Abstract
BACKGROUND
Increased knee pain at the time of ACL reconstruction may potentially predict more difficult rehabilitation, prolonged recovery, and/or be predictive of increased knee pain at two years.
HYPOTHESIS
We hypothesize that a bone bruise and/or other preoperative factors are associated with more knee pain/symptoms at the time of index ACL reconstruction, and the presence of a bone bruise would be associated with specific demographic and injury-related factors.
STUDY DESIGN
Prospective cohort.
METHODS
In 2007, the Multicenter Orthopaedic Outcomes Network (MOON) database began to prospectively collect surgeon-reported MRI bone bruise status. A multivariable analysis was performed to: 1) determine if a bone bruise, among other preoperative factors, is associated with more knee symptoms/pain, and 2) examine the association of factors related to bone bruise. To evaluate the association of a bone bruise with knee pain/symptoms, linear multiple regression models were fit using the continuous scores of the KOOS symptoms and pain subscales and the SF-36 bodily pain subscale as dependent variables. To examine the association between a bone bruise and risk factors, a logistic regression model was used in which the dependent variable was the presence or absence of a bone bruise.
RESULTS
Baseline data for 525 subjects was used for analysis, and a bone bruise was present in 419 (80%). The cohort is 58% male, median age 23 yrs. Median Marx activity level was 13. Factors associated with more pain were higher body mass index (p< 0.0001), female sex (p= 0.001), lateral collateral ligament injury (p=0.012), and older age (p= 0.038). Factors associated with more symptoms were a concomitant lateral collateral ligament injury (p=0.014), higher body mass index (p< 0.0001), and female sex (p< 0.0001). Bone bruise is not associated with symptoms/pain at the time of index ACL reconstruction. None of the factors included in the SF-36 bodily pain model were found to be significant. After controlling for other baseline factors, the following factors were associated with a bone bruise: younger age (p=0.034) and not jumping at the time of injury (p=0.006).
CONCLUSION
Following ACL injury, risk factors associated with a bone bruise are younger age and not jumping at the time of injury. Bone bruise is not associated with symptoms/pain at the time of index ACL reconstruction.
Keywords: bone bruise, ACL reconstruction, KOOS, MOON, knee pain/symptoms
INTRODUCTION
In patients who tear their anterior cruciate ligament (ACL), the consensus of opinion, whether ACL reconstruction (ACLR) or rehabilitation is chosen, is to reduce knee pain and symptoms, activate quadriceps, normalize range of motion, and promote normal gait.33 Achieving these parameters is believed to be a prerequisite for appropriate timing of ACLR to reduce risk of arthrofibrosis. Another commonly held belief is a “bone bruise” (BB) is associated with knee pain after ACL tear. However, in addition to a BB, which is observed in 80% of patients8, 30, 31, 34 with ACL tears, several demographic, mechanistic and intraarticular injuries to articular cartilage and meniscus could also be associated with increased knee pain and/or symptoms at ACLR.3–5, 23, 31, 33 Increased knee pain at the time of ACLR may potentially predict a more difficult rehabilitation, prolonged time to pain-free recovery, and/or be associated with more knee pain at two years after ACLR. Identifying whether a BB is associated with knee pain and symptoms could alter preoperative weight bearing protocols to decrease pain or delay ACLR or to allow sufficient healing time of BB with associated articular cartilage injury.19, 31
The BB observed in 80% of ACL tears30, 31, 34 predominantly occurs in the lateral femoral condyle and lateral tibial plateau. This BB has been associated with articular cartilage injuries11, 12, 19, 31, 33, 34 and meniscus tears.3, 4, 23, 31, 33 Marrow and subchondral injuries observed on magnetic resonance imaging (MRI) have been shown to resolve in a long-term follow-up of a longitudinal cohort.6, 13, 29 MRI and articular cartilage with bone biopsies of BB have demonstrated articular matrix pathologic changes and chondrocyte and osteocyte cell death.19 To evaluate which of these concurrent injuries, including the presence of a BB with an ACL tear, are associated with increased knee pain and/or symptoms at the time of ACLR requires multivariable analysis of a large cohort with sufficient sample size to analyze all the aforementioned factors.
Since the BB occurs at the time of injury to the ACL, it may herald other intraarticular injuries that simultaneously occurred. Further, examining the relationship between a BB and patient characteristics such as gender, age, and circumstances of the injury may yield insight into the mechanism of ACL injury.
The goals of this study are to determine if any preoperative factors including a BB are associated with knee symptoms and knee pain and to examine the association of baseline factors with the presence of BB occurrences. First, we hypothesized that the presence of a BB would be associated with increased knee pain/symptoms at the time of index ACLR. Second, the presence of a BB would be associated with specific demographic and injury-related factors.
MATERIALS AND METHODS
Study Design, and Setting
A multicenter prospective longitudinal cohort design was implemented. In this ongoing MOON cohort study, all subjects undergoing ACLR at seven sites (Cleveland Clinic, Ohio State University, Hospital for Special Surgery, Washington University at St. Louis, University of Iowa, University of Colorado, and Vanderbilt University) by 16 physicians (JTA, RDP, MHJ, CCK, DCF, RGM, RWW, RHB, MJM, AA, BRW, ECM, MLW, AFV, KPS, WRD) are targeted for enrollment. The recruitment period for the current study was between 12-1-2006 and 7-18-2008. The comparison for both hypotheses was the initial MRI findings (bone bruise) with preoperative factors that are associated with KOOS subscales measured at the time of surgery. Except for 16 patients without MRI, all patients meet inclusion criteria discussed below.
