechanisms. J Biol Chem. 2006;281:18539–48. doi: 10.1074/jbc.M601780200. [DOI] [PubMed] [Google Scholar]
- Romero-Lopez J, Moreno-Carretero MJ, Escriche-Jaime D, Corredera-Garcia E. Rapid-onset dystonia-parkinsonism: sporadic form. Rev Neurologia. 2008;47:638–40. [PubMed] [Google Scholar]
- Rosewich H, Thiele H, Ohlenbusch A, Maschke U, Altmuller J, Frommolt P, et al. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol. 2012;11:764–73. doi: 10.1016/S1474-4422(12)70182-5. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Augood SJ, Hewett JW, Leung JC, Sasaki H, Ozelius LJ, et al. TorsinA protein and neuropathology in early onset generalized dystonia with GAG deletion. Neurobiol Dis. 2003;12:11–24. doi: 10.1016/s0969-9961(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Roussigne M, Cayrol C, Clouaire T, Amalric F, Girard JP. THAP1 is a nuclear proapoptotic factor that links prostate-apoptosis-response-4 (Par-4) to PML nuclear bodies. Oncogene. 2003;22:2432–42. doi: 10.1038/sj.onc.1206271. [DOI] [PubMed] [Google Scholar]
- Roze E, Apartis E, Clot F, Dorison N, Thobois S, Guyant-Marechal L, et al. Myoclonus-dystonia: clinical and electrophysiologic pattern related to SGCE mutations. Neurology. 2008;70:1010–6. doi: 10.1212/01.wnl.0000297516.98574.c0. [DOI] [PubMed] [Google Scholar]
- Saunders-Pullman R, Raymond D, Senthil G, Kramer P, Ohmann E, Deligtisch A, et al. Narrowing the DYT6 dystonia region and evidence for locus heterogeneity in the Amish-Mennonites. Am J Med Genet A. 2007;143A:2098–105. doi: 10.1002/ajmg.a.31887. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Jabusch HC, Altenmuller E, Hagenah J, Bruggemann N, Lohmann K, et al. Etiology of musician's dystonia: familial or environmental? Neurology. 2009;72:1248–54. doi: 10.1212/01.wnl.0000345670.63363.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa M, Nomura Y, Nishiyama N. Autosomal dominant guanosine triphosphate cyclohydrolase I deficiency (Segawa disease) Ann Neurol. 2003;54(Suppl 6):S32–45. doi: 10.1002/ana.10630. [DOI] [PubMed] [Google Scholar]
- Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI, Jr, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: Bipolar disorder. Am J Hum Genet. 2003;73:49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Franco RA, Jr, Kuster JK, Mitchell AA, Fuchs T, Saunders-Pullman R, et al. Genetic evidence for an association of the TOR1A locus with segmental/focal dystonia. Mov Disord. 2010;25:2183–7. doi: 10.1002/mds.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharan P, Kramer BC, Walker RH, Olanow CW, Brin MF. Immunohistochemical localization and distribution of torsinA in normal human and rat brain. Brain Res. 2000;853:197–206. doi: 10.1016/s0006-8993(99)02232-5. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lee HY, Rawson J, Ojha S, Babbitt P, Fu YH, et al. Mutations in PNKD causing paroxysmal dyskinesia alters protein cleavage and stability. Hum Mol Genet. 2011;20:2322–32. doi: 10.1093/hmg/ddr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbing D, Asmus F, Konig IR, Tezenas du Montcel S, Vidailhet M, Sangla S, et al. Candidate gene studies in focal dystonia. Neurology. 2003;61:1097–101. doi: 10.1212/01.wnl.0000090560.20641.ab. [DOI] [PubMed] [Google Scholar]
- Silveira-Moriyama L, Gardiner AR, Meyer E, King MD, Smith M, Rakshi K, et al. Clinical features of childhood-onset paroxysmal kinesigenic dyskinesia with PRRT2 gene mutations. Dev Med Child Neurol. 2013;55:327–34. doi: 10.1111/dmcn.12056. [DOI] [PubMed] [Google Scholar]
- Skogseid IM, Malt UF, Roislien J, Kerty E. Determinants and status of quality of life after long-term botulinum toxin therapy for cervical dystonia. Eur J Neurol. 2007;14:1129–37. doi: 10.1111/j.1468-1331.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- Smith LA, Heersema PH. Periodic Dystonia. Mayo Clin Proc. 1941;16:4. [Google Scholar]
