Abstract
CD8+ T cells are critical for the control of many persistent viral infections, such as human immunodeficiency virus, hepatitis C virus, Epstein-Barr virus, and cytomegalovirus (CMV). In most infections, large CD8+-T-cell populations are induced early but then contract and are maintained thereafter at lower levels. In contrast, CD8+ T cells specific for murine CMV (MCMV) have been shown to gradually accumulate after resolution of primary infection. This unique behavior is restricted to certain epitopes, including an immunodominant epitope derived from the immediate-early 1 (IE1) gene product. To explore the mechanism behind this further, we measured CD8+-T-cell-mediated immunity induced by recombinant MCMV-expressing epitopes derived from influenza A virus or lymphocytic choriomeningitis virus placed under the control of an IE promoter. We observed that virus-specific CD8+-T-cell populations were induced and that these expanded gradually over time. Importantly, these CD8+ T cells provided long-term protection against challenge without boosting. These results demonstrate a unique pattern of accumulating T cells, which provide long-lasting immune protection, that is independent of the initial immunodominance of the epitope and indicates the potential of T-cell-inducing vaccines based on persistent vectors.
CD8+ T lymphocytes play a critical role in the control of persistent infections such as human immunodeficiency virus, hepatitis C virus, Epstein-Barr virus, and cytomegalovirus (CMV). Defining the nature of CD8+ T cells mediating immunological protection and the rules governing the maintenance of these cells is crucial not only for our understanding of the pathogenesis of such infections but also for the design of T-cell-based vaccines. It is clear that memory T-cell populations may persist in the absence of antigen or further triggering of their T-cell receptors (1, 41, 49). However, it is also clear that the biology of the inducing pathogen can strongly influence the magnitude and the quality of the resulting T-cell populations. Recent studies with major histocompatibility complex (MHC) class I peptide tetrameric complexes (tetramers) have revealed that human CD8+-T-cell populations specific for different pathogens may show considerable heterogeneity in phenotype (3). In murine models it has been shown that the recirculation pattern, the activation status, and ultimately the protective capacity of memory T cells may also be influenced by the nature of the primary infection (5, 10, 36, 60, 63). We examined in detail the unique T-cell responses induced by murine CMV (MCMV) infection and explored the protective capacity of CD8+ T cells generated in response to these pathogens.
MCMV has been used for decades as an animal model for analyzing the biology and immunology of human herpesvirus infections in general and human CMV (HCMV) in particular. Similar to its human counterpart, MCMV is not eliminated by the host, and persistent, lifelong, and usually latent infection is established (40). Primary infection and maintenance of MCMV latency are under immune control and CD8+ T cells have been shown to be most crucial (45). Holtappels et al. have shown that, after MCMV infection of irradiated bone marrow transplant recipients, CD8+ T cells with an effector memory phenotype accumulate in the lung (24, 25). We have extended these findings by a detailed longitudinal analysis of the phenotype and function of MCMV-specific CD8+ T cells demonstrating persistent accumulation of effector memory cells in several organs (29). These cells appear to encounter antigen in lymph nodes and undergo continuous activation and proliferation over many months.
The accumulation of virus-specific T cells was not uniform across all epitopes. Two epitopes, both of which were immunodominant during acute infection (derived from the immediate-early 1 [IE1/pp89] and m164 gene product) attracted “inflationary” responses, whereas three others were associated with stable, low-level memory responses (25, 29). In these experiments it was not possible to dissect whether the differences between epitopes related to their initial immunodominance or to other important aspects of CMV biology. Both human and murine CMVs possess a range of mechanisms to downregulate antigen presentation, but the genes concerned are not expressed immediately upon infection. Presentation of peptides from IE1/pp89 and m164 appear to escape these class I presentation interference effects (11, 21-23). Thus, during reactivation of the virus throughout latency, it is postulated that such peptides may be presented to CD8+ T cells, leading to continued proliferation and maintained effector function.
Here we evaluated this issue in more detail by using recombinant MCMVs expressing foreign viral epitopes derived from influenza A virus nucleoprotein (NP366-374) or lymphocytic choriomeningitis virus (LCMV) glycoprotein (GP33-41). These epitopes were C terminally fused to the IE2 gene. We tested the hypothesis that such viruses would prime epitope-specific responses which would expand during latency, regardless of their initial immunodominance and regardless of the mouse strain used. In addition, we examined the protective capacity of these populations in challenge experiments. The data provide important insight into the biology of host-CMV interactions and provide a novel perspective in the search for T-cell-based vaccines.
MATERIALS AND METHODS
Mice and animal experiments.
Mice were purchased from the Biomedical Services Unit, John Radcliffe Hospital, Oxford, United Kingdom, and from the Institute for Laboratory Animals, University of Zürich, Zürich, Switzerland. Animals were bred under specific-pathogen-free conditions and housed in a controlled conventional unit during the experiment. All animal experiments were performed with age-matched female BALB/c and C57BL/6 mice with permission of the home office according to the Animals (Scientific Procedures) Act 1986 (United Kingdom) or according to Swiss and Zürich law for the protection of animals, requiring the use of minimal numbers of animals. Mice were infected with a volume of 200 μl of virus in phosphate-buffered saline (PBS) either intravenously (i.v.; MCMV, LCMV, and vaccinia virus [VV]) or intraperitoneally (i.p.; VV) and with 20 μl of virus in PBS intranasally (i.n.; influenza virus). For i.n. influenza virus infection, a short period (<1 min) of ether anesthesia was applied. For subcutaneous immunization with peptide, 200 μg of peptide was solubilized in 100 μl of PBS and then emulsified in 100 μl of complete Freund adjuvant (CFA). For cytometry, a 50-μl blood sample was taken from the tail vein.
Viruses.
Bacterial artificial chromosome (BAC)-derived MCMV MW97.01 has previously been shown to be biologically equivalent to MCMV Smith strain ATCC VR-194 (recently assigned the new accession no. VR-1399) and is referred to here as MCMV (56). Recombinant VV expressing IE1/pp89 (Vac89) (27) was provided by U. H. Koszinowski, Department of Virology, Max von Pettenkofer Institute, Munich, Germany. MCMV was grown on mouse embryonic fibroblasts (MEF) and purified by sucrose cushion centrifugation according to established protocols (9). MCMV titers of virus stocks and organ homogenates were determined by virus plaque assays on MEF as described previously (9) by using centrifugal enhancement of infectivity. Influenza A virus H17 was provided by Keith Gould, Imperial College, London, United Kingdom. VV expressing the NP of influenza virus (VacNP) was provided by A. Townsend, Weatherall Institute of Molecular Medicine, Oxford, United Kingdom, and VV expressing the GP of LCMV (VacGP) by B. Moss, Laboratory of Viral Diseases, National Institutes of Health, Bethesda, Md. (18, 51). Recombinant VV were grown and plaqued on thimidine kinase-deficient (TK−) cells. Diluted and sonicated lysates of VV-infected TK− cells were used for infection.
The ovary protection assay was performed as described previously (8). Briefly, mice previously infected or immunized as indicated in the text were challenged with 106 to 107 PFU of recombinant VV i.p. Four days later, ovaries were homogenized and the VV titer was measured by virus plaque assay on TK− cells. Titers are expressed as the log10(PFU of VV/organ). The detection limit was 10 to 40 PFU of VV/ovary in all assays. The level of protection is given as the percentage of virus titer reduction in the respective experimental group compared to age-matched naive mice or mice infected or immunized with an unrelated virus and/or antigen.
Generation of recombinant MCMVs.
