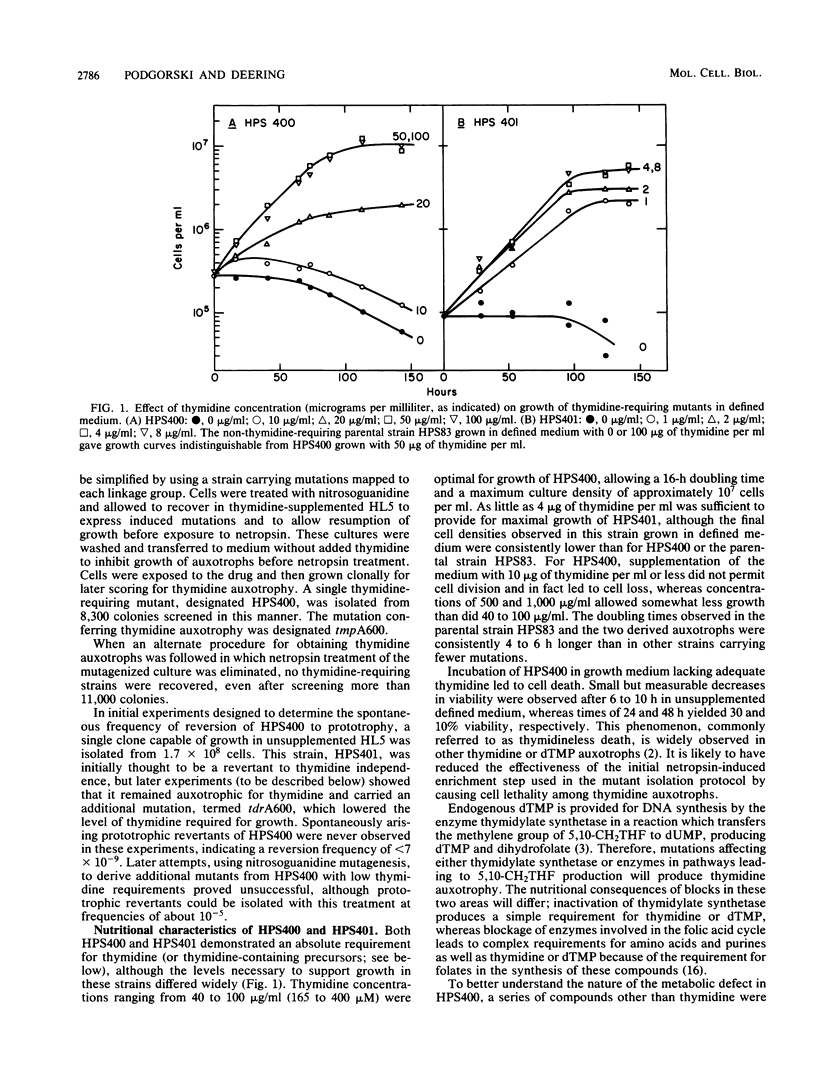
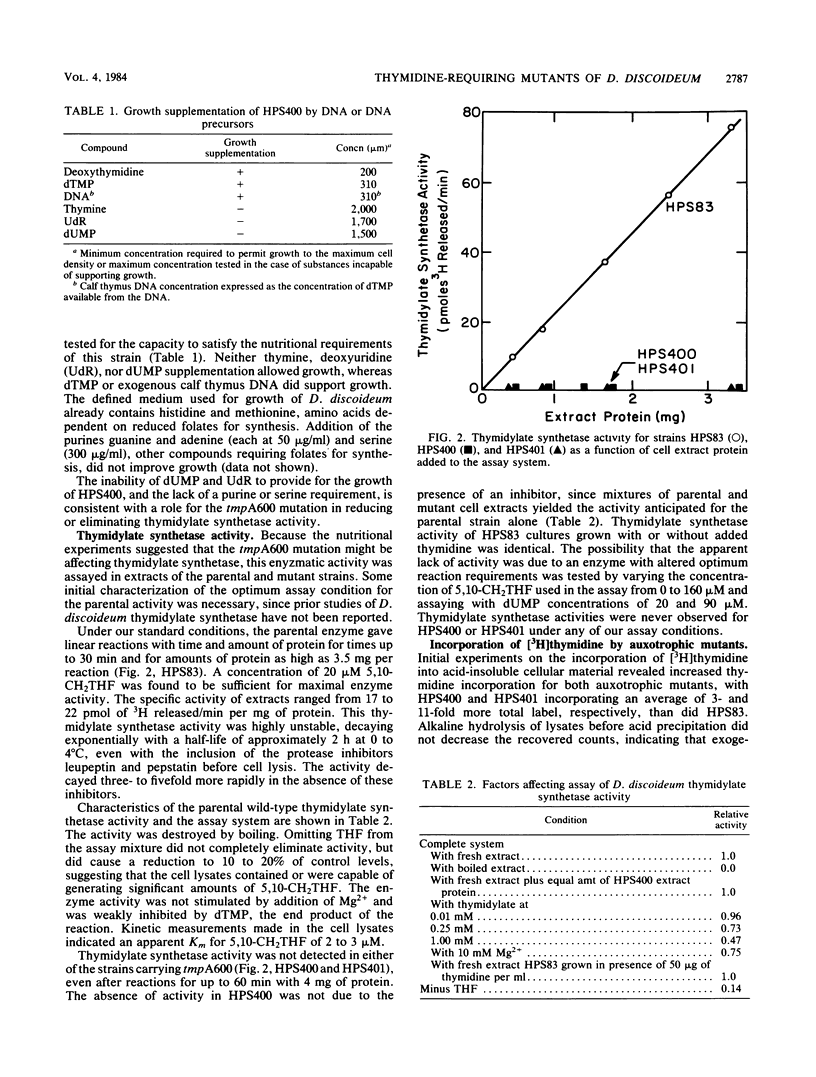
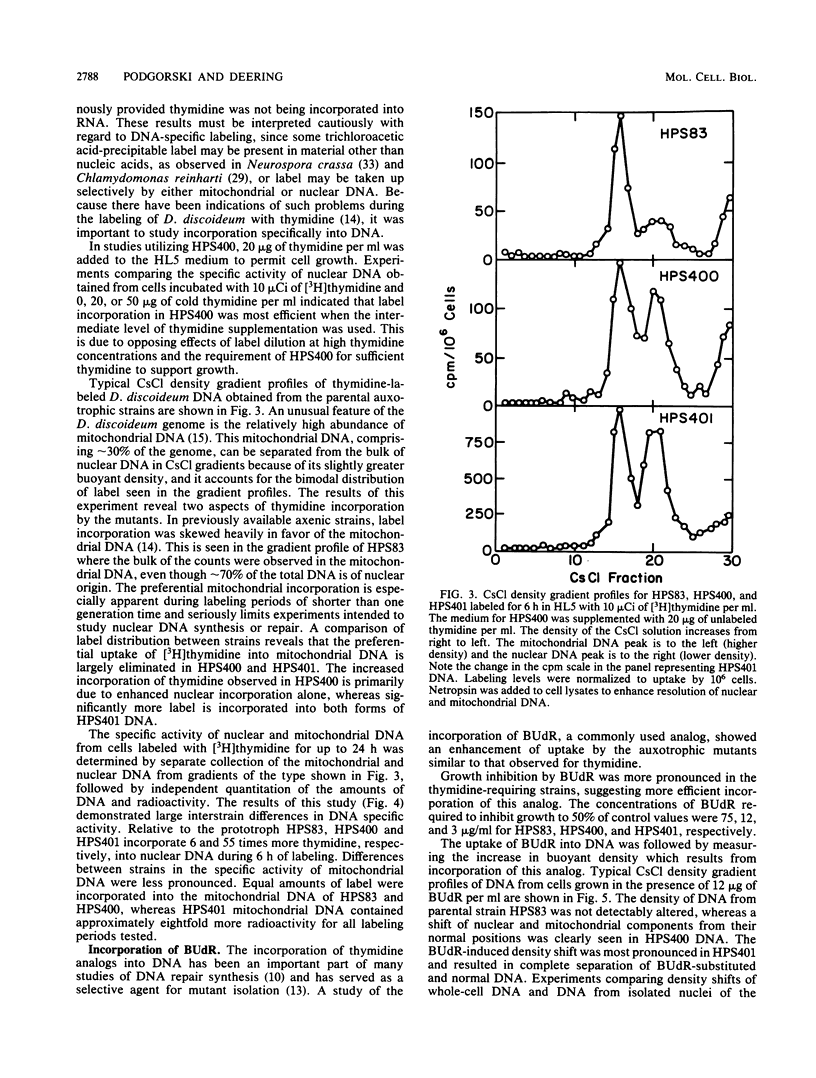
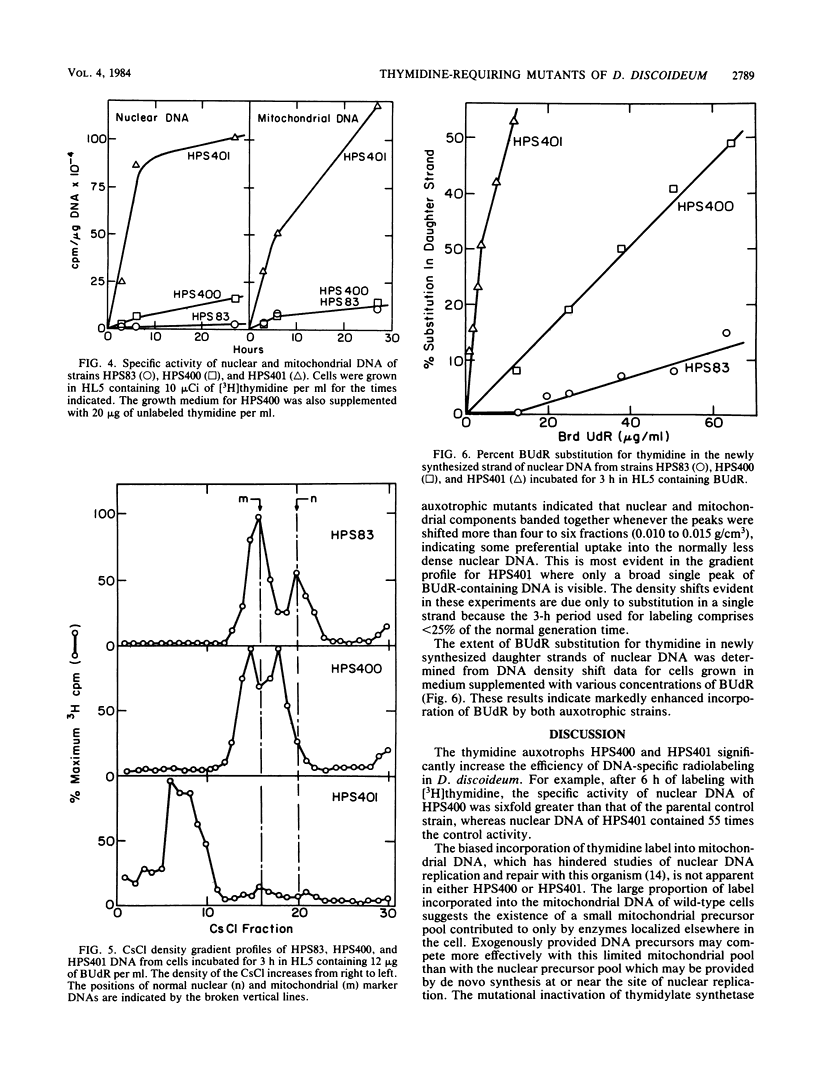
Abstract
Two thymidine auxotrophs of Dictyostelium discoideum were isolated which improve the efficiency of in vivo DNA-specific radiolabeling. Mutant HPS400 lacked detectable thymidylate synthetase activity, required 50 micrograms of thymidine per ml, and incorporated sixfold more [3H]thymidine into nuclear DNA than did a wild-type strain. Either dTMP or exogenously provided DNA also permitted growth of this strain. The second mutant, HPS401, was isolated from HPS400 and also lacked thymidylate synthetase activity, but required only 4 micrograms of thymidine per ml for normal growth and incorporated 55 times more thymidine label than did a control strain. Incorporation of the thymidine analog 5'-bromodeoxyuridine was also markedly increased in the mutants. Catalytic properties of the thymidylate synthetase of D. discoideum investigated in cell extracts were consistent with those observed for this enzyme in other organisms. These strains should facilitate studies of DNA replication and repair in D. discoideum which require short-term labeling, DNA of high specific activity, or elevated levels of substitution in DNA by thymidine analogs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Koyama H., Iwata K., Seno T. Selection of mammalian thymidine auxotrophic cell mutants defective in thymidylate synthase by their reduced sensitivity to methotrexate. Somatic Cell Genet. 1981 Sep;7(5):523–534. doi: 10.1007/BF01549656. [DOI] [PubMed] [Google Scholar]
