Abstract
Oltipraz (OPZ) is a well known inducer of NAD(P)H:quinone oxidoreductase (NQO1) along with other enzymes that comprise the nuclear factor E2-related factor 2 (Nrf2) battery of detoxification genes. However, OPZ treatment also induces expression of CYP2B, a gene regulated by the constitutive androstane receptor (CAR). Therefore, this study was designed to determine whether OPZ induces gene expression in the mouse liver through activation of CAR in addition to Nrf2. OPZ increased the mRNA expression of both Cyp2b10 and Nqo1 in C57BL/6 mouse livers. As expected, in livers from Nrf2−/− mice, OPZ induction of Nqo1 was reduced, indicating Nqo1 induction is dependent on Nrf2 activation, whereas Cyp2b10 induction was unchanged. The robust induction of Cyp2b10 by OPZ in wild-type mice was completely absent in CAR−/− mice, revealing a CAR-dependent induction by OPZ. OPZ also induced transcription of the human CYP2B6 promoter-reporter containing the phenobarbital (PB) responsive element in mouse liver using an in vivo transcription assay. Additionally, OPZ induced in vivo nuclear accumulation of CAR at 3 h but, as with PB, was unable to reverse androstanol repression of mouse CAR constitutive activity in transiently transfected HepG2 cells. In summary, OPZ induces expression of Cyp2b10 and Nqo1 via the activation of CAR and Nrf2, respectively.
The chemopreventive chemical oltipraz (OPZ) (4-methyl-5-(2-pyrazinyl)-1,2-dithiol-3-thione) belongs to a class of compounds known as dithiolethiones. The antitumorigenic activity of OPZ is generally ascribed to its strong induction of enzymes that mediate detoxication processes. Early studies revealed that OPZ increases expression of a number of Phase I and II enzymes, including gluta-thione-S-transferases (GSTs), NAD(P)H:quinone oxidoreductase (NQO1), microsomal epoxide hydrolase, aflatoxin aldehyde reductase, glucuronosyl transferases, as well as enzymes that increase glutathione levels, namely, glutathione reductase and glucose-6-phosphate dehydrogenase (Kensler et al., 1985; Ansher et al., 1986; Davidson et al., 1990; Morel et al., 1993; Primiano et al., 1996).
Induction of these metabolic enzymes by OPZ has been linked to the transcription factor nuclear factor E2–related factor 2 (Nrf2) and its activation of the antioxidant response element/electrophile response element (ARE/EpRE). Under normal conditions, Nrf2 is sequestered in the cytosol and marked for proteasomal degradation by the protein Kelch-like ECH-associated protein 1 (Keap1). This protein acts as an adapter for Cullin 3-based ubiquitin ligase (E3 ligase), as well as a “receptor” for electrophiles (Cullinan et al., 2004). In the absence of electrophiles/oxidative stress, Nrf2 is ubiquitinated and targeted for degradation by the 26S-proteasome, keeping cellular levels of Nrf2 low and preventing activation of its response element. Electrophilic binding to Keap1 is thought to disrupt the ubiquitination complex and inhibit Nrf2 degradation (Zhang et al., 2004). The increased cellular concentration of unbound Nrf2 in turn leads to ARE/EpRE activation (Zhang, 2006). It has been shown in Keap1 knockout mice that the loss of this Nrf2-Keap1 complex leads to Nrf2 nuclear accumulation and activation (Itoh et al., 2004). Further evidence of the role of Nrf2 in OPZ gene induction of detoxication enzymes was provided through experiments showing that loss of expression of Nrf2 abrogates both the chemoprotective activity of OPZ, as well as the inducibility of many known OPZ-induced genes (Kwak et al., 2001; Ramos-Gomez et al., 2001).
Although early investigation into the activity of OPZ focused on induction of detoxication enzymes like NQO1 and GST, OPZ also alters the expression and activity of certain cytochrome P450 enzymes. OPZ has been shown to induce the expression of CYP1A1, CYP1A2, CYP2B1, CYP2B2, and CYP2E1 (Maheo et al., 1998; Cherrington et al., 2003; Cho and Kim, 2003; Merrell et al., 2008). The induction of at least one of these isoforms, CYP1A1, has been characterized as aryl hydrocarbon receptor-dependent (Le Ferrec et al., 2002), which suggests that OPZ may activate multiple transcription factors.
