Abstract
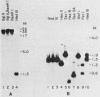
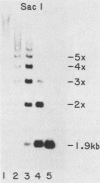
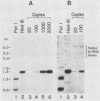
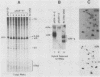
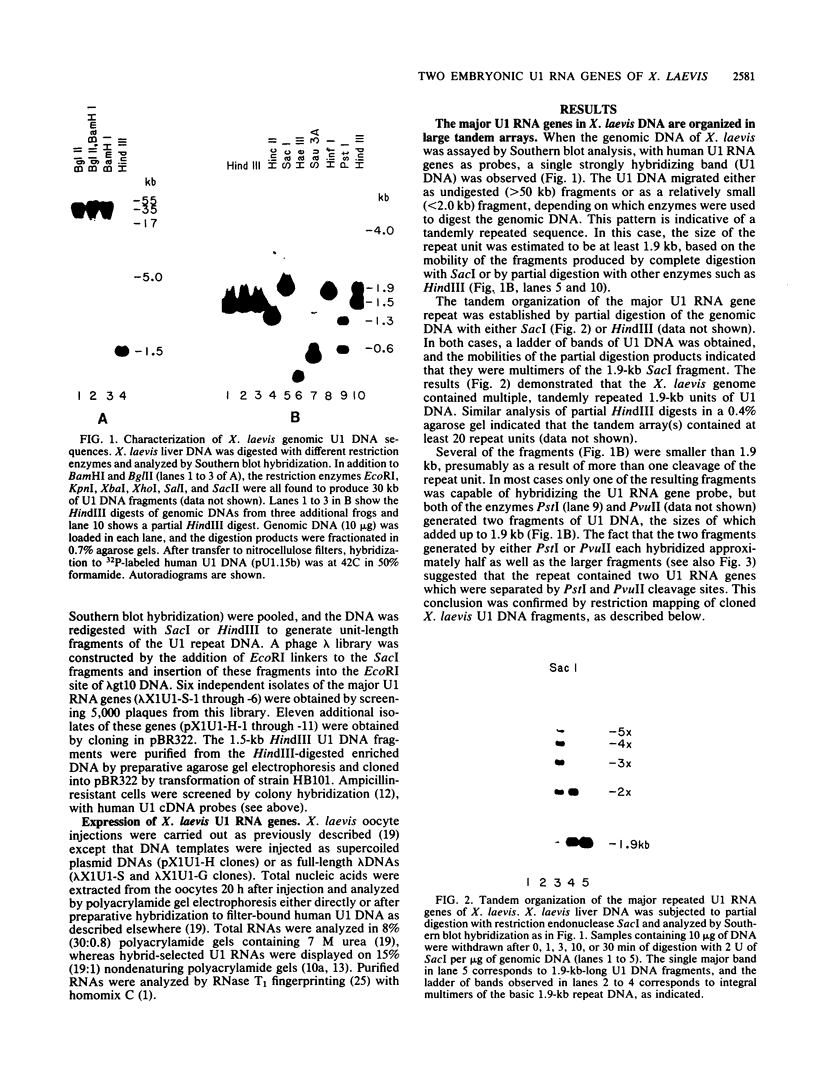
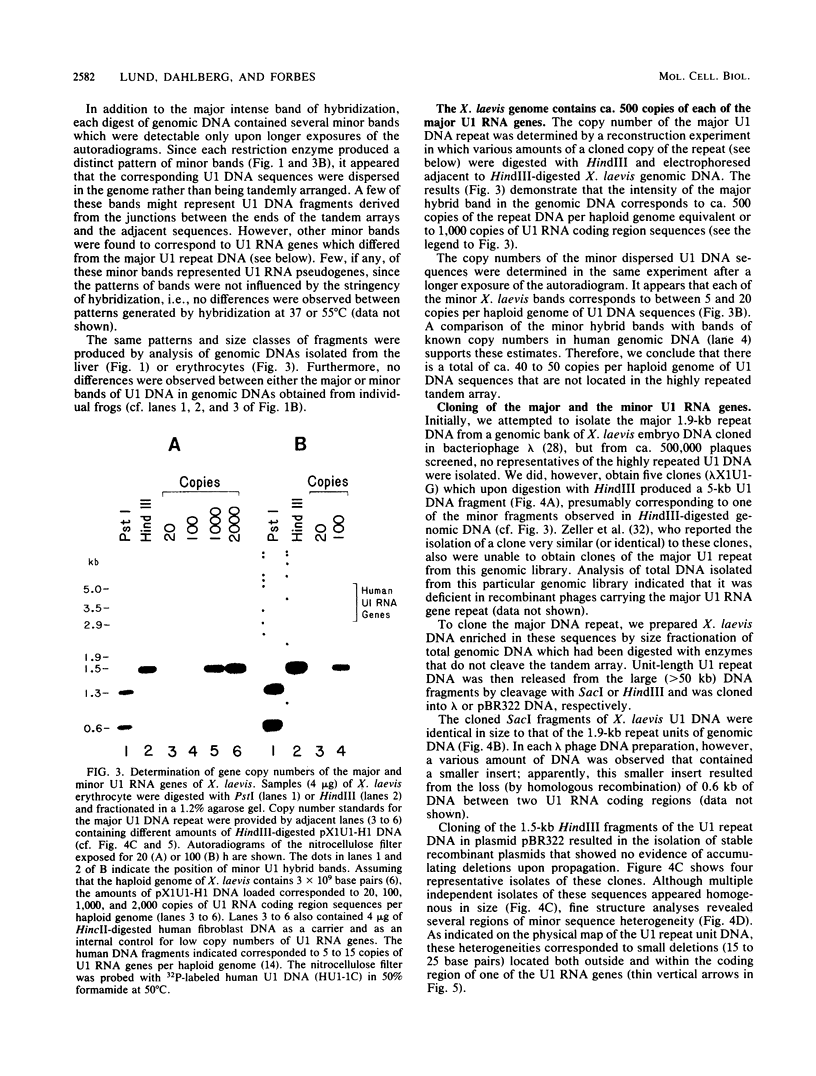
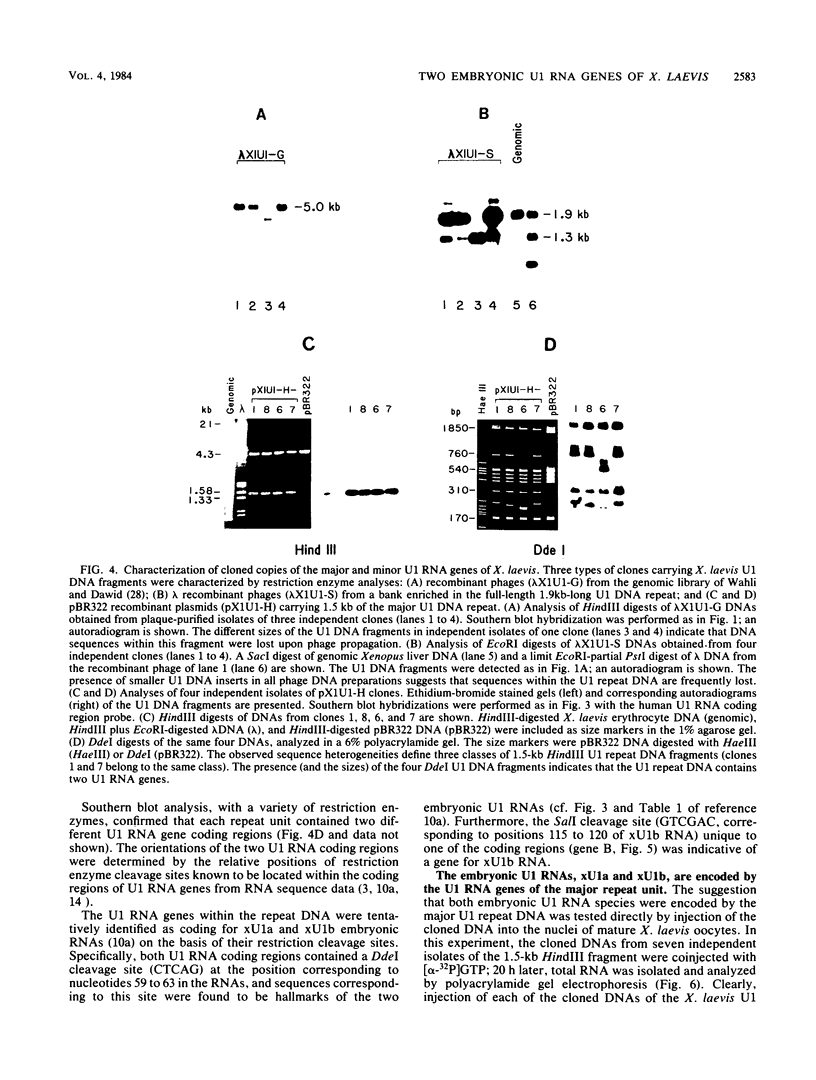
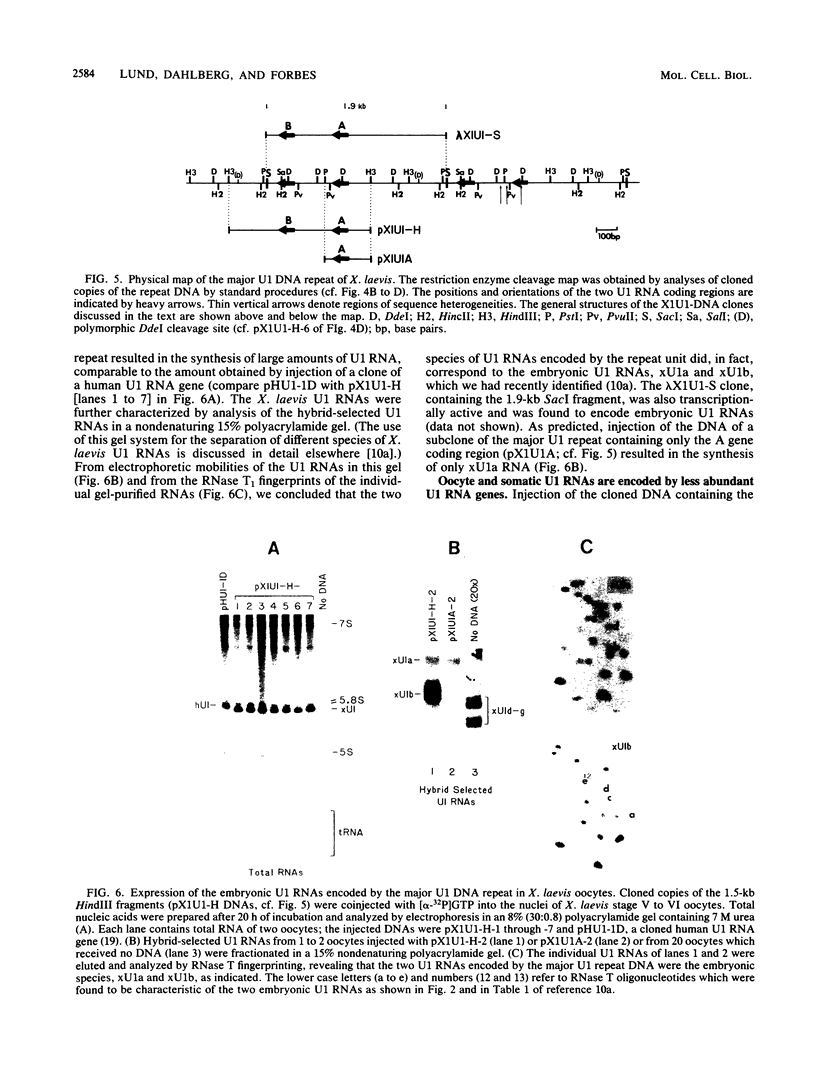
We have identified a large family of U1 RNA genes in Xenopus laevis that encodes two distinct species of U1 RNA. These genes are expressed primarily at the onset of transcription in the 4,000-cell embryo (D. J. Forbes, M. W. Kirschner, D. Caput, J. E. Dahlberg, and E. Lund, Cell 38:681-689, 1984). The two types of embryonic U1 RNA genes are interspersed and are organized in large tandem arrays. The basic 1.9-kilobase repeating unit contains a single copy of each of the embryonic genes and is reiterated ca. 500-fold per haploid genome. This repetitive U1 DNA accounts for more than 90% of all U1 DNA in X. laevis. In addition to this major family, there exist several minor families of dispersed U1 RNA genes, which presumably encode the oocyte and somatic species of X. laevis U1 RNA. Although the embryonic genes are normally inactive in stage VI oocytes, they are expressed when cloned copies are injected into oocyte nuclei.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Gallinaro H., Jacob M., Sri-Widada J., Jeanteur P. Nucleotide sequences of nuclear U1A RNAs from chicken, rat and man. Nucleic Acids Res. 1980 Sep 25;8(18):4143–4154. doi: 10.1093/nar/8.18.