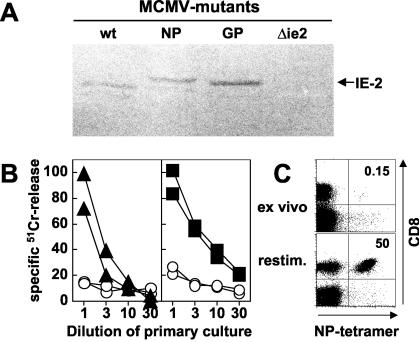
Recombinant MCMV with a C-terminal fusion of the LCMV-derived GP33-41 or the influenza virus-derived NP366-374 peptides (see below) to the nonessential ie2 gene of MCMV were generated by using a new mutagenesis procedure for the MCMV genome cloned as a BAC (pSM3fr) in Escherichia coli (57). It was shown that virus reconstituted from the BAC genome pSM3fr shows wild-type-like properties in vitro and in vivo (56). For the generation of the recombinant viruses MCMV-GP and MCMV-NP, two linear DNA fragments containing a kanamycin resistance gene, with homologies of about 40 nucleotides to the up- and downstream sequences of the stop codon of the ie2 gene and the GP33-41 or NP366-374 sequence, respectively, were generated by PCR. To generate the fragment containing the GP33-42 sequence (and an additional six flanking nucleotides on each side), the contiguous primers ie2-LCMV-5 (5′-GAACCACGGGTTCTTTCTCTTGACCAGAGACCTGGTGACCGTCAGGAAGAAGATTCAGGGTATCAAGGCTGTCTACAATTTTGCCACCTGTGGGATATGACGATTTATTCAACAAAGCCACG-3′)(GP33-41, underlined) and ie2-Kn-3 (5′-CTGTCCGATGAATAAAACCTCTTTATTTATTGATTAAAAACCATGACATACCTCGTGTCCTCGCCAGTGTTACAACCAATTAACC-3′) and the template plasmid pACYC177 (NEB) were used for PCR. To generate the fragment containing the NP366-374 sequence (and additional six flanking nucleotides on each side), the contiguous primers ie2-Flu-5 (5′-GAACCACGGGTTCTTTCTCTTGACCAGAGACCTGGTGACCGTCAGGAAGAAGATTCAGCAAATTGCTTCAAATGAAAACATGGATGCTATGGAATCATGACGATTTATTCAACAAAGCCACG-3′) (NP366-374, underlined) and ie2-Kn-3 (see above) and plasmid pACYC177 were used for PCR. The fragment was then transformed into DH10B for mutagenesis of the MCMV-BAC pSM3fr, which contains the whole MCMV genome (strain Smith). Mutagenesis was performed according to a recently published protocol that uses homologous recombination mediated by the recombination functions redαβ from bacteriophage λ (55). Correct fusion of the epitope sequences to the C terminus of the ie2 gene within the viral genomes was confirmed by restriction pattern analysis of the recombinant MCMV BAC with NsiI and by additional sequencing of the ie2-epitope fusion (data not shown). Expression of the IE2-epitope fusion was also confirmed by Western blot analysis with an IE2-specific mouse antiserum that was generously provided by Martin Messerle, University of Halle-Wittenburg (Fig. 2A) (38).
FIG. 2.
Efficient induction of specific CD8+ T cells after infection with recombinant MCMV. (A) Western blot analysis of expression of recombinant proteins from viruses in vitro. MEF were infected with wild-type (wt) MCMV, with MCMV-NP (NP), with MCMV-GP (GP) or, as a negative control, with a mutant MCMV lacking expression of IE2 (Δie2). To make IE2 expression detectable, protein transcription and translation were synchronized by the treatment of cells with cycloheximide for the first 3 h of infection and with actinomycin D 4 to 7 h postinfection. Protein expression was detected 7 h postinfection by using a mouse antiserum specific for IE2. The appropriate IE2 bands are marked on the figure. The NP-IE2 and GP-IE2 fusion proteins show a slightly higher molecular weight due to the additional NP and GP epitopes, respectively. (B) C57BL/6 mice were infected with 2 × 106 PFU of MCMV-GP (left panel) or MCMV-NP (right panel). At 10 days after infection, splenocytes were harvested, restimulated for 5 days in vitro, and then tested in a 5-h 51Cr-release assay on target cells pulsed with GP33 (▴), NP366 (▪), or an irrelevant peptide (○). Each line represents an individual mouse. The results for one of four experiments are shown. (C) NP tetramer staining of spleen cells from a mouse infected 10 days previously with MCMV-NP. The upper panel shows an ex vivo tetramer staining. For the lower panel, splenocytes from the same mouse were restimulated for 7 days in vitro with NP366-pulsed naive spleen cells. The numbers indicate the percentage of NP-specific cells of CD8+ T cells. Panels are gated on live lymphocytes without B cells. The results for one of seven mice are shown.
Peptides and tetramers.
The following peptides defining MCMV-, LCMV-, and influenza virus-derived cytotoxic-T-lymphocyte (CTL) epitopes were used: MCMV-IE1 (m123/pp89; pp89, H-2Ld restricted, YPHFMPTNL) (47), LCMV-GP (GP33-41, KAVYNFATC, H-2Db restricted) (44) or GP34-41, (AVYNFATC, H-2Kb restricted) (26), and influenza virus NP (NP366-374, ASNEMNDAM, H-2Db restricted) (51). Peptides were synthesized at a purity of >70% (Research Genetics, Huntsville, Ala.), diluted, and used at the indicated concentrations.
The tetramers used in the present study were produced as described previously (2). Briefly, recombinant H-2Ld, H-2Db, and H-2Kb heavy chains were expressed in E. coli (BL21), purified from inclusion bodies, biotinylated enzymatically, and refolded with human β2-microglobulin and the appropriate peptide. Plasmids encoding for the different MHC class I heavy chains were generously provided by Awen Gallimore (Oxford University, Oxford, United Kingdom) and John D. Altman (Stanford University, Stanford, Calif.). Refolded complexes were purified by high-pressure liquid chromatography and tetramerized by using phycoerythrin-labeled extravidin (Sigma-Aldrich, Poole, United Kingdom) at a molar ratio of 4:1. The specificity and sensitivity of the tetramers was regularly tested on CTL lines restimulated with the appropriate peptide.
Assessment of IFN-γ-secreting peptide-specific CD8+-T-lymphocyte frequencies.
Frequencies of gamma interferon (IFN-γ)-producing peptide-specific T cells in the spleen were quantified by intracellular cytokine staining (ICS) after stimulation with peptide according to the protocols of the supplier of reagents (Becton Dickinson, San Jose, Calif. [BD]). After 6 h of peptide stimulation (at 10−6 M), spleen cells were stained with allophycocyanin- or PerCP-labeled rat anti-mouse CD8 (clone 53-6.7), fixed, permeabilized, and then stained with fluorescein isothiocyanate (FITC)-labeled rat anti-mouse IFN-γ (clone XMG1.2) or with an FITC-labeled rat immunoglobulin G1 (IgG1) isotype control antibody. Samples were analyzed with a FACSCalibur (BD) by using CellQuest software. Gates were set according to the isotype control, and frequencies are given as the percentage of IFN-γ-secreting cells of total CD8+ T cells. Background staining for IFN-γ was <0.05% of CD8+ T cells after stimulation with irrelevant peptides and after stimulation of cells from naive mice.
Within the region of LCMV-GP, which is shared between MCMV-GP, VacGP, and the peptide GP33-41 (33KAVYNFATC41) used for immunization, two differentially restricted CD8+-T-cell epitopes exist in the H-2b haplotype: H-2Db-restricted GP33-41 and H-2Kb-restricted GP34-41 (26, 44). After in vitro stimulation with KAVYNFATC, specific CD8+ T cells with either MHC restriction produce IFN-γ; thus, these percentages are the sum of GP33- and GP34-specific cells.
Ex vivo detection and phenotypic characterization of antigen-specific T cells with MHC class I tetramer and flow cytometry.
A total of 50 μl of peripheral murine blood or 106 murine spleen cells was prepared in cold PBS containing 2% fetal calf serum, 0.2% NaN3, and 10 mM EDTA. Cells were stained for 20 min at 37°C with the respective tetramer. Other surface markers were then quantified by staining for 30 min at 4°C with the following monoclonal antibodies (purchased from BD): allophycocyanin- or PerCP-labeled rat anti-mouse CD8 (clone 53-6.7), PerCP- or FITC-labeled rat anti-mouse CD45R/B220 (clone RA3-6B2), FITC-labeled rat anti-mouse CD18 (clone C71/16), FITC-labeled rat anti-mouse CD431B11 (clone 1B11), FITC-labeled rat anti-mouse CD44 (PgP-1, clone IM7), and allophycocyanin- or FITC-labeled rat anti-mouse CD62L (MEL-14). FITC-labeled hamster anti-mouse CD69 (clone H1.2F3) was purchased from Serotec, Ltd., Oxford, United Kingdom. FITC-labeled rat IgG2a (BD) and hamster IgG (Serotec) isotype controls were used for gate settings. Analysis was performed as described above.