Little has been reported previously regarding the strong induction of the CYP2B genes by OPZ. The mechanism by which CYP2B genes are induced is well documented (Sueyoshi and Negishi, 2001). CYP2B inducers activate the constitutive androstane receptor (CAR), which is normally sequestered in the cytoplasm by the cytoplasmic CAR retention protein (Kobayashi et al., 2003). Treatment with CAR activators appears to induce nuclear accumulation through one of two mechanisms: 1) some CAR activators are ligands, such as TCPOBOP in mice and CITCO in humans, and act as direct activators of CAR nuclear translocation; and 2) other CAR activators, such as phenobarbital (PB) and phenytoin, do not appear to be actual ligands and are thought to promote CAR nuclear translocation indirectly (Jackson et al., 2004, 2006; Stanley et al., 2006). In both direct and indirect activation, CAR translocates to the nucleus where it heterodimerizes with the retinoid X receptor (RXR) α. The CAR/RXRα heterodimer then binds to a PB responsive enhancer module to drive expression of CYP2B genes, including Cyp2b1 in rats, Cyp2b10 in mice, and CYP2B6 in humans (Honkakoski and Negishi, 1997; Honkakoski et al., 1998). We have previously shown that OPZ induction of Cyp2b10 mRNA expression is significantly less in RXRα-deficient mice (Cherrington et al., 2003).
The present study was developed to determine whether, in addition to activating Nrf2, OPZ also activates the nuclear receptor CAR. We report here that OPZ strongly induces Cyp2b10 in a CAR-dependent manner, that OPZ treatment increases CAR nuclear accumulation, and that OPZ appears to be an indirect activator of CAR. This study shows an uncharacterized mechanism for OPZ-mediated gene induction through activation of the CAR.
Materials and Methods
Chemicals
OPZ was a gift of Dr. Ronald Lubet (National Cancer Institute, Bethesda, MD). D-Luciferin was synthesized by Dr. Eugene Mash (University of Arizona, Tuscon, AZ). 5α-Androstan-3α-ol (androstanol) was purchased from Steraloids (Newport, RI). PB was purchased from Mallinckrodt, Inc. (Paris, KY). Ketamine HCl and xylazine injectables were purchased from Associated Medical Supply (Scottsdale, AZ). Luciferin was obtained from Molecular Imaging Products Company (Ann Arbor, MI). All the other chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Animals
All the animals were housed and acclimated in a temperature-, light-, and humidity-controlled environment in cages with hardwood chips and were given Harlan Teklad Rodent Diet W (Harlan Laboratories, Madison, WI) and water ad libitum. All the animals were acclimated for at least 1 week before the experiments and were allowed water and standard chow ad libitum. Housing and experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals as determined by the U.S. National Institutes of Health. Male C57BL/6 mice were treated with either corn oil (5 ml/kg), OPZ (150 mg/kg, i.p. in corn oil), or PB (80 mg/kg, i.p. in saline) with an injection volume of 5 ml/kg. The choice of these doses was based on previous work from our laboratory (Cherrington et al., 2002), as well as the preponderance of published literature.
For the dose response, adult male C57BL/6 mice were treated with vehicle or OPZ once daily for 4 days (50, 100, 150, 200, and 250 mg/kg). Male Nrf2−/− mice (Aleksunes et al., 2006), CAR−/− mice (Wei et al., 2000), or C57BL/6 mice (n = 5 in each group) were treated with either corn oil (5 ml/kg), OPZ (150 mg/kg, p.o. in corn oil), or PB (80 mg/kg, i.p. in saline, 24 h) for 4 days with an injection volume of 5 ml/kg. For mRNA analysis, livers were excised 24 h following the final treatment, snap-frozen in liquid nitrogen, and stored at − 80°C until use. For nuclear extraction, livers were excised 3 h after OPZ treatment, and nuclear protein was isolated as previously described (Sueyoshi et al., 1995) and stored at − 80°C until Western blot analysis of CAR accumulation. To assess the degree of cytosolic contamination in the nuclear extracts, Western blotting was performed against glyceraldehyde-3-phosphate dehydrogenase protein, with minimal cytosolic contamination detected (data not shown).