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E. M., Brown D. D. Sequence studies of the 5 S DNA of Xenopus laevis. J Mol Biol. 1974 Nov 15;89(4):703–718. doi: 10.1016/0022-2836(74)90046-1. [DOI] [PubMed] [Google Scholar]
- Card C. O., Morris G. F., Brown D. T., Marzluff W. F. Sea urchin small nuclear RNA genes are organized in distinct tandemly repeating units. Nucleic Acids Res. 1982 Dec 11;10(23):7677–7688. doi: 10.1093/nar/10.23.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B. Deoxyribonucleic acid in amphibian eggs. J Mol Biol. 1965 Jul;12(3):581–599. doi: 10.1016/s0022-2836(65)80313-8. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Denison R. A., Van Arsdell S. W., Bernstein L. B., Weiner A. M. Abundant pseudogenes for small nuclear RNAs are dispersed in the human genome. Proc Natl Acad Sci U S A. 1981 Feb;78(2):810–814. doi: 10.1073/pnas.78.2.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison R. A., Weiner A. M. Human U1 RNA pseudogenes may be generated by both DNA- and RNA-mediated mechanisms. Mol Cell Biol. 1982 Jul;2(7):815–828. doi: 10.1128/mcb.2.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D. J., Kirschner M. W., Caput D., Dahlberg J. E., Lund E. Differential expression of multiple U1 small nuclear RNAs in oocytes and embryos of Xenopus laevis. Cell. 1984 Oct;38(3):681–689. doi: 10.1016/0092-8674(84)90263-0. [DOI] [PubMed] [Google Scholar]