Assessement of MCMV-specific cytotoxic activity in vitro.
CTL activity of spleen cells was determined by 51Cr-release assay as described previously (18). Effector spleen cells were restimulated for 5 days in vitro with peptide-pulsed (10−6 M) and gamma-irradiated (25 Gy) spleen cells. Serial dilutions of bulk cultures were incubated for 5 h with 51Cr-labeled and peptide-pulsed (10−6 M) EL4 target cells (H-2b), and the supernatant was assayed for released 51Cr. Spontaneous 51Cr-release and unspecific lysis of target cells pulsed with irrelevant peptides was <15% in all assays.
Calculation of correlation between frequencies of epitope-specific CD8+ T cells, level of protection, and time after priming.
Mice were primed with the indicated viruses and the frequency of epitope-specific CD8+ T cells was measured in the blood 0 to 3 days before challenge with recombinant VV. Four days after challenge the VV titers were determined in the ovaries of immunized and naive age-matched mice. The level of protection was calculated by subtracting the log10PFU of VV titer of the immunized mice from the mean log10PFU of VV titer of the naive group of mice. The frequency of epitope-specific CD8+ T cells before challenge was then correlated with the level of protection and the time since priming. In addition, the level of protection was correlated with the time since priming. Correlations were calculated by using Spearman's rank correlation on a logarithmic scale. The numbers of animals included into the calculations are indicated in Table 2.
TABLE 2.
Correlation between the frequency of epitope-specific CD8+ T cells, the level of protection measured by VV challenge, and the time after priming with MCMV-wt, MCMV-NP, or influenza virusa
| Priming (PFU) | n | Spearman rank correlation of factor vs factor
|
||
|---|---|---|---|---|
| Frequency of specific cells vs time after priming (P) | Frequency of specific cells vs level of pro- tection (P) | Time after priming vs level of pro- tection (P) | ||
| MCMV (103) | 40 | 0.841 (<0.001) | 0.521 (0.001) | 0.483 (0.002) |
| MCMV-NP | 23 | 0.702 (<0.001) | 0.507 (0.014) | 0.513 (0.012) |
| Influenza virus | 23 | −0.882 (<0.001) | 0.760 (<0.001) | −0.736 (<0.001) |
| Overall | 86 | 0.566 (<0.001) | ||
Mice were primed with the indicated virus, and the frequency of epitope-specific CD8+ T cells was monitored in the blood by tetramer staining. Frequencies used for calculation were measured 0 to 3 days before challenge. At 4 days after challenge, the VV titers were measured in the ovaries. The level of protection was calculated as described in Materials and Methods to correct for variation between different challenge experiments. Numbers indicate the correlation coefficients that were calculated with the Spearman's rank correlation using logarithmic scales for all variables. Two-tailed P values for each correlation are indicated in parentheses. Data from one to three experiments per virus and time point were included in the analysis.
RESULTS
Accumulation of IE1/pp89-specific CD8+ T cells and association with protection against in vivo challenge.
To evaluate the accumulation of MCMV-induced CD8+ T cells and its relationship to the size of the initial response, BALB/c mice were infected i.v. with a low dose (103 PFU) or a high dose (106 PFU) of MCMV. We then measured the frequency of CD8+ T cells specific for a dominant H-2Ld-restricted epitope derived from IE1 (IE1/pp89) in blood and spleen by staining cells with tetramers, and we characterized the phenotype and function of these cells longitudinally.
Previously, we have demonstrated that functional pp89-specific CD8+ T cells accumulate continuously after high-dose MCMV infection, reaching up to 20% of all CD8+ T cells (29) (Fig. 1A). This accumulation of memory T cells over time is not dependent on a large initial CD8+-T-cell expansion, since it was also evident after low-dose MCMV infection (Fig. 1A and Table 1). In this instance, a much lower initial peak of specific CD8+ T cells was generated, but subsequent accumulation during latency nevertheless was observed. Between 75 and 85% of these accumulating pp89-specific CD8+ T cells remained CD62Llo after both high- and low-dose infections in blood and spleen, a finding that is characteristic for effector memory cells. More than 90% of pp89-specific cells were immediately functional, as assessed by ICS (data not shown). The kinetics of accumulation in the blood closely reflect the total number of cells within the spleen (Table 1), with results similar to our previous observation demonstrating the accumulation of pp89-specific T cells also in the liver, lung, salivary gland, and lymph node (29).
FIG. 1.
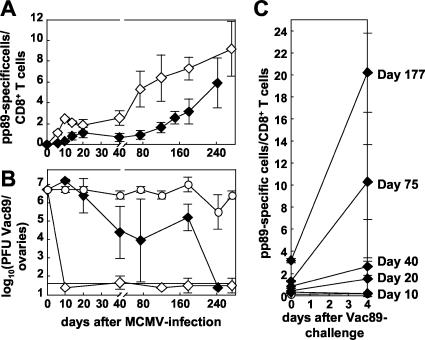
Protective immunity mediated by pp89-specific CD8+ T cells is dependent on the dose of infection and the timing of challenge. BALB/c mice were infected i.v. with 106 (◊) and 103 (⧫) PFU of MCMV or were left naive (○) as a negative control. (A) Thereafter, the pp89-specific CD8+-T-cell response was measured longitudinally in the blood. The percentage of CD8+ T cells staining with pp89 tetramer is plotted over time. Each line represents the mean of four to eight mice per experimental group. Error bars indicate the standard deviation (SD) within experimental groups. (B) At the indicated time points after MCMV infection, mice were challenged with Vac89 i.p., and the VV titers were measured in the ovaries 4 days later. Titers are given as the log10(PFU of Vac89/ovaries). The detection limit and the SD are indicated. Each line represents the mean of four or five mice per experimental group. The results from one of two similar experiments are shown; the second experiment was finished by day 120 after priming. To measure the expansion of specific T cells after challenge, the frequency of pp89-specific CD8+ T cells was determined by tetramer staining before and 4 days after challenge with Vac89 in the spleen at indicated time points after MCMV infection (Fig. 1C, blood, liver, and ovaries [data not shown]). The percentage of CD8+ T cells staining with pp89 tetramer is plotted over time. Each line represents the mean of three to four mice per experimental group. Error bars indicate the SD within experimental groups.
TABLE 1.
Total number of epitope-specific CD8+ T cells per spleen at different time points after infection with MCMV or influenza virus or after immunization with peptide GP33a
| Time postinfection (days) | Total no. of epitope-specific CD8+ T cells/spleen (104) ± SD with epitope:
|
|||||
|---|---|---|---|---|---|---|
| IE1/pp89 (PFU)
|
NP366
|
GP33
|
||||
| MCMV (106) | MCMV (103) | MCMV-NP | Influenza virus | MCMV-GP | GP33+CFA | |
| 8-11 | 56.8 ± 20 | 2.4 ± 0.3 | 2.5 ± 1.1 | 55.8 ± 28.5 | 1.1 ± 0.5 | 25.1 ± 9.7 |
| 18-22 | 31.3 ± 8.7 | 2.9 ± 0.8 | 2.9 ± 1.6 | 20.3 ± 10 | 2.0 ± 0.7 | |
| 35-45 | 26.5 ± 14.4 | 7.9 ± 2.8 | 6.9 ± 3.7 | 4.8 ± 0.9 | 10.4 ± 3.7 | 18.7 ± 6.4 |
| 100-160 | 59 ± 13.1 | 24.5 ± 6.9 | 12.7 ± 4.8 | 5.2 ± 2.2 | 15.5 ± 6.3 | 10.9 ± 2.6 |
| 250-350 | 115 ± 28 | 45.8 ± 22 | 9.2 ± 5.3 | 3.9 ± 0.5 | ||
Mice were infected or immunized with the indicated virus or antigen. At different time points thereafter the total numbers of epitope-specific CD8+ T cells per spleen were measured by fluorescence-activated cell sorting by using tetramer staining (IE1/pp 89 and NP366) or ICS (GP33). Mean numbers ± the SD are given for 4 to 12 mice per group. Data from one to three experiments with the same antigen or virus were pooled for calculation. Values of 106 and 103 indicate the virus dose in PFU used for infection.