RNA Isolation
Total RNA was isolated using RNAzol B reagent (Tel-Test Inc., Friendswood, TX) as per the manufacturer’s protocol. RNA concentration was determined by UV spectrophotometry, and its integrity was examined by ethidium bromide staining after agarose gel electrophoresis.
Branched DNA Signal Amplification Assay
Mouse Cyp2b10 and Nqo1 probe sets were used as previously described (Cherrington et al., 2003; Aleksunes et al., 2005). These oligonucleotide probes were diluted in lysis buffer supplied in the Quantigene HV Signal Screen Kit (Panomics, Inc., Freemont, CA). Reagents for analysis were supplied with the kit (i.e., lysis buffer, amplifier/label probe buffer, and substrate solution) or prepared in the laboratory (capture hybridization buffer). Total RNA (1 μg/μl, 10 μl) was added to each well of a 96-well plate containing 50 μl of capture hybridization buffer and 50 /μl of diluted probe set. mRNA was allowed to hybridize to each probe set overnight at 53°C. Subsequent hybridization steps were carried out as per the manufacturer’s protocol, and luminescence was measured with a Quantiplex 320 branched DNA (bDNA) luminometer (Bayer Diagnostics, Tarrytown, NY) interfaced with Quantiplex Data Management Software version 5.02 for analysis of luminescence from 96-well plates.
In Vivo Transcription Assay
Male C57BL/6 mice (20–25 g; Charles River Laboratories, Raleigh, NC) were administered 10 μg of naked plasmid DNA (CYP2B6 promoter luciferase reporter construct) as a rapid (5-s) tail vein injection in sterile saline at a dose volume equal to 10% of body weight. Eighteen hours later, animals were anesthetized with a mixture of ketamine (72 mg/kg) and xylazine (6 mg/kg). Mice were injected i.p. with luciferin (70 /μl of a 50-mg/ml stock solution) 5 min before imaging. A VersArray 1300B camera (Princeton Instruments, Trenton, NJ) thermoelectrically cooled to − 100°C, with an aperture of ƒ1, connected to a light-sealed imaging chamber was used for all the images. Images were acquired in gray scale, and pseudocolor maps were created with the WinView 32 program (Princeton Instruments). Color maps were superimposed over the light image of the mouse using Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA), and these images were considered time 0. Each animal (n = 3) was administered a single i.p. dose of either OPZ (150 mg/kg) or corn oil at a dose volume of 5 ml/kg, and imaging was repeated 24 h after dosing.
Western Blot Analysis
Nuclear extracts from control, PB-, and OPZ-treated C57BL/6 mice were resolved on a SDS-10% polyacrylamide gel and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). Blots were incubated 1 h at room temperature in blocking buffer containing 5% nonfat milk in Tris-buffered saline/Tween 20. After 3-h incubation with a rabbit anti-CAR polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), the membrane was incubated 1 h with a goat anti-rabbit IgG-horseradish peroxidase conjugate (Santa Cruz Biotechnology). Western blots were visualized using enhanced chemiluminescence advanced reagents (Amersham, Arlington Heights, IL) as per manufacturer’s protocol.
In Vitro Luciferase Assay
Cultured HepG2 cells were transiently transfected with a mouse CAR (mCAR) expression plasmid (mCAR/pCR3.0) (Sueyoshi et al., 1999) and a CYP2B6-tk-luciferase construct. The human CYP2B6 promoter luciferase reporter construct was obtained from Dr. Richard Kim (Vanderbilt University School of Medicine, Nashville, TN). The CYP2B6 promoter luciferase reporter construct contains a 1.7-kilobase fragment that maintains the core promoter (+39/−364) and the distal enhancer region (−1461/−2013) including the PB responsive element (PBREM) (R. Kim, unpublished data).
