Abstract
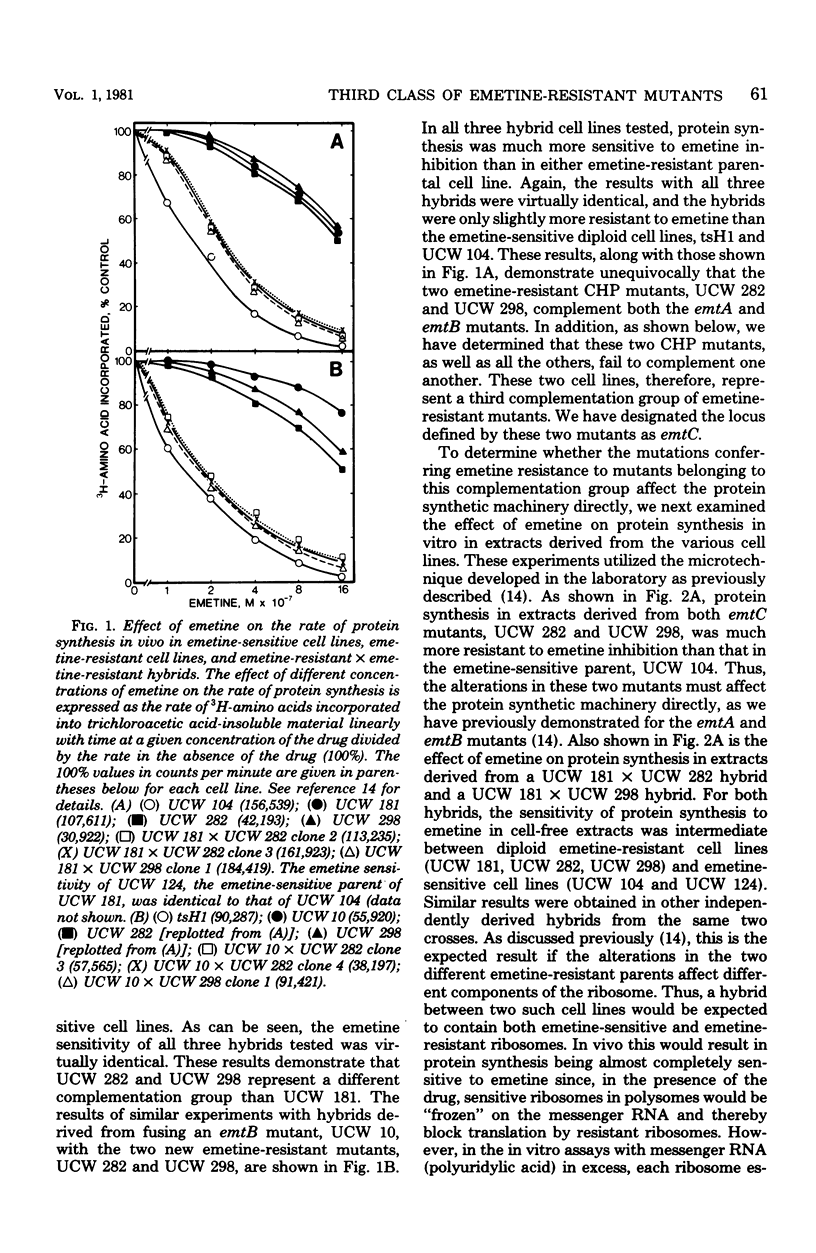
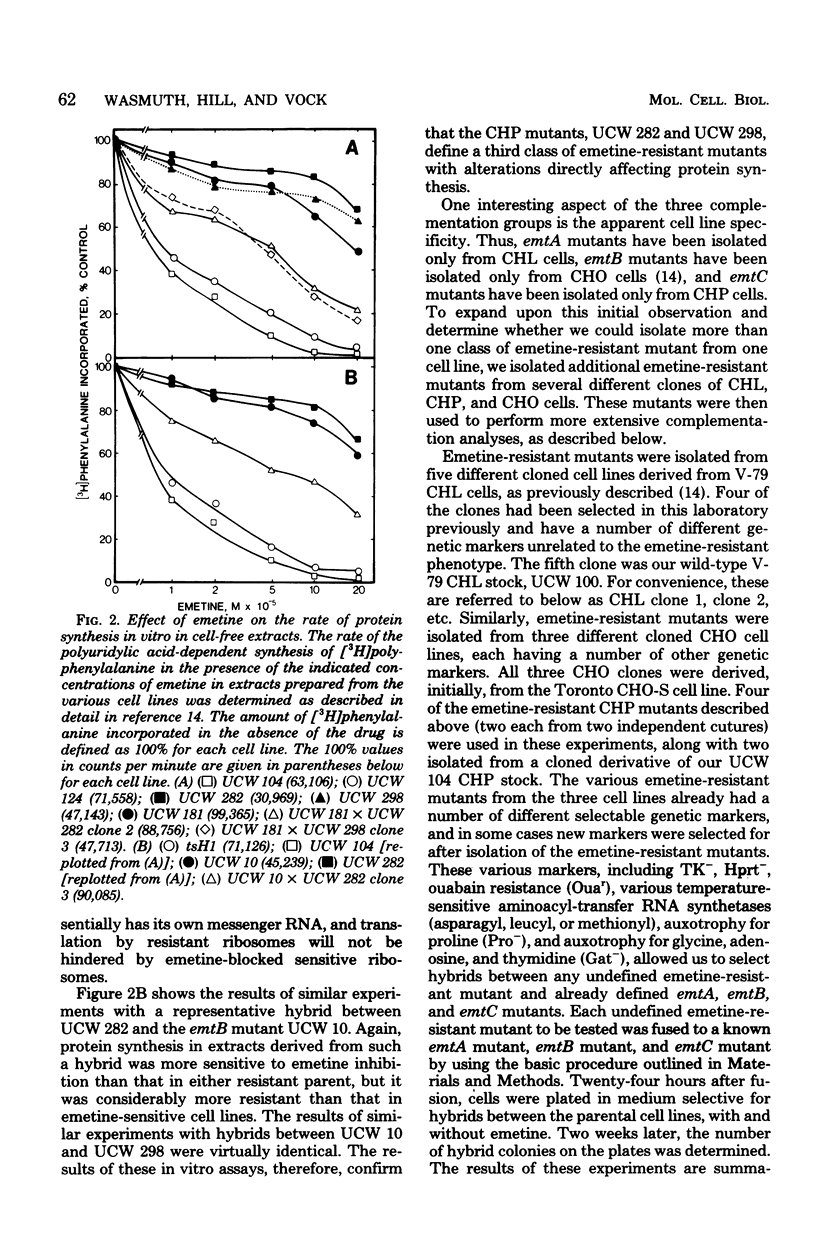
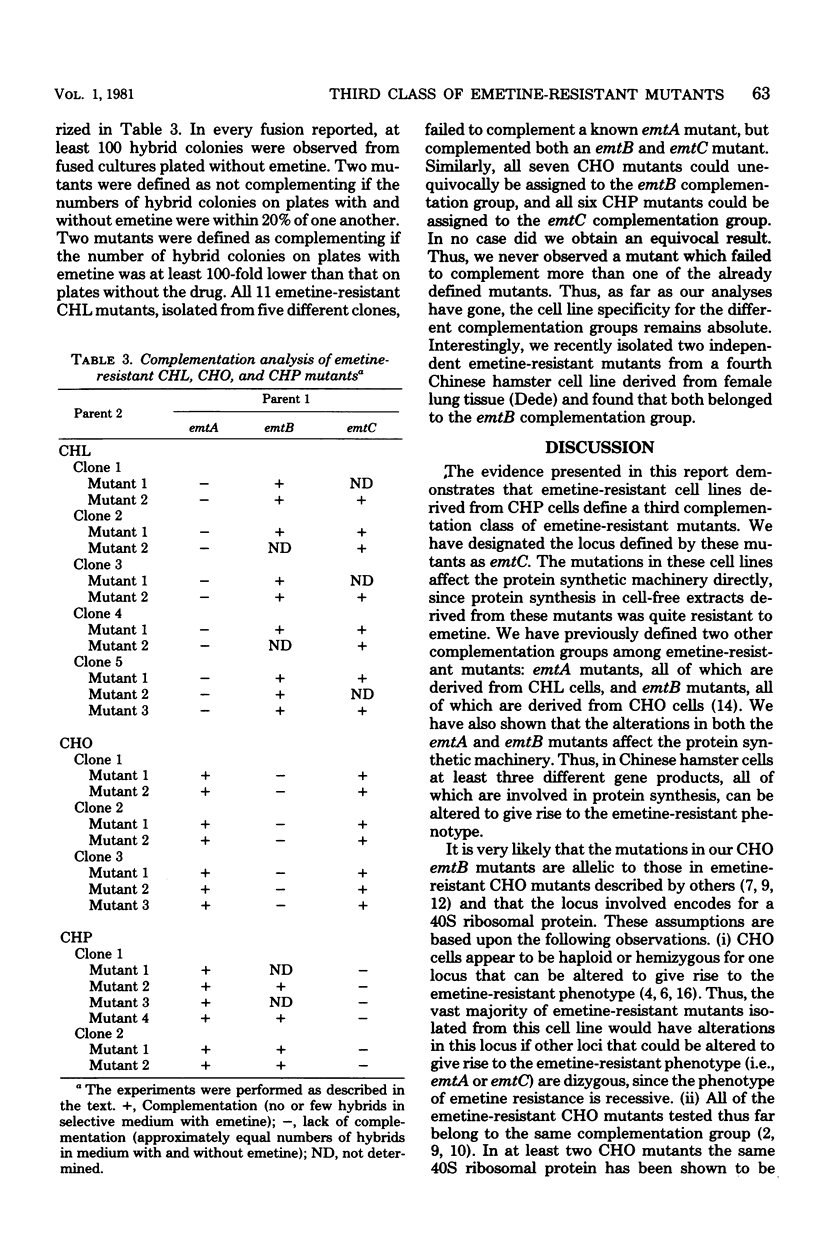
We have isolated emetine-resistant cell lines from Chinese hamster peritoneal fibroblasts and have shown that they represent a third distinct class or complementation group of emetine-resistant mutants, as determined by three different criteria. These mutants, like those belonging to the two other complementation groups we have previously defined, which were isolated from Chinese hamster lung and Chinese hamster ovary cells, have alterations that directly affect the protein biosynthetic machinery. So far, there is absolute cell line specificity with respect to the three complementation groups, in that all the emetine-resistant mutants we have isolated from Chinese hamster lung cells belong to one complementation group, all those we have isolated from Chinese hamster ovary cells belong to a second complementation group, and all those isolated from Chinese hamster peritoneal cells belong to a third complementation group. Thus, in cultured Chinese hamster cells, mutations in at least three different loci, designated emtA, emtB, and emtC, encoding for different components of the protein biosynthetic machinery, can give rise to the emetine-resistant phenotype.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boersma D., McGill S. M., Mollenkamp J. W., Roufa D. J. Emetine resistance in Chinese hamster cells is linked genetically with an altered 40S ribosomal subunit protein, S20. Proc Natl Acad Sci U S A. 1979 Jan;76(1):415–419. doi: 10.1073/pnas.76.