To test the in vivo protective capacity of pp89-specific CD8+ T cells, mice were challenged at various time points after MCMV infection with pp89-expressing Vac89 (usually 107 PFU) and, 4 days later, the VV titers were determined in the ovaries. Challenge with recombinant VV has been widely used to test the level of T-cell-mediated protective immunity in vivo (4, 5, 8, 28, 34, 60). The stringency of the assay varies with the genetic background of the respective recombinant VV, with the dose of VV challenge, and with the time interval between challenge and analysis.
Mice were completely protected against Vac89 challenge from day 10 until day 272 after infection with a high dose of MCMV (106 PFU, Fig. 1B). We observed no protection after challenge with an unrelated VV at any time point after priming, indicating that protection was pp89 specific and was not significantly influenced by natural or heterologous immunity (not shown). After infection with a low dose of 103 PFU MCMV, no significant protection was observed until day 20. Protection then increased until day 75 (when VV titer were 99% lower than in naive animals), and this level of protection was maintained until day 177. Using a lower dose of VV for challenge (106 PFU), four of four mice were completely protected against challenge on day 242 (Fig. 1B). Overall, for mice primed with 103 PFU of MCMV, the level of protection against challenge increased gradually over time after MCMV-infection and thus correlated with increasing frequencies of pp89-specific T cells (Table 2).
Protective capacity was associated with a marked expansion of pp89-specific CD8+ T cells in vivo after VV infection. These expansions were antigen specific, they occurred in spleen (Fig. 1C), blood, liver, lymph node, and ovaries (data not shown), and they showed a positive correlation with the in vivo protective capacity. Interestingly, the magnitude of the expansion seemed to correlate with the time after MCMV infection (Fig. 1C).
CD8+-T-cell induction by recombinant MCMVs.
To test the potential of MCMV to induce CD8+-T-cell responses against recombinant IE-expressed epitopes and to evaluate whether memory inflation would also occur in a different genetic background of the host, we generated two different recombinant viruses encoding two H-2Db-restricted murine CD8+-T-cell epitopes. They were derived from influenza A virus nucleoprotein (NP366-374, MCMV-NP) and from LCMV glycoprotein (GP33-41, MCMV-GP) and included two additional N- and C-terminal flanking amino acids. Recombinant epitopes were introduced into MCMV-IE2, which is dispensable for in vitro and in vivo viral replication and is expressed before MHC class I interference by MCMV becomes operational during the early (E) phase of viral replication (22, 40). In addition, IE2-transcripts have been found during MCMV latency (17).
MEF infected with MCMV-NP and MCMV-GP expressed IE2 similar to wild-type virus (MCMV-wt), when transcription and translation of viral IE proteins were synchronized with metabolic inhibitors. As expected, the IE2 protein of the recombinant viruses was slightly larger than of MCMV-wt due to the inserts (Fig. 2A). Replication kinetics and cell and tissue tropism of recombinant viruses was comparable to MCMV-wt in vitro and in vivo (not shown).
Ten days after infection of C57BL/6 mice with 2 × 106 PFU of MCMV-NP or MCMV-GP, ex vivo cytotoxic activity was not detectable by 51Cr-release assay (not shown) and tetramer staining was at or below the limit of detection (≤0.15% tetramer positive cells of CD8+ T cells; Fig. 2C, ex vivo). After 5 to 7 days of in vitro restimulation, cytotoxicity specific for recombinant epitopes and staining with the respective tetramer were strongly positive (Fig. 2B and C, restim.). These results demonstrate that priming of CD8+ T cells specific for recombinant epitopes expressed in MCMV-IE2 did occur early after infection, but the responses induced were initially of low magnitude.
Slow accumulation of NP-specific CD8+ T cells after infection with MCMV-NP and correlation with protective immunity.
We next analyzed NP-specific CD8+ T cells emerging over time after infection with influenza virus or MCMV-NP (13). We used several phenotypic markers to analyze activation status and presumptive recirculation pattern of NP-specific CD8+ T cells. In particular, CD62L expression was used to differentiate between “central” (i.e., lymphoid organ homing) memory CD8+ T cells (CD62Lhi) and effector (i.e., nonlymphoid organ homing) memory cells (CD62Llo) (29, 36, 42, 48). We compared the responses generated after persistent infection with recombinant MCMV with those elicited by the same epitope after natural, acute, and transient influenza virus infections.
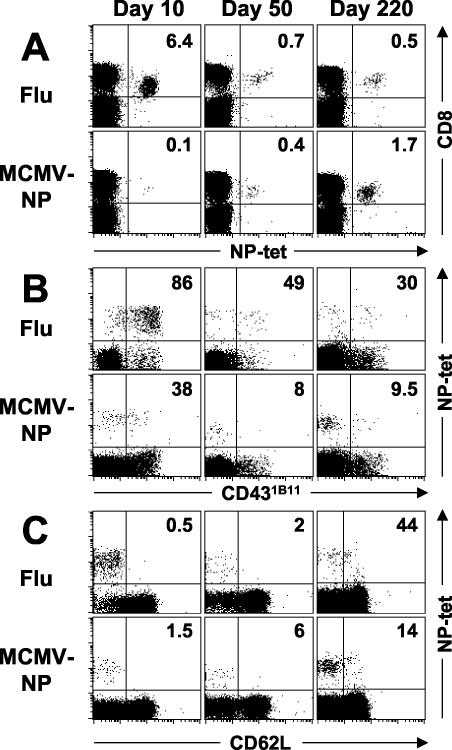
After influenza virus infection, NP-specific CD8+ T cells rapidly expanded, reaching a peak of 4 to 5% of CD8+ T cells after 8 to 10 days in the blood (Fig. 3A and 4A) and a total number of (5.6 ± 2.8) × 105 cells per spleen (Table 1). More than 95% of the cells were CD18 and CD44 positive but CD62Llo, and 75 to 85% produced IFN-γ ex vivo (Fig. 3C, ICS, CD18, and CD44 [not shown]). Early after i.n. influenza virus infection, NP-specific cells in the spleen were mostly low in expression of CD69 but high in expression of CD431B11 (Fig. 3B), which is compatible with recent activation, although the exact pattern of CD431B11 expression may potentially differ between mouse strains or infectious models (19, 37).
FIG. 3.
Phenotypic characterization of NP-specific CD8+ T cells after infection with MCMV-NP or influenza. C57BL/6 mice were infected i.v. with 2 × 106 PFU of MCMV-NP or i.n. with 100 hemagglutination units of influenza virus. Spleen cells were harvested at the indicated time points and then stained with NP tetramer (NP-tet), anti-CD8, or anti-B220 and with anti-CD62L. Panels are gated on live lymphocytes without B cells (A) or on live CD8+ lymphocytes (B). The numbers indicate the percentages of NP-specific cells among CD8+ T cells (A) or the percentages of NP-specific CD8+ T cells expressing CD431B11 (B) or CD62L (C). The results of one of three similar experiments are shown.
FIG. 4.