Participants
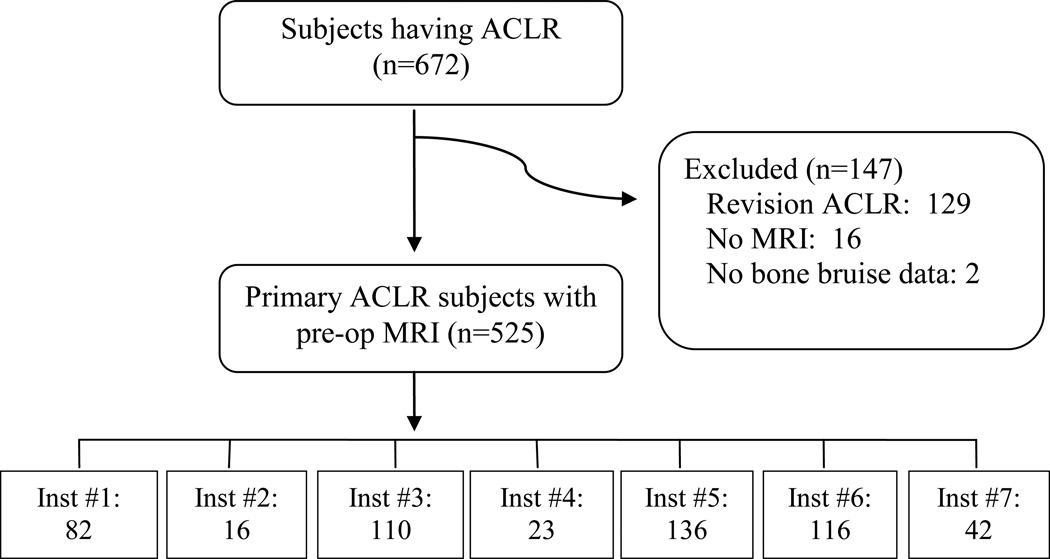
Subjects having primary ACLR that also had a preoperative MRI performed met inclusion criteria for study. The MOON surgeons prospectively added to surgeon evaluation forms in 2007 whether an MRI was obtained, whether a BB was present, and if present the location of BB as LFC (lateral femoral condyle), LTP (lateral tibial plateau), or other. Since a BB is recognizable on multiple types of MRI and the MRIs were obtained after ACL tears, the surgeons judged appropriateness of timing and reading of the MRIs. Participants undergoing primary ACLR without a preoperative MRI, as well as revision ACLR, were excluded (Figure 1).
Figure 1.
Study Flow Diagram – MOON ACLR Cohort.
Variables
To test the first hypothesis that the presence of a BB is associated with knee symptoms and knee pain at the time of ACLR, three subscales were utilized as continuous dependent variables: 1) Knee injury and Osteoarthritis Outcome Score (KOOS) pain subscale, 2) KOOS symptoms subscale, and 3) SF-36 bodily pain subscale. The KOOS pain subscale includes nine questions each scored on a 0 to 4 scale, and the possible raw score ranging from 0 to 36 is transformed to a 0 to 100 score where 100 constitutes no pain, and 0 is the worst score.28 The KOOS symptoms subscale consists of seven questions scored on a 0 to 4 scale, and the possible raw scores ranging from 0 to 28 are transformed into a 0 to 100 score where 100 is the best score and 0 is the worst. The bodily pain subscale of the SF-36 contains two items, one has six levels of responses, and the other has five that is recoded to a six-level item and transformed into a 0 to 100 score where 100 is the best health and 0 is the worst.18 The presence of a BB was captured as a discrete (yes/no) variable, location was recorded as a nominal variable with four categories: none, lateral tibial plateau, lateral femoral condyle, and other. The grading of the Lachman, MCL, and LCL was performed by each surgeon at the time of ACLR and they were classified according to IKDC criteria. For the first hypothesis (whether a BB is associated with knee symptoms or pain), a BB was considered as an exposure (independent variable); however, for our second hypothesis (association of a BB with demographics and intraartcular injuries), BB was treated as the outcome (dependent variable). Tables 1 and 2 include the exposures and potential confounders included in our analyses stratified by BB status.
Table 1.
Patient characteristics by bone bruise status.
| N | no | yes | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 106 | N = 419 | N = 525 | ||||||||
| Injury Chronicity weeks | 265 | 9.1 20.4 41.0 | (74.7±170.2) | 4.3 8.3 14.1 | (37.6±136.7) | 4.6 9.3 20.4 | (44.5±143.8) | |||
| Pain Chronicity weeks | 352 | 5.7 14.9 26.7 | (54.0±154.3) | 4.3 7.3 12.6 | (16.9± 65.2) | 4.4 7.9 14.1 | (22.9± 86.9) | |||
| Giving-way Episodes | 439 | 2.0 4.0 10.0 | (11.5±20.3) | 1.0 2.0 5.0 | (5.2± 9.9) | 1.0 3.0 6.0 | (6.6±13.1) | |||
| Reinjuries | 440 | 0.0 1.0 2.0 | (3.0±9.3) | 0.0 0.0 1.0 | (0.8±2.2) | 0.0 0.0 1.0 | (1.3±4.8) | |||
| Sex | 525 | |||||||||
| male | 60% | 57% | 58% | |||||||
| female | 40% | 43% | 42% | |||||||
| Age | 525 | 19 30 41 | (31±13) | 17 22 32 | (25±11) | 17 23 34 | (26±11) | |||
| Baseline BMI | 525 | 23.0 26.1 29.5 | (27.1± 5.8) | 22.1 24.4 27.6 | (25.6± 5.5) | 22.1 24.8 28.0 | (25.9± 5.6) | |||
| Activity at Injury | 465 | |||||||||
| Baseball/softball | 2% | 4% | 4% | |||||||
| Basketball | 14% | 20% | 19% | |||||||
| Football | 8% | 18% | 16% | |||||||
| Other Sport | 24% | 21% | 22% | |||||||
| Skiing | 9% | 7% | 8% | |||||||
| Soccer | 16% | 16% | 16% | |||||||
| Non-sport | 28% | 12% | 16% | |||||||
| Contact Injury | 525 | |||||||||
| no | 83% | 72% | 74% | |||||||
| yes | 17% | 28% | 26% | |||||||
| ”Pop” at Injury | 525 | |||||||||
| no | 29% | 34% | 33% | |||||||
| yes | 71% | 66% | 67% | |||||||
| Injured Jumping | 525 | |||||||||
| no | 65% | 76% | 74% | |||||||
| yes | 35% | 24% | 26% | |||||||
| Marx Activity Score | 521 | 4.0 12.0 16.0 | (9.6± 5.8) | 10.0 14.0 16.0 | (12.3± 4.8) | 9.0 13.0 16.0 | (11.7± 5.1) | |||
| KOOS Pain | 521 | 53 75 83 | (68±20) | 56 72 83 | (70±19) | 56 72 83 | (69±19) | |||
| KOOS Symptoms | 519 | 50 68 82 | (65±20) | 50 64 79 | (63±19) | 50 64 79 | (64±19) | |||
| SF36 Bodily Pain | 523 | 42.0 44.0 51.0 | (44.3± 8.9) | 41.0 44.0 52.0 | (45.7±10.2) | 41.0 44.0 52.0 | (45.4±10.0) | |||
Table 2.