- Barclay B. J., Kunz B. A., Little J. G., Haynes R. H. Genetic and biochemical consequences of thymidylate stress. Can J Biochem. 1982 Mar;60(3):172–184. doi: 10.1139/o82-023. [DOI] [PubMed] [Google Scholar]
- Bisson L. F., Thorner J. Exogenous dTMP utilization by a novel tup mutant of Saccharomyces cerevisiae. J Bacteriol. 1982 Oct;152(1):111–119. doi: 10.1128/jb.152.1.111-119.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Thorner J. Thymidylate synthetase from Saccharomyces cerevisiae. Purification and enzymic properties. J Biol Chem. 1981 Dec 10;256(23):12456–12462. [PubMed] [Google Scholar]
- Bisson L., Thorner J. Thymidine 5'-monophosphate-requiring mutants of Saccharomyces cerevisiae are deficient in thymidylate synthetase. J Bacteriol. 1977 Oct;132(1):44–50. doi: 10.1128/jb.132.1.44-50.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Jones K. C., James T. W. Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem. 1979 Jan 15;92(2):497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Deering R. A. Excision of pyrimidine dimers from nuclear deoxyribonucleic acid in ultraviolet-irradiated Dictyostelium discoideum. Mol Cell Biol. 1981 Feb;1(2):121–127. doi: 10.1128/mcb.1.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke J., Kessin R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1977 May;74(5):2157–2161. doi: 10.1073/pnas.74.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- Kammen H. O. A rapid assay for thymidylate synthetase. Anal Biochem. 1966 Dec;17(3):553–556. doi: 10.1016/0003-2697(66)90192-8. [DOI] [PubMed] [Google Scholar]