Briefly, HepG2 cells were cultured in Eagle’s minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen) according to the protocol provided by American Type Culture Collection (Manassas, VA). In 24-well plates, the CYP2B6-tk-luciferase plasmid (0.1 μg) was cotransfected with pRL-tk transfection control (0.001 μg) (Promega Corp., Madison, WI) into HepG2 cells using Transfectene (Qiagen, Hilden, Germany) with or without a mouse CAR expression plasmid (0.025 μg). Twenty-four hours after transfection, cells were cultured in the presence of dimethyl sulfoxide (DMSO; 0.2%), PB (1 mM), TCPOBOP (250 nM), or OPZ (5, 20, 50, 100 μM) with or without androstanol (10 μM). After 24 h of exposure, the media were removed and cells were lysed with 100 μl of passive lysis buffer. Luciferase activity was determined by the Dual-Glo Luciferase Assay per manufacturer’s protocol (Promega Corp.).
Statistical Analysis
Data are expressed as mean ± S.E. For multiple comparisons, an analysis of variance was performed followed by Duncan’s multiple range test. The level of significance was set at p ≤ 0.05.
Results
Induction of Cyp2b10 and Nqo1 Expression in C57BL/6 Mice
Nqo1 mRNA levels were significantly increased by OPZ (22-fold) but were not induced by administration of PB (Fig. 1). However, both PB and OPZ treatment resulted in robust increases in mRNA expression of Cyp2b10 compared with control.
Fig. 1.
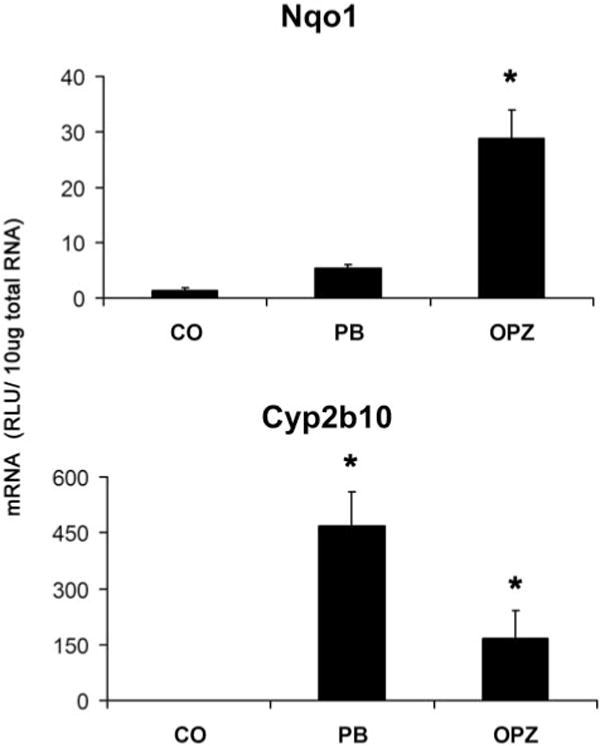
Nqo1 and Cyp2b10 mRNA induction. C57BL/6 mice were treated with corn oil, PB (80 mg/kg), or OPZ (150 mg/kg). Total RNA was isolated, and mRNA levels were analyzed by the bDNA signal amplification assay. mRNA levels are expressed as mean relative light units (RLU)/10 μg total RNA ± S.E.M. (n = 3); *, significantly different from vehicle (p ≤ 0.05).
Dose Response of Induction of Nqo1 and Cyp2b10 to OPZ Treatment
The increase of Nqo1 mRNA levels by OPZ (Fig. 2) reached significance at a dose of 100 mg/kg and continued to increase through the higher doses, reaching an induction of 6-fold by 250 mg/kg. Although Cyp2b10 induction by OPZ did not reach significance until 200 mg/kg, the induction was more profound, with 47- and 74-fold increases at 200 and 250 mg/kg.
Fig. 2.