Intraarticular factors by bone bruise status.
| no | yes | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 106 | N = 419 | N = 525 | |||||||
| Chondrosis Lateral Compartment | |||||||||
| No | 81% | 75% | 76% | ||||||
| Yes | 19% | 25% | 24% | ||||||
| Anterior Comp Chondrosis | |||||||||
| No | 75% | 83% | 81% | ||||||
| Yes | 25% | 17% | 19% | ||||||
| Medial Comp Chondrosis | |||||||||
| No | 70% | 79% | 77% | ||||||
| Yes | 30% | 21% | 23% | ||||||
| Medial Meniscus Status | |||||||||
| ≤50% excision | 26% | 8% | 12% | ||||||
| > 50% or complete excision | 8% | 3% | 4% | ||||||
| normal | 51% | 63% | 61% | ||||||
| repair | 11% | 19% | 18% | ||||||
| Tear not Tx | 3% | 7% | 6% | ||||||
| Lateral Meniscus Status | |||||||||
| ≤50% excision | 23% | 25% | 25% | ||||||
| > 50% or complete excision | 2% | 2% | 2% | ||||||
| normal | 60% | 52% | 53% | ||||||
| repair | 6% | 8% | 8% | ||||||
| Tear not Tx | 9% | 13% | 12% | ||||||
| Lachman | |||||||||
| < 5mm SSD | 22% | 24% | 24% | ||||||
| 6–10mm SSD | 52% | 61% | 59% | ||||||
| > 10mm SSD | 26% | 15% | 17% | ||||||
Data Sources and Measurement
Following documentation of informed consent, participants complete a 13-page questionnaire examining self-reported demographics, injury characteristics, sports participation history, comorbidities, and health status. Regarding the latter, the following validated instruments are included: SF-36 (version 2), the Knee injury and Osteoarthritis Outcome Score (KOOS) which includes the Western Ontario and McMaster Universities Arthritis Index (WOMAC), and Marx activity scale. This is administered on the day of surgery, and completed within two weeks of the surgery date.
The KOOS was developed as a patient-based assessment tool to evaluate sports injuries (ACL injuries, meniscus tears and mild OA) in young and middle-aged athletes as an extension of the WOMAC.26, 28 The outcome instrument is comprised of 42 questions covering five subscales: pain (nine items), symptoms (seven items), activities of daily living (17 items), sport and recreation function (five items), and knee related quality of life (four items).28 Each subscale is summed and transformed to a zero (worst possible) to 100 (best possible) score. The KOOS has undergone extensive validation for the assessment of ACL injuries, meniscectomies, tibial osteotomies and post-traumatic OA.10, 25, 27, 28, 35 A change in score of eight points represents a real change in condition.26 The minimum clinically important difference for SF-36 is reported to be 12% (12 points) over the transformed 100 point scale score.1
The Marx activity scale was developed as a short, patient-reported activity assessment that estimates the peak activity over the past year to account for variability related to seasonal trends in sports or injury.21 It is a validated tool that quantifies the frequency of running, cutting, decelerating, and pivoting activities, which are difficult for those with ACL deficiency. It contains four items that ask about frequency of participation in running, cutting, decelerating, and pivoting; items are scored using a five-point scale quantifying the frequency of participation in each activity -- less than one time per month (0), one time per month (1), one time per week (2), 2 or 3 times per week (3), and 4 or more times in a week (4). The metric is reported as the sum of scores from the four categories ranging from 0–16. For example, a young, competitive, high school athlete practicing four times per week in football, basketball, or soccer would score a 16. Conversely, a recreational jogger three times per week would score a 3.
Following ACLR, surgeons complete a 49-page questionnaire which includes sections on past history of knee injury and/or surgery on both knees, the results of the general knee examination done under anesthesia, recording of all intraarticular injuries and treatments to the meniscus and articular cartilage, and the surgical technique used for the ACLR. Classification of the general knee examination findings follows the recommendations of the updated 1999 IKDC guidelines.16, 17 Surgeon documentation of articular cartilage injury is recorded on the modified Outerbridge classification.7, 20 Meniscus injuries are classified by size, location and partial versus complete tears, and treatment is recorded as not treated, repair, or extent of resection.9
Completed data forms are mailed from the participating sites to the data-coordinating center. Data from both the patient and surgeon questionnaires are scanned with Teleform™ software (Cardiff Software, Inc., Vista, CA) utilizing optical character recognition to avoid manual data entry, and the scanned data is verified and then exported to a database.14, 24 Both patient and surgeon questionnaires have a matched, barcode unique ID on each page in order to de-identify the data and to aid in data merging.
Study Size
Sample size considerations guided variable selection in order to generate a model as complex as the data would allow without overfitting the data using the ratio m/10 as the minimum acceptable ratio for reliable models. Hence, sample size estimates are based on model complexity where m is the effective sample and m/10 parameters (predictors plus nonlinearities and interactions) are possible in the model.15 The effective sample size to model the presence of a BB as the dependent variable equals 106 (the number of subjects without a BB).
Quantitative Variables & Statistical Methods
The following categorical variables were reduced due to low prevalence categories. Articular cartilage variables were grouped by compartment (medial, lateral, anterior), and severity of chondromalacia was dichotomized into grade II chondromalacia or higher being positive for chondrosis in that compartment. Activities at injury variables were collapsed into a new variable with three levels: non-sport injury (n=73), contact sport injury (football, n=74), and non-contact sport injury (n=318). The latter, non-contact sport injuries, included those injured playing baseball/softball (n=18), basketball (n=87), soccer (n=76), skiing (n=35), and other sports (n=102). Lateral collateral ligament (LCL) injury was less common than medial collateral ligament (MCL) injury and was dichotomized into normal and abnormal (n=14). MCL injuries were collapsed into a three-level variable: normal, grade I (n=23), grades II/III (n= 24).
To evaluate the association of a BB with knee pain and symptoms (hypothesis one) multivariable linear multiple regression models were fit using the continuous scores of the KOOS symptoms subscale, KOOS pain subscale, and the SF-36 bodily pain subscale as the dependent variable. Independent variables included in these models were age, sex, body mass index (BMI), injury chronicity, medial meniscus status and treatment, lateral meniscus status and treatment, collateral ligament injury, laxity by Lachman exam, and chondrosis in the medial, lateral, and anterior compartments.