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma D., McGill S., Mollenkamp J., Roufa D. J. Emetine resistance in Chinese hamster cells. Analysis of ribosomal proteins prepared from mutant cells. J Biol Chem. 1979 Jan 25;254(2):559–567. [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Evidence obtained by induced mutation frequency analysis for functional hemizygosity at the emt locus in CHO cells. Somatic Cell Genet. 1979 Jan;5(1):51–65. doi: 10.1007/BF01538786. [DOI] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Linkage of genetic markers emt and chr in Chinese hamster cells. Somatic Cell Genet. 1980 Mar;6(2):215–224. doi: 10.1007/BF01538797. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Chan D. Y., Siminovitch L. Evidence for functional hemizygosity at the Emtr locus in CHO cells through segregation analysis. Cell. 1978 Aug;14(4):1007–1013. doi: 10.1016/0092-8674(78)90354-9. [DOI] [PubMed] [Google Scholar]
- Gupta R. S. Random segretation of multiple genetic markers from CHO-CHO hybrids: evidence for random distribution of functional hemizygosity in the genome. Somatic Cell Genet. 1980 Jan;6(1):115–125. doi: 10.1007/BF01538700. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Mutants of CHO cells resistant to the protein synthesis inhibitor emetine: genetic and biochemical characterization of second-step mutants. Somatic Cell Genet. 1978 Jan;4(1):77–94. doi: 10.1007/BF01546494. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Mutants of CHO cells resistant to the protein synthesis inhibitors, cryptopleurine and tylocrebrine: genetic and biochemical evidence for common site of action of emetine, cryptopleurine, tylocrebine, and tubulosine. Biochemistry. 1977 Jul 12;16(14):3209–3214. doi: 10.1021/bi00633a026. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The isolation and preliminary characterization of somatic cell mutants resistant to the protein synthesis inhibitor-emetine. Cell. 1976 Oct;9(2):213–219. doi: 10.1016/0092-8674(76)90112-4. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The molecular basis of emetine resistance in Chinese hamster ovary cells: alteration in the 40S ribosomal subunit. Cell. 1977 Jan;10(1):61–66. doi: 10.1016/0092-8674(77)90140-4. [DOI] [PubMed] [Google Scholar]
- Haralson M. A., Roufa D. J. A temperature-sensitive mutation affecting the mammalian 60 S ribosome. J Biol Chem. 1975 Nov 25;250(22):8618–8623. [PubMed] [Google Scholar]
- Reichenbecher V. E., Jr, Caskey C. T. Emetine-resistant Chinese hamster cells. The identification of an electrophoretically altered protein of the 40 S ribosomal subunit. J Biol Chem. 1979 Jul 25;254(14):6207–6210. [PubMed] [Google Scholar]
- Skup D., Millward S. Highly efficient translation of messenger RNA in cell-free extracts prepared from L-cells. Nucleic Acids Res. 1977 Oct;4(10):3581–3587. doi: 10.1093/nar/4.10.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J. J., Chu L. Y. Linkage in cultured Chinese hamster cells of two genes, emtB and leuS, involved in protein synthesis and isolation of cell lines with mutations in three linked genes. J Cell Biol. 1980 Dec;87(3 Pt 1):697–702. doi: 10.1083/jcb.87.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J. J., Hill J. M., Vock L. S. Biochemical and genetic evidence for a new class of emetine-resistant Chinese hamster cells with alterations in the protein biosynthetic machinery. Somatic Cell Genet. 1980 Jul;6(4):495–516. doi: 10.1007/BF01539152. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Duff C., Campbell C. E. Marker segregation without chromosome loss at the emt locus in Chinese hamster cell hybrids. Somatic Cell Genet. 1980 Mar;6(2):199–213. doi: 10.1007/BF01538796. [DOI] [PubMed] [Google Scholar]