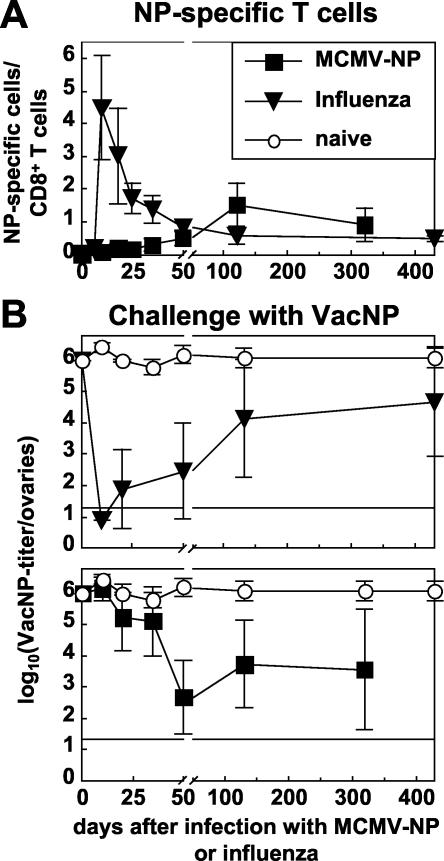
Increasing protective CD8+-T-cell-mediated immunity after infection with MCMV-NP. C57BL/6 mice were infected with 2 × 106 PFU of MCMV-NP i.v. (▪) or with 100 hemagglutination units of influenza virus i.n. (▾), or they were left naive (○) as a control. (A) Thereafter, the NP-specific CD8+-T-cell response was measured longitudinally in the blood. The percentage of CD8+ T cells staining with NP tetramer is plotted over time. Each line represents the mean of four to five mice per experimental group. Error bars indicate the SD within experimental groups. (B) At indicated time points after infection with influenza virus (upper panel) or MCMV-NP (lower panel), mice were challenged i.p. with VacNP, and the VV titers were measured in the ovaries 4 days later. Titers are given as the log10(PFU of VacNP/ovaries). The detection limit and the SD are indicated. Each line represents the mean of four or five mice per experimental group. The experiment was repeated with comparable results.
Thereafter, NP-specific CD8+-T-cell populations contracted by 90 to 95% and were maintained at a stable memory level of 0.5% of CD8+ T cells in the blood and (3.9 to 5.2 [±2.2]) × 104 NP-specific cells per spleen (Table 1). As demonstrated previously, within a year after influenza virus infection a substantial proportion of NP-specific CD8+ T cells (40 to 60%) regained expression of CD62L, a finding typical for resting or central memory cells (Fig. 3C, day 220) (52).
After infection with recombinant MCMV-NP, a completely different pattern of CD8+-T-cell expansion emerged. Up until day 20, the frequency of NP-specific CD8+ T cells was generally below or just at the limit of detection by tetramer or ICS, reaching (2.5 to 2.9 [±1.6]) × 104 NP-specific cells per spleen (Table 1 and Fig. 3A and 4A [ICS not shown]). If NP-specific cells were measurable by tetramer staining early after MCMV infection (and not below the limit of detection), they expressed high levels of CD18 and CD44 but they were consistently low in CD69, CD431B11, and CD62L, suggesting previous contact with antigen but a lower level of activation than after influenza virus infection (not shown and Fig. 3B and C).
Slowly but steadily, the frequencies of NP-specific T cells increased over time, reaching a level of 1.5% of CD8+ T cells by day 120 ([12.7 ± 4.8] × 104 cells/spleen). Thereafter, we observed no further increase but rather fluctuating T-cell frequencies ranging between 0.7 and 1.8% ([9.15 ± 5.3] × 104 cells/spleen). In the memory phase, 85 to 90% of NP-specific CD8+ T cells maintained a CD62Llo effector memory phenotype (Fig. 3C, day 220) and produced IFN-γ ex vivo (ICS results not shown).
To compare the level of protection generated after infection with influenza virus with that generated after MCMV-NP infection, mice were challenged at different time points with 5 × 106 PFU VacNP i.p. Four days later, we measured the VacNP titer in the ovaries. These three viruses only share one CTL epitope (NP366-374).
As expected from the frequency of functional NP-specific T cells, mice were highly protected against VacNP challenge during the first 50 days after influenza virus infection (Fig. 4B). Thereafter, protection was partially and gradually lost in parallel to the loss of NP-specific memory cells resulting, as expected, in a negative correlation between the time after influenza virus infection (priming) and the level of protection: c = −0.736, where c is the correlation coefficient (P < 0.001 [Table 2]). However, on average, VacNP titers were still 96% reduced compared to naive mice 430 days after influenza virus infection.
In contrast, over the first 35 days mice infected with MCMV-NP were not protected against VacNP challenge. Only by day 50, when NP-specific CD8+ T cells had accumulated sufficiently, was a significant level of protection (99.97%) reached, a level comparable to that of mice infected with influenza virus (Fig. 4B). A high level of protection was maintained thereafter, correlating roughly with the frequency of NP-specific CD8+ T cells (c = 0.507, P = 0.014), although there was considerable variation between individual mice late after priming. Nevertheless, the level of protection was positively correlated with the time since priming with MCMV-NP (c = 0.513, P = 0.012; Table 2).
Protective CD8+-T-cell-mediated immunity after infection with MCMV-GP.
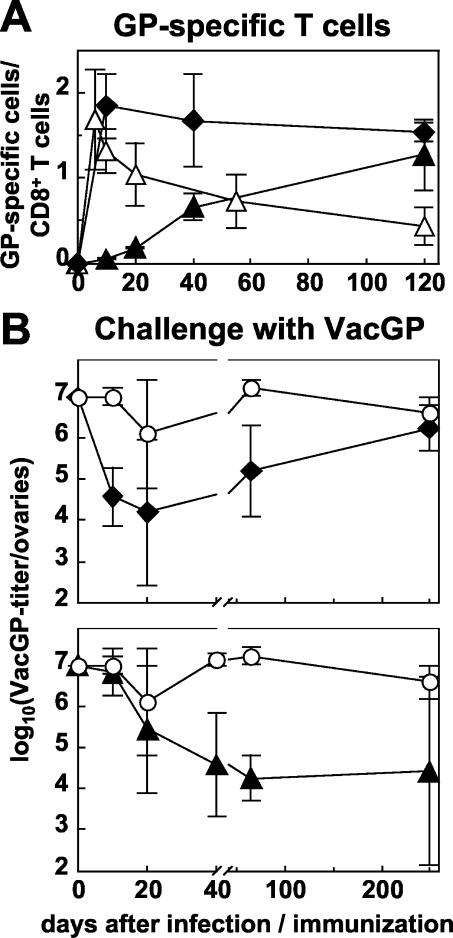
We used the same system to analyze immunity generated against a second antigen, in this case derived from LCMV. We compared the GP-specific CD8+-T-cell response after infection with MCMV-GP, after infection with VV recombinant for LCMV-GP (VacGP), and after immunization with GP33-41 peptide emulsified in CFA (Fig. 5A). The response generated by VacGP was maximal on day 6 and then dropped to a stable memory level of 0.4% of CD8+ T cells. After immunization with GP33 in CFA, GP-specific T cells expanded to 1.9% within 10 days and were then maintained at a nearly constant level for 120 days, reflecting the granulomatous inflammation and delayed antigen release provoked by CFA. However, the total numbers of GP-specific cells per spleen dropped from (25 ± 9.7) × 104 on day 10 to (10.9 ± 2.6) × 104 on day 120 (Table 1). In contrast, after infection with MCMV-GP, GP-specific CD8+ T cells also showed the pattern of “memory inflation” as demonstrated previously for NP-specific T cells after infection with MCMV-NP. By day 120, ca. 1.3% of CD8+ T cells were GP specific in the spleen. Overall, the total number of GP-specific cells per spleen increased from (1.1 ± 0.5) × 104 on day 10 to (15.5 ± 2.3) × 104 on day 120 (Table 1).
FIG. 5.