To examine the association between a BB and risk factors (hypothesis two), a logistic regression model was used in which the dependent variable was the presence or absence of a BB, and independent variables were age, BMI, sex, contact injury, jumping at the time of injury, a ‘pop’ at time of injury, activity at injury, and the Marx activity score.
We did not assume linearity of covariate effects but only assumed smoothed relationships, using restricted cubic regression splines. Missing values of predictor variables were imputed using multiple imputation incorporating predictive mean matching and flexible additive imputation models as implemented in the aregImpute function available in the Hmisc package in R.2 Data reduction methods used to preserve degrees of freedom in models included pooling of low prevalence categories, variable grouping, and hierarchical clustering (using squared Spearman rank correlation coefficients as the similarity matrix) to identify colinear variables that could be deleted from the model. Statistical analysis was performed with free open source R statistical software (www.r-project.org).
RESULTS
There were 672 subjects undergoing ACLR with baseline data collected between 12-1-2006 and 7-18-2008 examined for eligibility, and 145 revision cases were excluded. Of the remaining 543 primary cases, 525 were confirmed eligible and analyzed (Figure 1). Patient characteristics including KOOS subscales and the SF-36 bodily pain subscale are listed in Table 1 stratified by BB status. Intraarticular findings and results of the Lachman exam are listed in Table 2 stratified by BB status.
Risk Factors Associated with Knee Pain and Symptoms on the KOOS Score
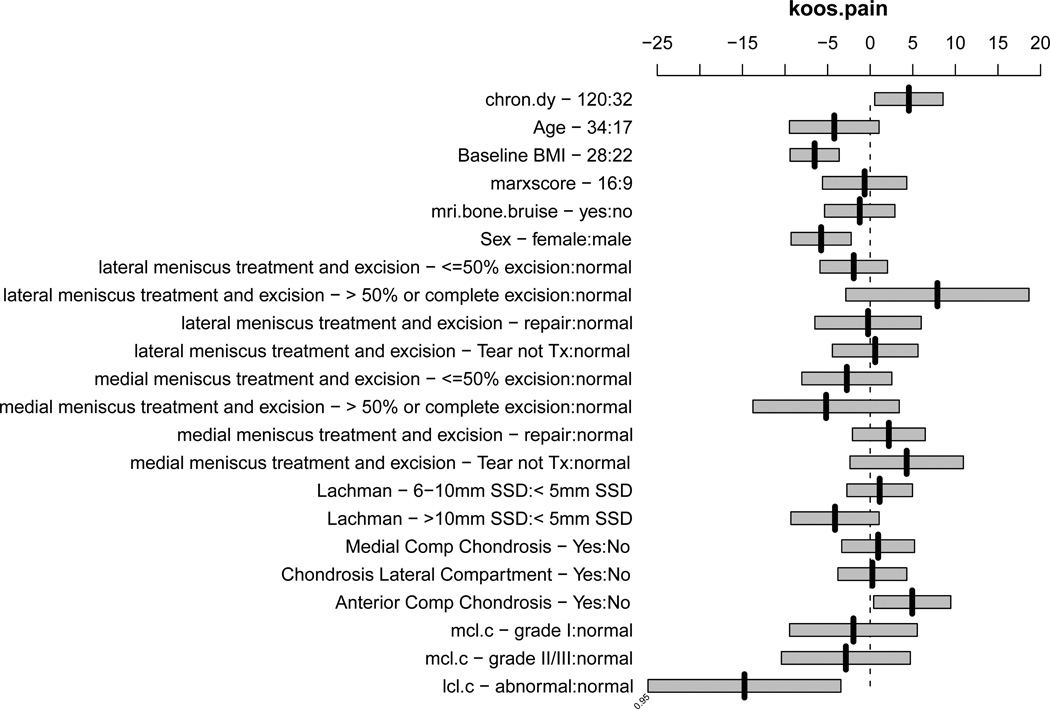
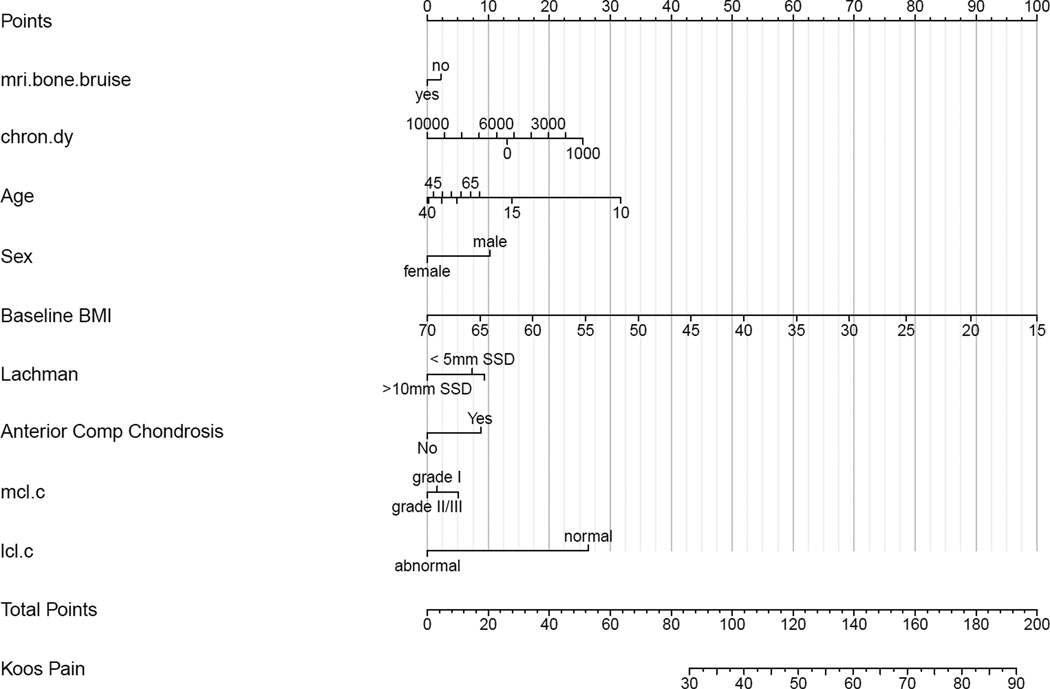
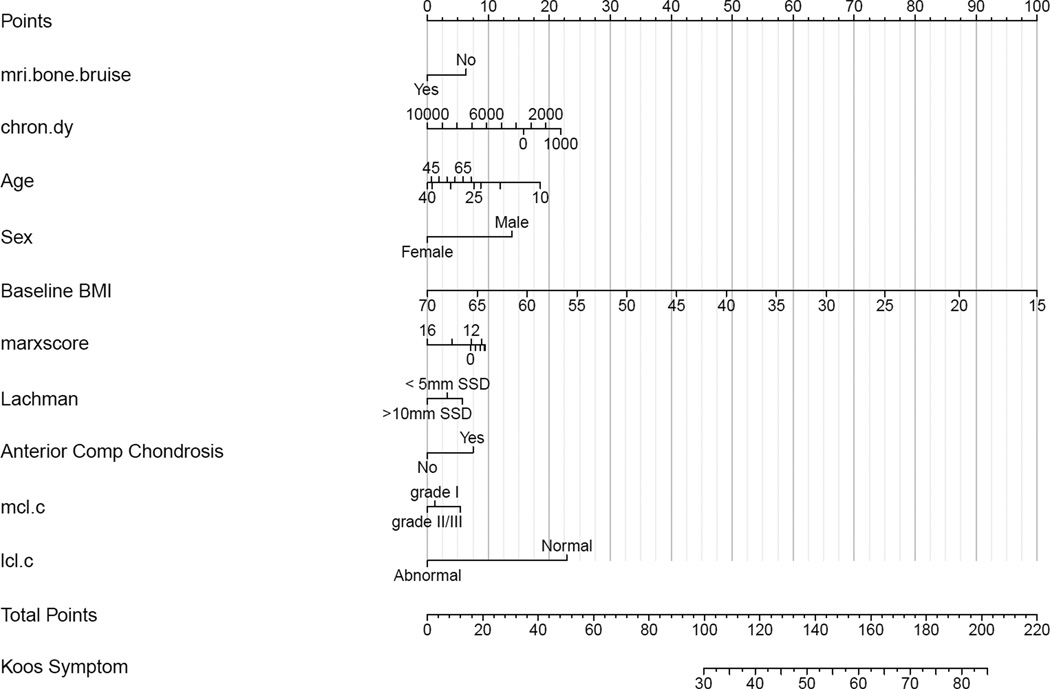
The presence of a BB as yes/no or by location was not associated with knee pain/symptoms as measured by the KOOS pain and symptoms subscales, nor the SF-36 bodily pain subscale. Factors associated with having more pain on the KOOS subscale were higher BMI (p< 0.0001), female sex (p= 0.001), an LCL injury (p=0.012), and older age (p= 0.038), which are adjusted for injury chronicity, medial meniscus status and treatment, lateral meniscus status and treatment, laxity by Lachman exam, MCL injury, and chondrosis in the medial, lateral, and anterior compartments (Figure 2). The presence of chondrosis in the anterior compartment was associated with less pain (p=0.031). A summary of effects is given in Appendix I, using interquartile ranges (IQR) for continuous