- Forbes D. J., Kornberg T. B., Kirschner M. W. Small nuclear RNA transcription and ribonucleoprotein assembly in early Xenopus development. J Cell Biol. 1983 Jul;97(1):62–72. doi: 10.1083/jcb.97.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford P. J., Southern E. M. Different sequences for 5S RNA in kidney cells and ovaries of Xenopus laevis. Nat New Biol. 1973 Jan 3;241(105):7–12. doi: 10.1038/newbio241007a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Bostock C., Robertson M., Christie S., Mitchen J. L., Dahlberg J. E. U1 small nuclear RNA genes are located on human chromosome 1 and are expressed in mouse-human hybrid cells. Mol Cell Biol. 1983 Dec;3(12):2211–2220. doi: 10.1128/mcb.3.12.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. True genes for human U1 small nuclear RNA. Copy number, polymorphism, and methylation. J Biol Chem. 1984 Feb 10;259(3):2013–2021. [PubMed] [Google Scholar]
- Manser T., Gesteland R. F. Human U1 loci: genes for human U1 RNA have dramatically similar genomic environments. Cell. 1982 May;29(1):257–264. doi: 10.1016/0092-8674(82)90110-6. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Brown D. T., Lobo S., Wang S. S. Isolation and characterization of two linked mouse U1b small nuclear RNA genes. Nucleic Acids Res. 1983 Sep 24;11(18):6255–6270. doi: 10.1093/nar/11.