Increasing protective CD8+-T-cell-mediated immunity after infection with MCMV-GP. C57BL/6 mice were infected i.v. with 2 × 106 PFU of MCMV-GP (▴) or i.v. with 5 × 106 of PFU VacGP (▵) or were immunized s.c. with the peptide GP33 and CFA (⧫), or they were left naive (○) as a control. (A) Thereafter, the GP-specific CD8+-T-cell response was measured longitudinally in the spleen by ICS. The percentage of CD8+ T cells staining for intracellular IFN-γ after stimulation with GP33-41 is plotted over time. Each line represents the mean of three to four mice per experimental group. Error bars indicate the SD within experimental groups. (B) At indicated time points after immunization with GP33+CFA (upper panel) or after infection with MCMV-GP (lower panel), mice were challenged i.p. with VacGP, and the VV titers were measured in the ovaries 4 days later. Titers are given as the log10(PFU of VacGP/ovaries). The detection limit was 1.6, and the SD is indicated. Each line represents the mean of four to five mice per experimental group.
Mice infected previously with MCMV-GP or immunized with GP33+CFA were then challenged with VacGP, and the VV titer was measured in the ovaries. GP33+CFA induced partial protection from 10 day until day 65 of ca. 99% compared to naive mice. By day 250 however, protection was lost completely (Fig. 5B, upper panel). In contrast, protection generated by infection with MCMV-GP developed more slowly within 40 days (99.7%) but was then maintained with considerable variation between individual mice (Fig. 5B, lower panel).
DISCUSSION
In this study we have demonstrated that the pattern of slowly accumulating CD8+-T-cell responses after CMV infection is not restricted to certain T-cell epitopes or to a particular strain of inbred mice. It can occur in immunodominant and in subdominant epitopes independently of the dose of infection and even T cells specific for recombinant epitopes accumulate over time. These T cells are of the effector memory phenotype and provide, as they accumulate, long-term protective immunity. These findings have important implications for our understanding of the immunological long-term control of CMV infection in mice and, potentially, humans. In addition, these results provide a first proof of the principle for the use of persisting vectors to maintain or even increase protective CD8+-T-cell responses without external antigen reexposure.
Efficient induction of protective CD8+-T-cell responses by cross-priming with nonreplicating antigens has proven to be a difficult task to achieve despite constant refinement of vectors and immunization protocols and despite recent improvements in our understanding of how innate immune mechanisms direct and enhance the adaptive immune response (7). It is even more challenging to develop practical immunization regimes capable of maintaining T-cell-mediated protection over prolonged periods of time, and it has been suggested that persistent antigen is required (5, 34). Our results support and extend these findings: CD8+-T-cell-mediated protection against challenge with recombinant VV decreased late after infection with live influenza virus correlating with the loss of effector memory cells. This was despite a very potent primary CD8+-T-cell response, which was quantitatively at least 20-fold higher than after infection with MCMV-NP (NP-specific CD8+ T cells/spleen on day 8: 5.6 × 105 versus 2.5 × 104). Similarly, protective immunity was lost late after priming with the synthetic GP33 peptide, despite the use of a very potent adjuvant, inducing inflammation and slow antigen release (15). In addition, protection against challenge with LCMV is known to decrease rapidly 80 days after priming with recombinant VV expressing LCMV-GP (5, 34). These different priming conditions, although very efficient initially, share the feature of transient antigen delivery to CD8+ T cells. Conversely, protective immunity was weak initially but improved over time after priming with recombinant MCMV. The acquisition of protection was associated with the emergence of epitope-specific effector memory T cells.
The cells generated by MCMV infection have the characteristics of “effector” memory T cells, possessing rapid effector function ex vivo and more importantly, providing protection in vivo. The phenotype shown is probably similar to the “mature” memory phenotype associated with HCMV-specific T-cell populations in humans (3, 16, 30). These cells remain low in CD62L and may therefore effectively patrol and protect nonlymphoid organs. Indeed, in addition to accumulation in blood and spleen as demonstrated here, the accumulation of memory T cells in other organs (liver, salivary gland, lung, ovary, etc.) has been demonstrated in BALB/c mice (24, 25, 29). Similarly, after rechallenge with recombinant VVs, very striking expansions of virus specific CD8+ T cells occur rapidly in the spleen and in the liver (up to 40% of infiltrating CD8+ T cells [data not shown]), as well as clearly protecting the ovary. It has been demonstrated that the protective capacity of an anti-Listeria response induced by vaccination is strongly linked to the CD62L expression and thus recruitment and redistribution characteristics of CD8+ T cells (36). Recent data has, however, suggested that in other situations the protective capacity of central memory T cells generated from TCR-transgenic mice is even more significant compared to effector memory cells, and this has been linked to an increased proliferative capacity of central memory cells (60). Although the CD8+-T-cell populations after MCMV infection (pp89, NP, and GP specific) were not pure, they are largely effector memory (>75%) and nevertheless did show strong protective capacity and massive expansion upon challenge (Fig. 1C), suggesting that these experiments may strongly depend on the exact nature of the priming and its duration and on the exact nature of the challenge.
The cells induced by MCMV, which expand during latency, retain effector function in the long term, as judged by IFN-γ production measured by ICS or ELISPOT (24, 25, 29; data not shown). This is true even after initial MCMV-infection during immunosuppression for bone marrow transplantation (24). However, a significant variability in the protective capacity of MCMV-primed cells was noted which was more pronounced than after influenza virus infection. Further functional analysis may be required to identify any specific cellular defects which might account for a lack of protection despite increased frequencies. Nevertheless, there was a reasonable correlation between the precence of a MCMV-induced population of T cells and protection against challenge (Table 2).
Although formal experimental proof is lacking, a reasonable explanation for this unique pattern of accumulating CD8+ T cells mediating long-term protection is persistent or repetitive exposure to antigen. This idea is supported by recent virologic studies in MCMV: transcription of IE1 and, somewhat less frequently, of IE2 (where the recombinant epitopes were inserted) occurs randomly during MCMV latency, and the frequency of these events seems to be dependent on the amount of latent MCMV genomes (17, 35, 46). These are the first steps of any reactivation event of MCMV. Whether or not the reactivation proceeds further to transcription of early and late genes is likely to be under additional control mechanisms and does usually not occur. CD8+ T cells have been shown to be most crucial to maintaining MCMV latency (45). Therefore, CD8+ T cells specific for IE1- and IE2-derived epitopes will encounter their cognate antigen repetitively during MCMV latency, leading to endogenous boosting.
Moreover, MCMV-dependent MHC class I downregulation is not yet operational during the IE phase of viral reactivation attempts contributing to efficient presentation of these epitopes (22). Interestingly, similar accumulations of CD8+ T cells specific for an epitope derived from m164 were found, although m164 is not expressed under the control of an IE promoter (23, 25, 29). It is possible that recombinant epitopes inserted in this and potentially other genes may show similar patterns of immunogenicity.
There are several possible explanations why accumulation of IE1/pp89-specific cells in BALB/c mice was more pronounced than accumulation of NP- or GP-specific cells in C57BL/6. First, differences between these strains of mice particularly concerning natural killer cell activity could contribute to a reduced amount of latent MCMV genomes after primary infection and to enhanced control of MCMV latency by natural killer cells in C57BL/6 (14, 46). Second, the processing of the natural epitope (IE1/pp89), which has been selected during long-lasting coevolution of virus and host is probably more efficient than the processing of recombinant epitopes. Third, Grzimek et al. have shown that the frequency of reactivation events is higher for IE1 than for IE2 in the lungs of latently infected mice (17).
Clearly, we have not used specifically developed vaccine vectors, adjuvants, or sophisticated immunization regimes in our control groups, which would certainly improve the efficiency of CD8+-T-cell induction. Nevertheless, we used the original pathogen (influenza) in a major part of the present study, and most modern vaccines are still less efficient at inducing immune responses than the natural pathogens (6). However, we have not selected the recombinant MCMV for high expression of recombinant epitopes in vivo, for their optimal processing, or for the expression of additional immunostimulatory effects, and we have used a similar straightforward immunization protocol for priming with recombinant MCMV. In addition, our recombinant viruses still express the whole “machinery” of immune deviation and subversion, particularly concerning MHC class I function (20, 22). It seems quite likely that deletion of some of these genes will lead to an attenuated viral phenotype and might even increase their immunogenicity. As a relevant example, deletion of m152, which is responsible for retention of MHC class I peptide complexes in the cis-Golgi compartment, led to drastically improved MCMV control by CD8+ T cells in vivo (33). It will therefore be important to demonstrate whether attenuated MCMVs retain the characteristics of protective memory T-cell accumulation described in the present study.