variables with the 95% confidence intervals (CI) for the mean effects, which are plotted in Figure 2. A partial effects plot is shown in Appendix II. The presence of an LCL injury was associated with both a statistically and clinically meaningful increase in pain with a mean effect of −14.1 (95%CI: −25.2, −3.0). While sex, age, and BMI were statistically significant, none of the respective point estimates (−5.9, −4.1, and −6.5) represents a clinically meaningful difference (8 points). Using the nomogram in Figure 3, one can estimate the cumulative effects of these predictors. For instance, letting the other variables default to the value of category contributing no points to the total (left-most value/category), summing the points for a male with a BMI of 20 (~10 points for male + 89 for BMI = 99 total points) compared to a female with BMI of 25 (0 points for female + ~78 for BMI = 78 total points), the predicted KOOS pain scores are 43 and 30, respectively, which would be considered a clinically meaningful difference.
Figure 2.
Plot of effects of predictors in the model for KOOS pain subscale using interquartile ranges for continuous variables with bars representing the 95% confidence interval for the mean effect. For example, the effect of raising BMI from its first quartile (22) to its third quartile (28) is to lower the mean KOOS pain score by 6.5 points. For the KOOS pain subscale a score of 100 indicates normal without pain whereas a score of zero indicates the worst pain. Thus, lowering the KOOS pain score indicates an increase in pain. Abbreviations in order presented: chron.dy = chronicity in days, bmi = body mass index, Itx.ex - < −50% = lateral meniscus tear less than or equal to 50% excision, Itx.ex - > 50% or complete = lateral meniscus tear great than 50% or complete excision, Itx.ex – tear not tx = lateral meniscus tear not treated, mtx = medial meniscus tear, lach.ssd.c – 6–10mm = Lachman physical examination 6–10mm vs. less than 5mm side-to-side difference, lach.ssd.c - >10mm SSD = Lachman physical examination greater than 10mm side-to-side difference, mcl.c = medial collateral ligament, lcl.c = lateral collateral ligament.
Figure 3.
Nomogram for model predicting KOOS pain scores. The nomogram is used to predict an individual patient’s KOOS score as follows. First, each variable is marked and the points for each are derived by viewing the point total on the top of the nomogram. Then the points are totaled and placed on the bottom total points scale. After placing the total points, view down to obtain KOOS subscale score.