- Kessin R. H., Williams K. L., Newell P. C. Linkage analysis in Dictyostelium discoideum using temperature-sensitive growth mutants selected with bromodeoxyuridine. J Bacteriol. 1974 Sep;119(3):776–783. doi: 10.1128/jb.119.3.776-783.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielman J. K., Deering R. A. Ultraviolet light-induced inhibition of cell division and DNA synthesis in axenically grown repair mutants of Dictyostelium discoideum. Photochem Photobiol. 1980 Aug;32(2):149–156. doi: 10.1111/j.1751-1097.1980.tb04002.x. [DOI] [PubMed] [Google Scholar]
- Luk D. C., Bick M. D. Determination of 5'-bromodeoxyuridine in DNA by buoyant density. Anal Biochem. 1977 Feb;77(2):346–349. doi: 10.1016/0003-2697(77)90247-0. [DOI] [PubMed] [Google Scholar]
- Martinelli J. E., Chaykovsky M. A convenient preparation of tetrahydropteridines and tetrahydrofolate analogs. Synthesis of tetrahydro-3',5'-dichloromethotrexate. Prep Biochem. 1980;10(2):161–166. doi: 10.1080/00327488008061731. [DOI] [PubMed] [Google Scholar]
- Michrina C. A., Deering R. A. Thymidine kinase activity in Dictyostelium discoideum. J Gen Microbiol. 1980 Jul;119(1):263–266. doi: 10.1099/00221287-119-1-263. [DOI] [PubMed] [Google Scholar]
- Podgorski G., Deering R. A. Quantitation of induced mutation in Dictyostelium discoideum: characterization and use of a methanol-resistance mutation assay. Mutat Res. 1980 Dec;74(6):459–468. doi: 10.1016/0165-1161(80)90176-4. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman S. T., Greenberg G. R. A temperature-sensitive thy mutant blocked in the synthesis of thymidylate synthetase. J Biol Chem. 1971 Apr 25;246(8):2609–2617. [PubMed] [Google Scholar]