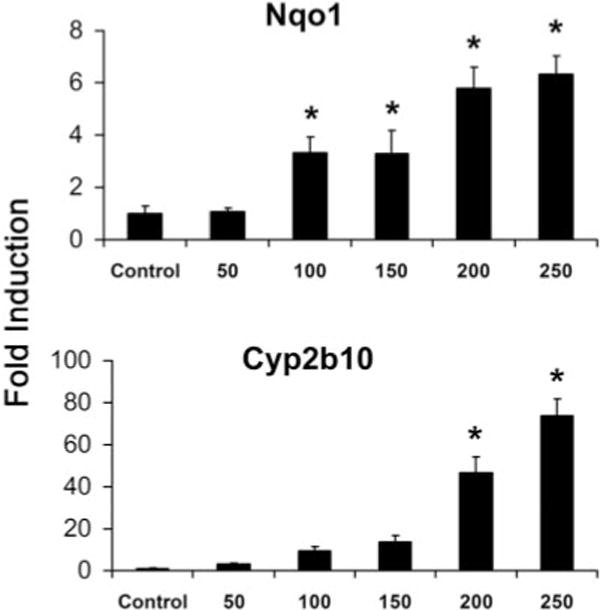
Dose response of induction of Nqo1 and Cyp2b10 to OPZ treatment. Total RNA was isolated from adult male C57BL/6 mice after treatment of OPZ (50, 100, 150, 200, 250 mg/kg, i.p., once daily, 4 days). The mRNA levels of Cyp2b10 and Nqo1 were determined by the bDNA signal amplification assay. The data are presented as mean -fold induction ± S.E.M. (n = 5–6); *, significantly different from vehicle (p ≤ 0.05).
Induction of Cyp2b10 and Nqo1 Expression in Nrf2- and CAR-Deficient Mice
To determine the involvement of Nrf2 and CAR in the induction of Cyp2b10 and Nqo1, Nrf2- and CAR-null mice were used. As an indication of Nrf2-mediated induction, treatment of wild-type mice with PB and OPZ resulted in 2.5- and 3.6-fold increases in Nqo1 mRNA, respectively (Fig. 3). This induction of Nqo1 was ablated in the Nrf2−/− animals, as has been previously noted for OPZ (Kwak et al., 2001). The absence of Nrf2 had no effect on the induction of Cyp2b10 mRNA, as the robust induction by PB and OPZ was maintained in Nrf2−/− animals.
Fig. 3.
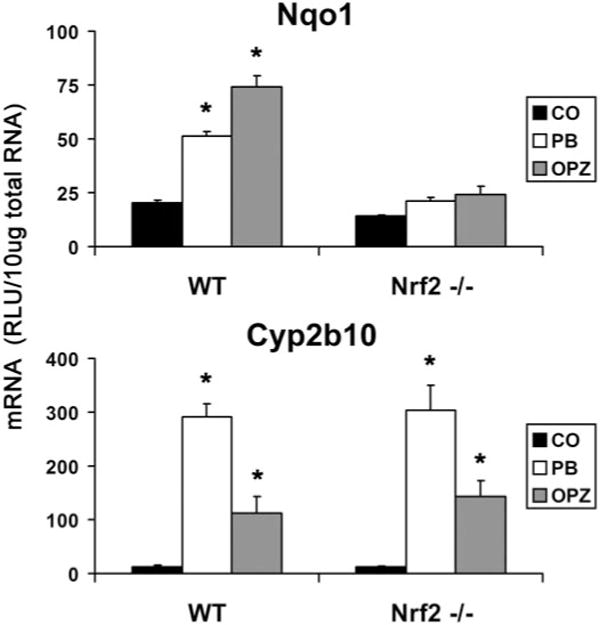
Induction of Cyp2b10 and Nqo1 mRNA in wild-type and Nrf2-null mice. Total RNA was isolated from wild-type and Nrf2−/− mice treated with OPZ (150 mg/kg) and PB (80 mg/kg) and analyzed by the bDNA signal amplification assay. mRNA levels are expressed as mean relative light units (RLU)/10 μg total RNA ± S.E.M. (n = 5); *, significantly different from vehicle (p ≤ 0.05).
Treatment of wild-type mice with PB or OPZ resulted in a greater than 30- and 10-fold increase in Cyp2b10 mRNA, respectively (Fig. 4). This robust induction was completely absent in the CAR−/− animals treated with PB and OPZ, as has been previously noted for PB (Wei et al., 2000). In contrast, the induction of Nqo1 mRNA was maintained in CAR−/− animals.
Fig. 4.