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W., Zeller R. Xenopus laevis U2 snRNA genes: tandemly repeated transcription units sharing 5' and 3' flanking homology with other RNA polymerase II transcribed genes. EMBO J. 1983;2(11):1883–1891. doi: 10.1002/j.1460-2075.1983.tb01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monstein H. J., Hammarström K., Westin G., Zabielski J., Philipson L., Pettersson U. Loci for human U1 RNA: structural and evolutionary implications. J Mol Biol. 1983 Jun 25;167(2):245–257. doi: 10.1016/s0022-2836(83)80334-9. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Burgess R. R., Dahlberg J. E., Lund E. Transcription of a gene for human U1 small nuclear RNA. Cell. 1982 May;29(1):265–274. doi: 10.1016/0092-8674(82)90111-8. [DOI] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982 Oct;30(3):675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Nojima H., Kornberg R. D. Genes and pseudogenes for mouse U1 and U2 small nuclear RNAs. J Biol Chem. 1983 Jul 10;258(13):8151–8155. [PubMed] [Google Scholar]
- Peterson R. C., Doering J. L., Brown D. D. Characterization of two xenopus somatic 5S DNAs and one minor oocyte-specific 5S DNA. Cell. 1980 May;20(1):131–141. doi: 10.1016/0092-8674(80)90241-x. [DOI] [PubMed] [Google Scholar]
- Rice P. W., Dahlberg J. E. A gene between polA and glnA retards growth of Escherichia coli when present in multiple copies: physiological effects of the gene for spot 42 RNA. J Bacteriol. 1982 Dec;152(3):1196–1210. doi: 10.1128/jb.152.3.1196-1210.