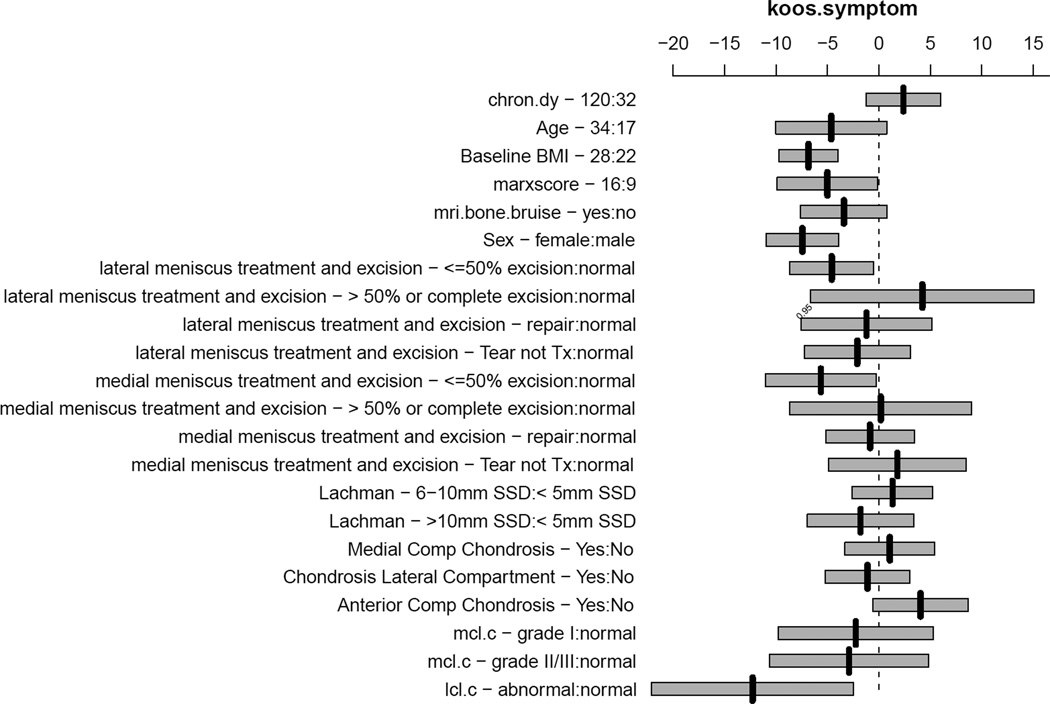
Factors associated with having more symptoms on the KOOS subscale were a concomitant LCL injury (p=0.014), higher BMI (p< 0.0001), and female sex (p< 0.0001) which are adjusted for age, injury chronicity, medial meniscus status and treatment, lateral meniscus status and treatment, MCL injury, laxity by Lachman exam, and chondrosis in the medial, lateral, and anterior compartments. A summary of effects is given in Appendix IV, using IQR for continuous variables with the 95% confidence intervals for the mean effects, which are plotted in Figure 4. The presence of an LCL injury was associated with both a statistically and clinically meaningful increase in symptoms with a mean effect of −12.4 (95%CI: −22.2, −2.6). While sex and BMI were statistically significant, the respective point estimates (−6.9 and −7.4) do not represent a clinically meaningful difference on the KOOS. However, using the nomogram in Figure 5, one can estimate the cumulative effects of the predictors on symptoms. For instance, summing the points for a 20 year old male with a BMI of 20 and a normal LCL (~ 14 points for male + ~ 10 points for age + 23 points for LCL + 87 for BMI = 134 total points) compared to a 20 year old female with a BMI of 25 and a normal LCL (0 points for female + 10 points for age + 23 points for LCL + ~ 75 for BMI = 108 total points), the predicted KOOS symptoms scores are 51 and 36, respectively, which would be considered a clinically meaningful difference.
Figure 4.
Plot of effects of predictors in the model for KOOS symptoms subscale using interquartile ranges for continuous variables with bars representing the 95% confidence interval for the mean effect. For example, the effect of raising BMI from its first quartile (22) to its third quartile (28) is to lower the mean KOOS symptoms score by 7 points. For the KOOS symptoms subscale a score of 100 indicates normal without symptoms and a score of zero indicates the worst symptoms. Thus, lowering the KOOS symptom score indicates an increase in symptoms. Abbreviations is order presented: chron.dy = chronicity in days, bmi = body mass index, Itx.ex - <−50% = lateral meniscus tear less than or equal to 50% excision, Itx.ex - > 50% or complete = lateral meniscus tear great than 50% or complete excision, Itx.ex – tear not tx = lateral meniscus tear not treated, mtx = medial meniscus tear, lach.ssd.c – 6–10mm = Lachman physical examination 6–10mm vs. less than 5mm side-to-side difference, lach.ssd.c - >10mm SSD = Lachman physical examination greater than 10mm side-to-side difference, mcl.c = medial collateral ligament, lcl.c = lateral collateral ligament.
Figure 5.
Nomogram for model predicting KOOS symptoms score. The nomogram is used to predict an individual patient’s KOOS score as follows. First, each variable is marked and the points for each are derived by viewing the point total on the top of the nomogram. Then the points are totaled and placed on the bottom total points scale. After placing the total points, view down to obtain KOOS subscale score.
None of the factors included in the SF-36 bodily pain model were found to be significant; the summary effects are listed in Appendix IV, and plotted in Appendix V.
Risk Factors Associated with a Bone Bruise
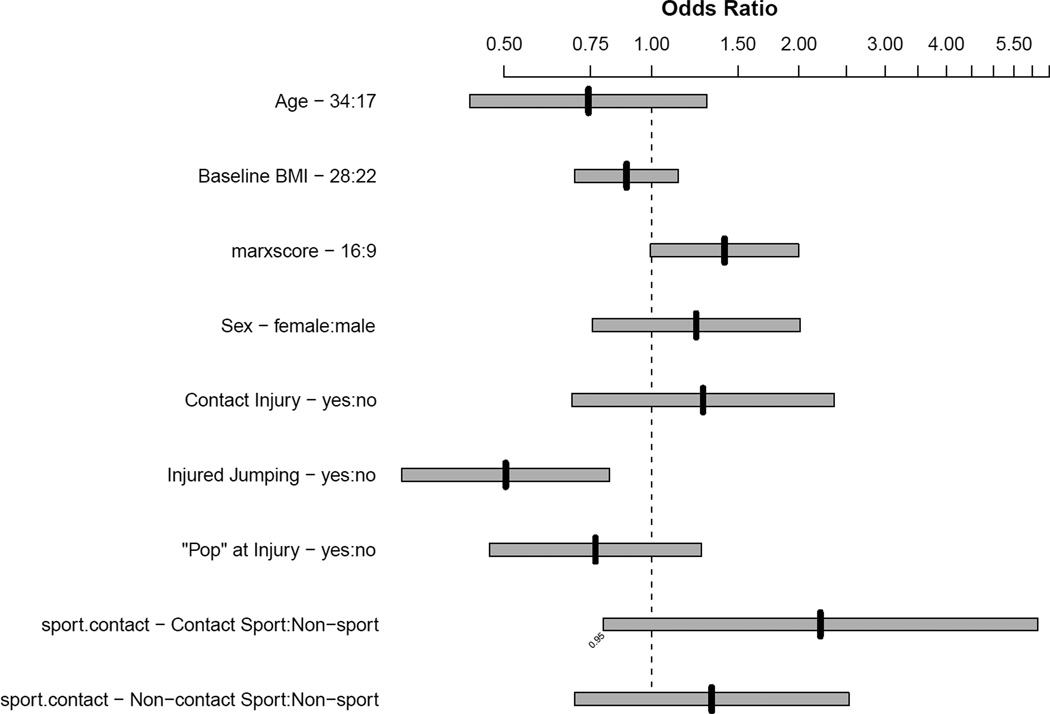
The number of parameters included in the model examining risk factors associated with a BB was limited by the effective sample size (106). Hence, to reduce the degrees of freedom to 10, eight variables felt to be the most relevant were included in the model, which were age, BMI, sex, contact injury, jumping at the time of injury, a ‘pop’ at time of injury, activity at injury, and the Marx activity score. Factors associated with having a BB were younger age (p=0.034) and not jumping at the time of injury (p=0.006). A table including the point estimates for each parameter is provided in Appendix VI, and Figure 6 is a plot of the odds ratios from the final model.
