Abstract
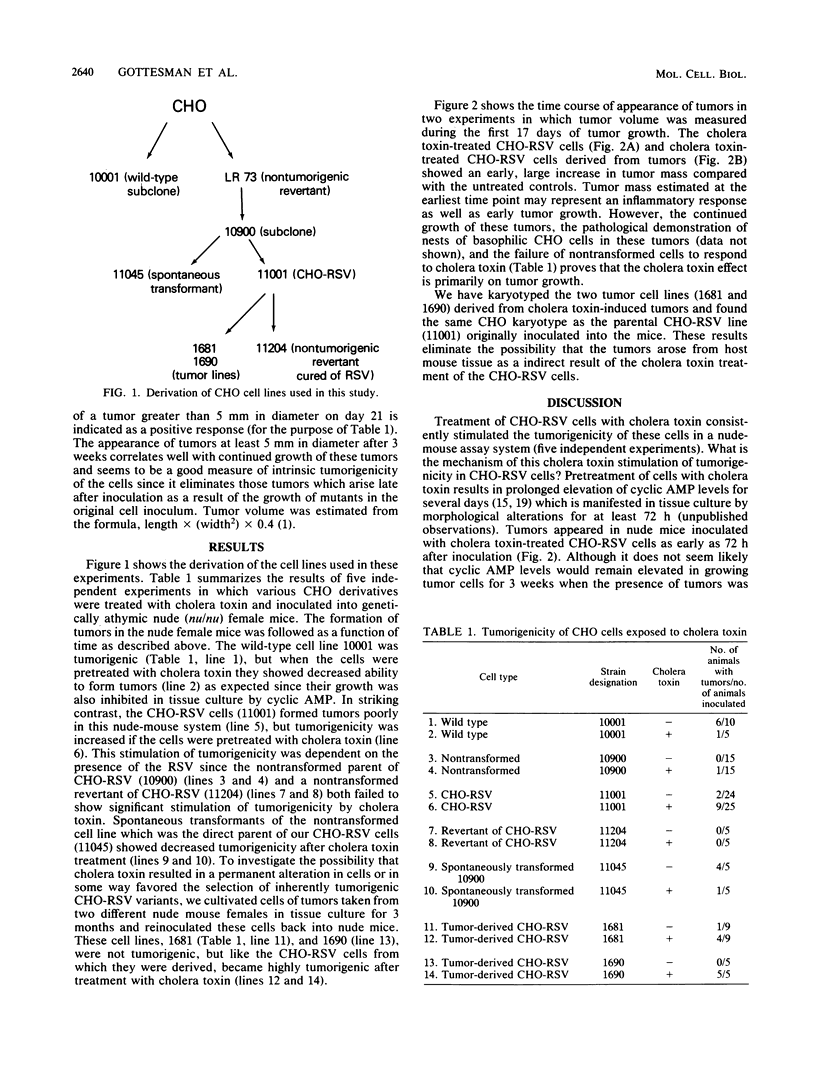
Chinese hamster ovary cells transformed by Rous sarcoma virus form tumors poorly in nude mice. Tumorigenicity was markedly stimulated by pretreatment of the cells with cholera toxin, which raises cyclic AMP levels and activates cyclic AMP-dependent protein kinase. Increased tumorigenicity was manifested by a severalfold increase in the rate of tumor formation, as well as earlier appearance and more rapid growth of tumors. In contrast, spontaneously transformed Chinese hamster ovary cells showed decreased tumorigenicity after cholera toxin treatment. The activation of tumorigenic potential in Rous sarcoma virus-transformed Chinese hamster ovary cells by cholera toxin correlated with increased phosphorylation of the viral oncogene product pp60src and stimulation of its tyrosine kinase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attia M. A., Weiss D. W. Immunology of spontaneous mammary carcinomas in mice. V. Acquired tumor resistance and enhancement in strain A mice infected with mammary tumor virus. Cancer Res. 1966 Aug;26(8):1787–1800. [PubMed] [Google Scholar]
- Croy B. A., Osoba D. Use of genetically defined nude mice in the study of the mixed leukocyte reaction. J Immunol. 1974 Nov;113(5):1626–1634. [PubMed] [Google Scholar]
- Eisinger M., Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evain D., Gottesman M., Pastan I., Anderson W. B. A mutation affecting the catalytic subunit of cyclic AMP-dependent protein kinase in CHO cells. J Biol Chem. 1979 Aug 10;254(15):6931–6937. [PubMed] [Google Scholar]
- Gottesman M. M., LeCam A., Bukowski M., Pastan I. Isolation of multiple classes of mutants of CHO cells resistant to cyclic AMP. Somatic Cell Genet. 1980 Jan;6(1):45–61. doi: 10.1007/BF01538695. [DOI] [PubMed] [Google Scholar]
- Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978 Nov;15(3):801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Gutmann N. S., Rae P. A., Schimmer B. P. Altered cyclic AMP-dependent protein kinase activity in a mutant adrenocortical tumor cell line. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):451–460. doi: 10.1002/jcp.1040970320. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada S., Imada M. Increase of a surface glycoprotein by cyclic AMP in Chinese hamster ovary cells. Dependence on cell-cell interaction. J Biol Chem. 1982 Aug 10;257(15):9108–9113. [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Li A. P., O'Neill J. P., Kawashima K., Hsie A. W. Correlation between changes in intracellular level of cyclic AMP, activation of cyclic AMP-dependent protein kinase, and the morphology of Chinese hamster ovary cells in culture. Arch Biochem Biophys. 1977 Jul;182(1):181–187. doi: 10.1016/0003-9861(77)90297-1. [DOI] [PubMed] [Google Scholar]