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop D. R., Kristo P., Stumph W. E., Tsai M. J., O'Malley B. W. Structure and expression of a chicken gene coding for U1 RNA. Cell. 1981 Mar;23(3):671–680. doi: 10.1016/0092-8674(81)90430-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B. Isolation of two closely related vitellogenin genes, including their flanking regions, from a Xenopus laevis gene library. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1437–1441. doi: 10.1073/pnas.77.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L., Gurdon J. B. Cytoplasmic regulation of 5S RNA genes in nuclear-transplant embryos. EMBO J. 1983;2(9):1613–1619. doi: 10.1002/j.1460-2075.1983.tb01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Nagasu N., Itoh Y., Tani T., Okano K., Koga N., Okada N., Ohshima Y. Structural analysis of gene loci for rat U1 small nuclear RNA. Nucleic Acids Res. 1983 Mar 25;11(6):1791–1801. doi: 10.1093/nar/11.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington W. M., Schlissel M., Brown D. D. Developmental regulation of Xenopus 5S RNA genes. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):879–884. doi: 10.1101/sqb.1983.047.01.101. [DOI] [PubMed] [Google Scholar]
- Zeller R., Carri M. T., Mattaj I. W., De Robertis E. M. Xenopus laevis U1 snRNA genes: characterisation of transcriptionally active genes reveals major and minor repeated gene families. EMBO J. 1984 May;3(5):1075–1081. doi: 10.1002/j.1460-2075.1984.tb01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]