In relation to humans, recent studies have shown that very large numbers of functional HCMV-specific CD8+ T cells are present in seropositive individuals long after resolution of the primary infection (16, 30, 53, 58, 61). Indeed, such responses in the elderly may comprise a significant proportion of the total lymphocyte pool (43, 59). In addition, certain activation markers such as CD69 are overrepresented in HCMV-specific CD8+ T cells, suggesting recent activation by antigen (12). However, since primary infection is usually clinically silent, we know little about the longitudinal evolution of these massive HCMV-specific CD8+-T-cell pools over time. Large-scale studies are needed to evaluate to what extent the principle of “memory inflation” in mice also applies to certain HCMV-specific T-cell responses in humans. However, some indications of possible, memory inflation from small-scale cross-sectional studies already exist (31).
The most likely explanation for these massive HCMV-specific T-cell populations in healthy seropositive individuals is that HCMV, like MCMV, constantly initiates reactivation during clinically latent infection (17, 35). This leads to a repetitive (or possibly constant) triggering of HCMV-specific memory T cells, which both prevents reactivation and leads to the accumulation of T cells over time. Whether memory inflation is needed to maintain virologic control or whether a stable level of memory T cells would perform the same function remains speculative. Understanding this requires very detailed analysis of the extremely low viral load of MCMV or HCMV and its distribution over time.
The pattern of CD8+-T-cell responses demonstrated here is unique. To exploit this type of immune response for the development of a safe vaccine, such a vector would have to be engineered to allow latent infection with regulated recombinant protein expression without the possibility of productive reactivation. Herpesvirus-based vaccines already exist in the form of vaccines directed against varicella-zoster virus (50, 54), and in that case the benefits and the risks of reactivation need to be carefully balanced (32, 39). Whether we will be able to harness the biology of these viruses to generate safe, successful recombinant vaccines for complex infectious diseases such as human immunodeficiency virus, hepatitis C virus, tuberculosis, and malaria, as well as for tumors such as melanoma, is a challenge for the future (62). In addition, it will be important to establish, in careful long-term experiments, whether any of the current DNA-based regimes possess the potential to recruit antiviral effector cells over extended periods of time. On the basis of the evidence presented here, we feel, however, that for T-cell-based approaches, attenuated but persistent vectors such as recombinant herpesviruses should be seriously evaluated.
Acknowledgments
We thank A. Gallimore and E. Jones (Oxford, United Kingdom) for help with the production of the tetramers and M. Messerle (Halle, Germany) for the IE2-specific mouse antiserum.
This study was supported by the Wellcome Trust, the British Medical Association, and the Swiss National Science Foundation. U.K. and A.O. were supported by the Schweizerische Stiftung für Medizinisch-Biologische Stipendien and the Novartis Foundation. U.H.K., M.W., and H.H. were supported by the Deutsche Forschungsgemeinschaft and the Bayrische Forschungsstiftung (for M.W. through Forschungsverbund, Neue Strategien der Immuntherapie, individual project I5).
REFERENCES
- 1.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54-60. [DOI] [PubMed] [Google Scholar]
- 2.Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. (Erratum, 280:1821, 1998.) [DOI] [PubMed] [Google Scholar]
- 3.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., and T. M. Kundig. 1994. In vivo versus in vitro assays for assessment of T- and B-cell function. Curr. Opin. Immunol. 6:320-326. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., T. M. Kundig, H. Hengartner, and R. M. Zinkernagel. 1997. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T-cell memory without “memory T cells”? Proc. Natl. Acad. Sci. USA 94:640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmann, M. F., R. M. Zinkernagel, and A. Oxenius. 1998. Immune responses in the absence of costimulation: viruses know the trick. J. Immunol. 161:5791-5794. [PubMed] [Google Scholar]
- 7.Bendelac, A., and R. Medzhitov. 2002. Adjuvants of immunity: harnessing innate immunity to promote adaptive immunity. J. Exp. Med. 195:F19-F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder, D., and T. M. Kündig. 1991. Antiviral protection by CD8+ versus CD4+ T cells: CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent interleukins. J. Immunol. 146:4301-4307. [PubMed] [Google Scholar]
- 9.Brune, W., H. Hengel, and U. H. Koszinowski. 1999. A mouse model for cytomegalovirus infection, p. 19.7.1-19.7.13. In Current protocols in immunology. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 10.Cerwenka, A., T. M. Morgan, and R. W. Dutton. 1999. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J. Immunol. 163:5535-5543. [DOI] [PubMed] [Google Scholar]
- 11.Del Val, M., K. Munch, M. J. Reddehase, and U. H. Koszinowski. 1989. Presentation of CMV immediate-early antigen to cytolytic T lymphocytes is selectively prevented by viral genes expressed in the early phase. Cell 58:305-315. [DOI] [PubMed] [Google Scholar]
- 12.Dunn, H. S., D. J. Haney, S. A. Ghanekar, P. Stepick-Biek, D. B. Lewis, and H. T. Maecker. 2002. Dynamics of CD4 and CD8 T cell responses to cytomegalovirus in healthy human donors. J. Infect. Dis. 186:15-22. [DOI] [PubMed] [Google Scholar]
- 13.Eichelberger, M. C., M. Wang, W. Allan, R. G. Webster, and P. C. Doherty. 1991. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J. Gen. Virol. 72:1695-1698. [DOI] [PubMed] [Google Scholar]
- 14.Farrell, H. E., M. A. Degli-Esposti, and N. J. Davis-Poynter. 1999. Cytomegalovirus evasion of natural killer cell responses. Immunol. Rev. 168:187-197. [DOI] [PubMed] [Google Scholar]
- 15.Freund, J., E. R. Stern, and T. M. Pisani. 1947. Isoallergic encephalomyelitis and radiculitis in guinea pigs after one injection of brain and mycobacteria in water-in-oil emulsion. J. Immunol. 57:179-194. [PubMed] [Google Scholar]
- 16.Gillespie, G. M., M. R. Wills, V. Appay, C. O'Callaghan, M. Murphy, N. Smith, P. Sissons, S. Rowland-Jones, J. I. Bell, and P. A. Moss. 2000. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J. Virol. 74:8140-8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grzimek, N. K., D. Dreis, S. Schmalz, and M. J. Reddehase. 2001. Random, asynchronous, and asymmetric transcriptional activity of enhancer-flanking major immediate-early genes ie1/3 and ie2 during murine cytomegalovirus latency in the lungs. J. Virol. 75:2692-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hany, M., S. Oehen, M. Schulz, H. Hengartner, M. Mackett, D. H. L. Bishop, and R. M. Zinkernagel. 1989. Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein. Eur. J. Immunol. 19:417-424. [DOI] [PubMed] [Google Scholar]
- 19.Harrington, L. E., M. Galvan, L. G. Baum, J. D. Altman, and R. Ahmed. 2000. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J. Exp. Med. 191:1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hengel, H., W. Brune, and U. H. Koszinowski. 1998. Immune evasion by cytomegalovirus: survival strategies of a highly adapted opportunist. Trends Microbiol. 6:190-197. [DOI] [PubMed] [Google Scholar]
- 21.Hengel, H., U. Reusch, G. Geginat, R. Holtappels, T. Ruppert, E. Hellebrand, and U. H. Koszinowski. 2000. Macrophages escape inhibition of major histocompatibility complex class I-dependent antigen presentation by cytomegalovirus. J. Virol. 74:7861-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hengel, H., U. Reusch, A. Gutermann, H. Ziegler, S. Jonjic, P. Lucin, and U. H. Koszinowski. 1999. Cytomegaloviral control of MHC class I function in the mouse. Immunol. Rev. 168:167-176. [DOI] [PubMed] [Google Scholar]