Figure 6.
Association of risk factors with having a bone bruise.
DISCUSSION
The results of all three validated, patient-reported outcome scales (KOOS Pain, KOOS Symptoms, SF-36 bodily pain) refute our first hypothesis that a BB is associated with pain or symptoms at index ACLR. To our knowledge, this is the first multivariable modeling to include the majority of “knee injuries” which occur concurrently at ACL injury and are plausible sources of pain/symptoms. However, after adjusting for demographic and mechanistic factors, as well as meniscus and articular cartilage injuries, only concomitant LCL injury was both statistically significant and clinically relevant. The finding of chondrosis in the anterior compartment being associated with less pain, mean effect of 5.02 (95% CI: 0.49, 9.55) does not have an obvious clinical or biological explanation. Alternative explanations include Type I error and an unmeasured third variable that is associated with anterior chondrosis and knee pain. Other plausible variables not examined in the current study, such as cytokines and neurogenic chemical pain mediators, could be explored in future research. Higher BMI and female sex are associated with increased pain and symptoms, and for the clinician, the nomogram can be used to calculate the expected pain and symptoms at ACLR for an individual patient.
For the second hypothesis, both younger age and not jumping at the time of injury were significantly associated with the presence of a BB, while gender, Marx activity level, BMI and other mechanistic factors were not. A prior prospective study likewise found a significant association between jumping and a BB.31 At 12-year follow-up of this cohort, all of the BB marrow changes had resolved, and there was no difference in IKDC scores among those with and without a BB at index.13
There are several study limitations, foremost is inherent to the cross-sectional design using only baseline data from a prospective cohort, hence, causal inferences cannot be made. Two-year follow-up of this cohort is planned to determine the relationship between the presence of a BB on MRI and outcomes after ACLR. Temporal trends that are difficult to account for may influence pain and symptoms. For instance, a BB could be a cause of symptoms after the initial injury and then resolve with time before index ACLR. To that end, the standard clinical guideline followed by the MOON group (regain active range of motion, quadriceps function, and normal gait as a prerequisite to perform ACLR)33 likely introduces some lag time. Patients at the time of index ACLR still have clinically relevant levels of pain and symptoms as reflected in their baseline scores. Regarding our second hypothesis, the model was limited by the effective sample size of 106 subjects without a BB; consequently, only eight variables were examined. Rationale for inclusion of these parameters is based both on clinical knowledge and existing evidence.5, 22, 23, 32 The multicenter design (7 sites and 16 surgeons) and large sample size (n=525) seem generalizable to fellowship-trained sports medicine doctors in an academic setting and, potentially, to a broader population of ACL tears.
In the short-term, the presence of a BB on MRI concurrent with an ACL tear is not associated with patient-reported outcomes of pain and symptoms at time of index ALCR; subjects with higher BMI and females report more pain and symptoms. The key findings of the study are presented in nomograms, which have several advantages, including ease of interpretation compared to regression equations, and they can be used to calculate knee pain and symptoms for individual subjects. Finally, the presence of a BB is associated with younger age and not jumping at the time of injury. Future planned two-year follow-up of this cohort will evaluate the relationship between a BB and patient-reported outcomes after ACLR.
Supplementary Material
Acknowledgments
Funding acknowledgements: This project was supported in part by NIH grants #K23 AR052392-04 (Dunn), #1 R01AR053684 (Spindler), an AOSSM-MTF Career Development Award Supplement (Dunn), and the Vanderbilt Sports Medicine Research Fund. Unrestricted educational grants were also provided by DonJoy and Smith & Nephew Endoscopy.