- Pollard J. W., Stanners C. P. Characterization of cell lines showing growth control isolated from both the wild type and a leucyl-tRNA synthetase mutant of Chinese hamster ovary cells. J Cell Physiol. 1979 Mar;98(3):571–585. doi: 10.1002/jcp.1040980315. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. B. Cholera toxin stimulates division of 3T3 cells. J Cell Physiol. 1979 Mar;98(3):469–474. doi: 10.1002/jcp.1040980305. [DOI] [PubMed] [Google Scholar]
- Roth C. W., Richert N. D., Pastan I., Gottesman M. M. Cyclic AMP treatment of Rous sarcoma virus-transformed Chinese hamster ovary cells increases phosphorylation of pp60src and increases pp60src kinase activity. J Biol Chem. 1983 Sep 10;258(17):10768–10773. [PubMed] [Google Scholar]
- Roth C. W., Singh T., Pastan I., Gottesman M. M. Rous sarcoma virus transformed cells are resistant to cyclic AMP. J Cell Physiol. 1982 Apr;111(1):42–48. doi: 10.1002/jcp.1041110108. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Strang G., Courtenay-Luck N. Cyclic AMP: a mitogenic signal for Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4392–4396. doi: 10.1073/pnas.78.7.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Simantov R., Sachs L. Temperature sensitivity of cyclic adenosine 3':5'-monophosphate-binding proteins and the regulation of growth and differentiation in neuroblastoma cells. J Biol Chem. 1975 May 10;250(9):3236–3242. [PubMed] [Google Scholar]
- Singh T. J., Roth C., Gottesman M. M., Pastan I. H. Characterization of cyclic AMP-resistant Chinese hamster ovary cell mutants lacking type I protein kinase. J Biol Chem. 1981 Jan 25;256(2):926–932. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Purkis P., Fentiman I. S. Cholera toxin and analogues of cyclic AMP stimulate the growth of cultured human mammary epithelial cells. J Cell Physiol. 1980 Mar;102(3):317–321. doi: 10.1002/jcp.1041020306. [DOI] [PubMed] [Google Scholar]
- Thion C., Green M. Cyclic AMP-amplified replication of RNA tumour virus-like particles in Chinese hamster ovary cells. Nat New Biol. 1973 Aug 22;244(138):227–231. doi: 10.1038/newbio244227a0. [DOI] [PubMed] [Google Scholar]