- Soeder A, Kluger BM, Okun MS, Garvan CW, Soeder T, Jacobson CE, et al. Mood and energy determinants of quality of life in dystonia. J Neurol. 2009;256:996–1001. doi: 10.1007/s00415-009-5060-3. [DOI] [PubMed] [Google Scholar]
- Song CH, Fan X, Exeter CJ, Hess EJ, Jinnah HA. Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia. Neurobiol Dis. 2012;48:66–78. doi: 10.1016/j.nbd.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlein OK, Villain M, Korenke C. The PRRT2 mutation c.649dupC is the so far most frequent cause of benign familial infantile convulsions. Seizure. 2012;21:740–2. doi: 10.1016/j.seizure.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957–68. doi: 10.1016/j.cell.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Stojanovic M, Cvetkovic D, Kostic VS. A genetic study of idiopathic focal dystonias. J Neurol. 1995;242:508–11. doi: 10.1007/BF00867421. [DOI] [PubMed] [Google Scholar]
- Suls A, Dedeken P, Goffin K, Van Esch H, Dupont P, Cassiman D, et al. Paroxysmal exercise-induced dyskinesia and epilepsy is due to mutations in SLC2A1, encoding the glucose transporter GLUT1. Brain. 2008;131(Pt 7):1831–44. doi: 10.1093/brain/awn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin J, Durr A, Bonnet AM, Gil R, Vidailhet M, Lucking CB, et al. Levodopa-responsive dystonia. GTP cyclohydrolase I or parkin mutations? Brain. 2000;123(Pt 6):1112–21. doi: 10.1093/brain/123.6.1112. [DOI] [PubMed] [Google Scholar]
- Teo JT, van de Warrenburg BP, Schneider SA, Rothwell JC, Bhatia KP. Neurophysiological evidence for cerebellar dysfunction in primary focal dystonia. J Neurol Neurosurg Psychiatry. 2009;80:80–3. doi: 10.1136/jnnp.2008.144626. [DOI] [PubMed] [Google Scholar]
- Trender-Gerhard I, Sweeney MG, Schwingenschuh P, Mir P, Edwards MJ, Gerhard A, et al. Autosomal-dominant GTPCH1-deficient DRD: clinical characteristics and long-term outcome of 34 patients. J Neurol Neurosurg Psychiatry. 2009;80:839–45. doi: 10.1136/jnnp.2008.155861. [DOI] [PubMed] [Google Scholar]
- Tuschl K, Clayton PT, Gospe SM, Jr, Gulab S, Ibrahim S, Singhi P, et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet. 2012;90:457–66. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitti RJ, Maraganore DM. Adult onset familial cervical dystonia: report of a family including monozygotic twins. Mov Disord. 1993;8:489–94. doi: 10.1002/mds.870080413. [DOI] [PubMed] [Google Scholar]
- Van Hove JL, Steyaert J, Matthijs G, Legius E, Theys P, Wevers R, et al. Expanded motor and psychiatric phenotype in autosomal dominant Segawa syndrome due to GTP cyclohydrolase deficiency. J Neurol Neurosurg Psychiatry. 2006;77:18–23. doi: 10.1136/jnnp.2004.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddy HM, Fletcher NA, Harding AE, Marsden CD. A genetic study of idiopathic focal dystonias. Ann Neurol. 1991;29:320–4. doi: 10.1002/ana.410290315. [DOI] [PubMed] [Google Scholar]
- Wang JL, Cao L, Li XH, Hu ZM, Li JD, Zhang JG, et al. Identification of PRRT2 as the causative gene of paroxysmal kinesigenic dyskinesias. Brain. 2011;134(Pt 12):3493–501. doi: 10.1093/brain/awr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TT, Granata A, Schiavo G. TorsinA and DYT1 dystonia: a synaptopathy? Biochem Soc Trans. 2010;38:452–6. doi: 10.1042/BST0380452. [DOI] [PubMed] [Google Scholar]
- Waters CH, Faust PL, Powers J, Vinters H, Moskowitz C, Nygaard T, et al. Neuropathology of lubag (x-linked dystonia parkinsonism) Mov Disord. 1993;8:387–90. doi: 10.1002/mds.870080328. [DOI] [PubMed] [Google Scholar]