Footnotes
Presented at 2009 Annual Meeting of AOSSM
REFERENCES
- 1.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001 Aug;45(4):384–391. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Harrell FE, Buyse M, Emberson JR, Altman DG. Ensuring trial validity by data quality assurance and diversification of monitoring methods. Clin Trials. 2008;5(1):49–55. doi: 10.1177/1740774507087554. [DOI] [PubMed] [Google Scholar]
- 3.Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE. Treatment of anterior cruciate ligament injuries, part 2. Am J Sports Med. 2005 Nov;33(11):1751–1767. doi: 10.1177/0363546505279922. [DOI] [PubMed] [Google Scholar]
- 4.Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE. Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med. 2005 Oct;33(10):1579–1602. doi: 10.1177/0363546505279913. [DOI] [PubMed] [Google Scholar]
- 5.Bowers AL, Spindler KP, McCarty EC, Arrigain S. Height, weight, and BMI predict intraarticular injuries observed during ACL reconstruction: evaluation of 456 cases from a prospective ACL database. Clin J Sport Med. 2005 Jan;15(1):9–13. doi: 10.1097/00042752-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Costa-Paz M, Muscolo DL, Ayerza M, Makino A, Aponte-Tinao L. Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy. 2001 May;17(5):445–449. doi: 10.1053/jars.2001.23581. [DOI] [PubMed] [Google Scholar]
- 7.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997 Aug;13(4):456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 8.Davies NH, Niall D, King LJ, Lavelle J, Healy JC. Magnetic resonance imaging of bone bruising in the acutely injured knee--short-term outcome. Clin Radiol. 2004 May;59(5):439–445. doi: 10.1016/j.crad.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Dunn WR, Wolf BR, Amendola A, et al. Multirater agreement of arthroscopic meniscal lesions. Am J Sports Med. 2004 Dec;32(8):1937–1940. doi: 10.1177/0363546504264586. [DOI] [PubMed] [Google Scholar]
- 10.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003 Aug;48(8):2178–2187. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 11.Faber KJ, Dill JR, Amendola A, Thain L, Spouge A, Fowler PJ. Occult osteochondral lesions after anterior cruciate ligament rupture. Six-year magnetic resonance imaging follow-up study. Am J Sports Med. 1999 Jul-Aug;27(4):489–494. doi: 10.1177/03635465990270041301. [DOI] [PubMed] [Google Scholar]
- 12.Fang C, Johnson D, Leslie MP, Carlson CS, Robbins M, Di Cesare PE. Tissue distribution and measurement of cartilage oligomeric matrix protein in patients with magnetic resonance imaging-detected bone bruises after acute anterior cruciate ligament tears. J Orthop Res. 2001 Jul;19(4):634–641. doi: 10.1016/S0736-0266(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 13.Hanypsiak BT, Spindler KP, Rothrock CR, et al. Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med. 2008 Apr;36(4):671–677. doi: 10.1177/0363546508315468. [DOI] [PubMed] [Google Scholar]
- 14.Hardin JM, Woodby LL, Crawford MA, Windsor RA, Miller TM. Data collection in a multisite project: Teleform. Public Health Nurs. 2005 Jul-Aug;22(4):366–370. doi: 10.1111/j.0737-1209.2005.220410.x. [DOI] [PubMed] [Google Scholar]
- 15.Harrell FE., Jr . Regression Modeling Strategies. New York: Springer; 2001. [Google Scholar]
- 16.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001 Sep-Oct;29(5):600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 17.Irrgang JJ, Ho H, Harner CD, Fu FH. Use of the International Knee Documentation Committee guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1998;6(2):107–114. doi: 10.1007/s001670050082. [DOI] [PubMed] [Google Scholar]
- 18.John E. Ware J, Mark Kosiniski, James E. Dewey. How to Score Version 2 of the SF-36® Health Survey. Lincoln, RI: Quality Metric Incorporated; 2000. [Google Scholar]
- 19.Johnson DL, Urban WP, Jr, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998 May-Jun;26(3):409–414. doi: 10.1177/03635465980260031101. [DOI] [PubMed] [Google Scholar]
- 20.Marx RG, Connor J, Lyman S, et al. Multirater agreement of arthroscopic grading of knee articular cartilage. Am J Sports Med. 2005 Nov;33(11):1654–1657. doi: 10.1177/0363546505275129. [DOI] [PubMed] [Google Scholar]
- 21.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 22.Paul JJ, Spindler KP, Andrish JT, Parker RD, Secic M, Bergfeld JA. Jumping versus nonjumping anterior cruciate ligament injuries: a comparison of pathology. Clin J Sport Med. 2003 Jan;13(1):1–5. doi: 10.1097/00042752-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Piasecki DP, Spindler KP, Warren TA, Andrish JT, Parker RD. Intraarticular injuries associated with anterior cruciate ligament tear: findings at ligament reconstruction in high school and recreational athletes. An analysis of sex-based differences. Am J Sports Med. 2003 Jul-Aug;31(4):601–605. doi: 10.1177/03635465030310042101. [DOI] [PubMed] [Google Scholar]
- 24.Quan KH, Vigano A, Fainsinger RL. Evaluation of a data collection tool (TELEform) for palliative care research. J Palliat Med. 2003 Jun;6(3):401–408. doi: 10.1089/109662103322144718. [DOI] [PubMed] [Google Scholar]
- 25.Roos E. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati Knee Rating System in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2000 May-Jun;28(3):436–438. [PubMed] [Google Scholar]
- 26.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roos EM, Ostenberg A, Roos H, Ekdahl C, Lohmander LS. Long-term outcome of meniscectomy: symptoms, function, and performance tests in patients with or without radiographic osteoarthritis compared to matched controls. Osteoarthritis Cartilage. 2001 May;9(4):316–324. doi: 10.1053/joca.2000.0391. [DOI] [PubMed] [Google Scholar]
- 28.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998 Aug;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 29.Rosen MA, Jackson DW, Berger PE. Occult osseous lesions documented by magnetic resonance imaging associated with anterior cruciate ligament ruptures. Arthroscopy. 1991;7(1):45–51. doi: 10.1016/0749-8063(91)90077-b. [DOI] [PubMed] [Google Scholar]
- 30.Speer KP, Spritzer CE, Bassett FH, 3rd, Feagin JA, Jr, Garrett WE., Jr Osseous injury associated with acute tears of the anterior cruciate ligament. Am J Sports Med. 1992 Jul-Aug;20(4):382–389. doi: 10.1177/036354659202000403. [DOI] [PubMed] [Google Scholar]
- 31.Spindler KP, Schils JP, Bergfeld JA, et al. Prospective study of osseous, articular, and meniscal lesions in recent anterior cruciate ligament tears by magnetic resonance imaging and arthroscopy. Am J Sports Med. 1993 Jul-Aug;21(4):551–557. doi: 10.1177/036354659302100412. [DOI] [PubMed] [Google Scholar]
- 32.Spindler KP, Warren TA, Callison JC, Jr, Secic M, Fleisch SB, Wright RW. Clinical outcome at a minimum of five years after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 2005 Aug;87(8):1673–1679. doi: 10.2106/JBJS.D.01842. [DOI] [PubMed] [Google Scholar]
- 33.Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008 Nov 13;359(20):2135–2142. doi: 10.1056/NEJMcp0804745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vellet AD, Marks PH, Fowler PJ, Munro TG. Occult posttraumatic osteochondral lesions of the knee: prevalence, classification, and short-term sequelae evaluated with MR imaging. Radiology. 1991 Jan;178(1):271–276. doi: 10.1148/radiology.178.1.1984319. [DOI] [PubMed] [Google Scholar]
- 35.W-Dahl A, Toksvig-Larsen S, Roos EM. A 2-year prospective study of patient-relevant outcomes in patients operated on for knee osteoarthritis with tibial osteotomy. BMC Musculoskelet Disord. 2005;6:18. doi: 10.1186/1471-2474-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.