- 23.Holtappels, R., N. K. Grzimek, C. O. Simon, D. Thomas, D. Dreis, and M. J. Reddehase. 2002. Processing and presentation of murine cytomegalovirus pORFm164-derived peptide in fibroblasts in the face of all viral immunosubversive early gene functions. J. Virol. 76:6044-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtappels, R., M. F. Pahl-Seibert, D. Thomas, and M. J. Reddehase. 2000. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62Llo memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J. Virol. 74:11495-11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtappels, R., D. Thomas, J. Podlech, and M. J. Reddehase. 2002. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J. Virol. 76:151-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudrisier, D., B. Kessler, S. Valitutti, C. Horvath, J. C. Cerottini, and I. F. Luescher. 1998. The efficiency of antigen recognition by CD8+ CTL clones is determined by the frequency of serial TCR engagement. J. Immunol. 161:553-562. [PubMed] [Google Scholar]
- 27.Jonjic, S., M. del Val, G. M. Keil, M. J. Reddehase, and U. H. Koszinowski. 1988. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J. Virol. 62:1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kägi, D., P. Seiler, J. Pavlovic, B. Ledermann, K. Bürki, R. M. Zinkernagel, and H. Hengartner. 1995. The roles of perforin- and fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur. J. Immunol. 25:2356-2362. [DOI] [PubMed] [Google Scholar]
- 29.Karrer, U., S. Sierro, M. Wagner, A. Oxenius, H. Hengel, U. H. Koszinowski, R. E. Phillips, and P. Klenerman. 2003. Memory inflation: continous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022-2029. [DOI] [PubMed] [Google Scholar]
- 30.Khan, N., M. Cobbold, R. Keenan, and P. A. Moss. 2002. Comparative analysis of CD8+ T cell responses against human cytomegalovirus proteins pp65 and immediate early 1 shows similarities in precursor frequency, oligoclonality, and phenotype. J. Infect. Dis. 185:1025-1034. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu, H., A. Vargas, S. Sierro, and P. Klenerman. 2003. Population analysis of antiviral CD8+ T cell responses using class I peptide tetramers. Clin. Exp. Immunol. 184:9-12. [DOI] [PMC free article] [PubMed]
- 32.Krause, P. R., and D. M. Klinman. 2000. Varicella vaccination: evidence for frequent reactivation of the vaccine strain in healthy children. Nat. Med. 6:451-454. [DOI] [PubMed] [Google Scholar]
- 33.Krmpotic, A., M. Messerle, I. Crnkovic-Mertens, B. Polic, S. Jonjic, and U. H. Koszinowski. 1999. The immunoevasive function encoded by the mouse cytomegalovirus gene m152 protects the virus against T-cell control in vivo. J. Exp. Med. 190:1285-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kundig, T. M., M. F. Bachmann, S. Oehen, U. W. Hoffmann, J. J. Simard, C. P. Kalberer, H. Pircher, P. S. Ohashi, H. Hengartner, and R. M. Zinkernagel. 1996. On the role of antigen in maintaining cytotoxic T-cell memory. Proc. Natl. Acad. Sci. USA 93:9716-9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurz, S. K., M. Rapp, H. P. Steffens, N. K. Grzimek, S. Schmalz, and M. J. Reddehase. 1999. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J. Virol. 73:482-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauvau, G., S. Vijh, P. Kong, T. Horng, K. Kerksiek, N. Serbina, R. A. Tuma, and E. G. Pamer. 2001. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science 294:1735-1739. [DOI] [PubMed] [Google Scholar]
- 37.Marshall, D. R., S. J. Turner, G. T. Belz, S. Wingo, S. Andreansky, M. Y. Sangster, J. M. Riberdy, T. Liu, M. Tan, and P. C. Doherty. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA 98:6313-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messerle, M., G. M. Keil, and U. H. Koszinowski. 1991. Structure and expression of murine cytomegalovirus immediate-early gene 2. J. Virol. 65:1638-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mills, J. 2000. Varicella-zoster vaccine: the bad news may be good. Nat. Med. 6:381-382. [DOI] [PubMed] [Google Scholar]
- 40.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 41.Murali-Krishna, K., L. L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286:1377-1381. [DOI] [PubMed] [Google Scholar]
- 42.Oehen, S., and K. Brduscha-Riem. 1998. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J. Immunol. 161:5338-5346. [PubMed] [Google Scholar]
- 43.Olsson, J., A. Wikby, B. Johansson, S. Lofgren, B. O. Nilsson, and F. G. Ferguson. 2000. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121:187-201. [DOI] [PubMed] [Google Scholar]
- 44.Pircher, H. P., K. Bürki, R. Lang, H. Hengartner, and R. M. Zinkernagel. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342:559-561. [DOI] [PubMed] [Google Scholar]
- 45.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Luccaronin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddehase, M. J., M. Balthesen, M. Rapp, S. Jonjic, I. Pavic, and U. H. Koszinowski. 1994. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J. Exp. Med. 179:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddehase, M. J., J. B. Rothbard, and U. H. Koszinowski. 1989. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature 337:651-653. [DOI] [PubMed] [Google Scholar]
- 48.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 49.Swain, S. L., H. Hu, and G. Huston. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science 286:1381-1383. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi, M., T. Otsuka, Y. Okuno, Y. Asano, and T. Yazaki. 1974. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet i:1288-1290. [DOI] [PubMed] [Google Scholar]
- 51.Townsend, A., J. Rothbard, F. M. Gotch, G. Bahadur, D. Wraith, and A. J. McMichael. 1986. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44:959-968. [DOI] [PubMed] [Google Scholar]
- 52.Tripp, R. A., S. Hou, and P. C. Doherty. 1995. Temporal loss of the activated L-selectin low phenotype for virus-specific CD8+ T cells. J. Immunol. 154:5870-5975. [PubMed] [Google Scholar]
- 53.Vargas, A. L., F. Lechner, M. Kantzanou, R. E. Phillips, and P. Klenerman. 2001. Ex vivo analysis of phenotype and TCR usage in relation to CD45 isoform expression on cytomegalovirus-specific CD8+ T lymphocytes. Clin. Exp. Immunol. 125:432-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vazquez, M., P. S. LaRussa, A. A. Gershon, S. P. Steinberg, K. Freudigman, and E. D. Shapiro. 2001. The effectiveness of the varicella vaccine in clinical practice. N. Engl. J. Med. 344:955-960. [DOI] [PubMed] [Google Scholar]
- 55.Wagner, M., A. Gutermann, J. Podlech, M. J. Reddehase, and U. H. Koszinowski. 2002. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J. Exp. Med. 196:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner, M., S. Jonjic, U. H. Koszinowski, and M. Messerle. 1999. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J. Virol. 73:7056-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner, M., Z. Ruzsics, and U. H. Koszinowski. 2002. Herpesvirus genetics has come of age. Trends Microbiol. 10:318-324. [DOI] [PubMed] [Google Scholar]
- 58.Weekes, M. P., A. J. Carmichael, M. R. Wills, K. Mynard, and J. G. Sissons. 1999. Human CD28-CD8+ T cells contain greatly expanded functional virus-specific memory CTL clones. J. Immunol. 162:7569-7577. [PubMed] [Google Scholar]
- 59.Weekes, M. P., M. R. Wills, K. Mynard, R. Hicks, J. G. Sissons, and A. J. Carmichael. 1999. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28− CD8+ T-cell population. Immunology 98:443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 3:225-234. [DOI] [PubMed] [Google Scholar]
- 61.Wills, M. R., A. J. Carmichael, M. P. Weekes, K. Mynard, G. Okecha, R. Hicks, and J. G. Sissons. 1999. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhigh CD8+ T cells comprise both naive and memory cells. J. Immunol. 162:7080-7087. [PubMed] [Google Scholar]
- 62.Zinkernagel, R. M. 2002. Immunity, immunopathology, and vaccines against HIV? Vaccine 20:1913-1917. [DOI] [PubMed] [Google Scholar]
- 63.Zinkernagel, R. M., M. F. Bachmann, T. M. Kundig, S. Oehen, H. Pircher, and H. Hengartner. 1996. On immunological memory. Annu. Rev. Immunol. 14:333-367. [DOI] [PubMed] [Google Scholar]