- Weber YG, Kamm C, Suls A, Kempfle J, Kotschet K, Schule R, et al. Paroxysmal choreoathetosis/spasticity (DYT9) is caused by a GLUT1 defect. Neurology. 2011;77:959–64. doi: 10.1212/WNL.0b013e31822e0479. [DOI] [PubMed] [Google Scholar]
- Weber YG, Storch A, Wuttke TV, Brockmann K, Kempfle J, Maljevic S, et al. GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest. 2008;118:2157–68. doi: 10.1172/JCI34438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wider C, Melquist S, Hauf M, Solida A, Cobb SA, Kachergus JM, et al. Study of a Swiss dopa-responsive dystonia family with a deletion in GCH1: redefining DYT14 as DYT5. Neurology. 2008;70(Pt 2):1377–83. doi: 10.1212/01.wnl.0000275527.35752.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RA, Winkler S, Lohmann K, Klein C. Whispering dysphonia in an Australian family (DYT4): a clinical and genetic reappraisal. Mov Disord. 2011;26:2404–8. doi: 10.1002/mds.23866. [DOI] [PubMed] [Google Scholar]
- Willemsen MA, Verbeek MM, Kamsteeg EJ, de Rijk-van Andel JF, Aeby A, Blau N, et al. Tyrosine hydroxylase deficiency: a treatable disorder of brain catecholamine biosynthesis. Brain. 2010;133(Pt 6):1810–22. doi: 10.1093/brain/awq087. [DOI] [PubMed] [Google Scholar]
- Winter P, Kamm C, Biskup S, Kohler A, Leube B, Auburger G, et al. DYT7 gene locus for cervical dystonia on chromosome 18p is questionable. Mov Disord. 2012;27:1819–21. doi: 10.1002/mds.25219. [DOI] [PubMed] [Google Scholar]
- Xiao J, Uitti RJ, Zhao Y, Vemula SR, Perlmutter JS, Wszolek ZK, et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71:458–69. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Zhao Y, Bastian RW, Perlmutter JS, Racette BA, Tabbal SD, et al. The c.-237_236GA>TT THAP1 sequence variant does not increase risk for primary dystonia. Mov Disord. 2011;26:549–52. doi: 10.1002/mds.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiromerisiou G, Houlden H, Scarmeas N, Stamelou M, Kara E, Hardy J, et al. THAP1 mutations and dystonia phenotypes: Genotype phenotype correlations. Mov Disord. 2012;27:1290–4. doi: 10.1002/mds.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Gay DA, Pachter JS, Cleveland DW. Autoregulated changes in stability of polyribosome-bound beta-tubulin mRNAs are specified by the first 13 translated nucleotides. Mol Cell Biol. 1988a;8:1224–35. doi: 10.1128/mcb.8.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Machlin PS, Cleveland DW. Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature. 1988b;334:580–5. doi: 10.1038/334580a0. [DOI] [PubMed] [Google Scholar]
- Zanotti-Fregonara P, Vidailhet M, Kas A, Ozelius LJ, Clot F, Hindie E, et al. [123I]-FP-CIT and [99mTc]-HMPAO single photon emission computed tomography in a new sporadic case of rapid-onset dystonia-parkinsonism. J Neurol Sci. 2008;273:148–51. doi: 10.1016/j.jns.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374:640–3. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Belluscio L, Hen R. G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci. 2000;20:RC91. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirn B, Grundmann K, Huppke P, Puthenparampil J, Wolburg H, Riess O, et al. Novel TOR1A mutation p.Arg288Gln in early-onset dystonia (DYT1) J Neurol Neurosurg Psychiatry. 2008;79:1327–30. doi: 10.1136/jnnp.2008.148270. [DOI] [PubMed] [Google Scholar]
- Zlotogora J. Autosomal recessive, DYT2-like primary torsion dystonia: a new family. Neurology. 2004;63:1340. doi: 10.1212/wnl.63.7.1340-a. [DOI] [PubMed] [Google Scholar]
- Zoons E, Dijkgraaf MG, Dijk JM, van Schaik IN, Tijssen MA. Botulinum toxin as treatment for focal dystonia: a systematic review of the pharmaco-therapeutic and pharmaco-economic value. J Neurol. 2012;259:2519–26. doi: 10.1007/s00415-012-6510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