- Schwartz M. Thymidine phosphorylase from Escherichia coli. Methods Enzymol. 1978;51:442–445. doi: 10.1016/s0076-6879(78)51061-6. [DOI] [PubMed] [Google Scholar]
- Skoog L., Bjursell G. Nuclear and cytoplasmic pools of deoxyribonucleoside triphosphates in Chinese hamster ovary cells. J Biol Chem. 1974 Oct 25;249(20):6434–6438. [PubMed] [Google Scholar]
- Swinton D. C., Hanawalt P. C. In vivo specific labeling of Chlamydomonas chloroplast DNA. J Cell Biol. 1972 Sep;54(3):592–597. doi: 10.1083/jcb.54.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartell R. M., Larson J. E., Wells R. D. Netropsin. A specific probe for A-T regions of duplex deoxyribonucleic acid. J Biol Chem. 1974 Nov 10;249(21):6719–6731. [PubMed] [Google Scholar]
- Welker D. L., Deering R. A. Interactions between radiation-sensitive mutations in double-mutant haploids of Dictyostelium discoideum. Mol Gen Genet. 1979 Jan 2;167(3):265–270. doi: 10.1007/BF00267418. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Mitchell H. K. Mutants affecting thymidine metabolism in Neurospora crassa. J Bacteriol. 1969 Oct;100(1):383–389. doi: 10.1128/jb.100.1.383-389.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Gorman J. W., Gorman J. A., Bock R. M. Indirect selection for auxotrophic mutants of Saccharomyces cerevisiae using the antibiotic netropsin. Mutat Res. 1976 Jun;35(3):423–428. doi: 10.1016/0027-5107(76)90204-9. [DOI] [PubMed] [Google Scholar]