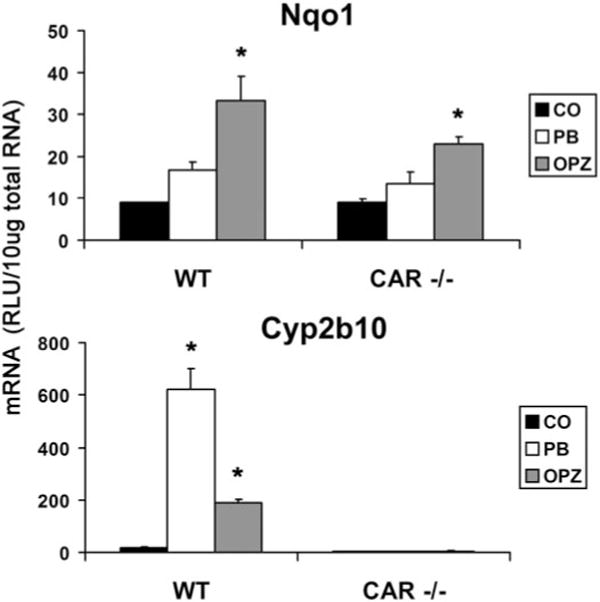
Induction of Cyp2b10 and Nqo1 mRNA in wild-type and CAR-null mice. Total RNA was isolated from wild-type and CAR−/− mice treated with OPZ (150 mg/kg) and PB (80 mg/kg) and analyzed by the bDNA signal amplification assay. mRNA levels are expressed as mean relative light units (RLU)/10 μg total RNA ± S.E.M. (n = 5); *, significantly different from vehicle (p ≤ 0.05).
Real-Time in Vivo Activation of the Human CYP2B6 Promoter in Mice
The ability of OPZ to activate transcription of the human CYP2B6 promoter containing the PBREM was determined by transfecting a human CYP2B6 promoter-reporter luciferase reporter via rapid i.v. hydrodynamic injection via the tail vein and a real-time in vivo imaging assay before and after dosing. OPZ treatment resulted in robust activation of the human CYP2B6 promoter as seen in Fig. 5.
Fig. 5.
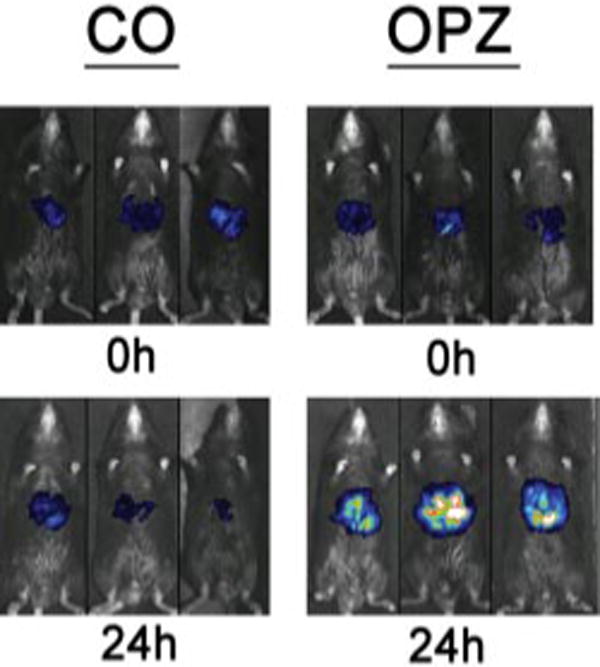
In vivo activation of the human CYP2B6 promoter-luciferase reporter construct in mouse liver after vehicle or OPZ administration. Male C57Bl/6 mice were injected with 10 μg of naked plasmid DNA in a rapid (5-s) tail vein injection in sterile saline in a volume equal to 10% of body weight. Following a 16-h recovery period, animals were anesthetized with a solution of ketamine (72 mg/kg) and xylazine (6 mg/kg) and injected with luciferin i.p. At time 0, mice were injected with corn oil vehicle (5 ml/kg, i.p.) or OPZ (150 mg/kg). Images were collected at 0 and 24 h following vehicle or OPZ administration.
Nuclear Accumulation of CAR in PB- and OPZ-Treated Mice
To determine the ability of OPZ to induce nuclear accumulation of CAR, C57BL/6 mice were treated with corn oil (5 ml/kg), PB (80 mg/kg), and OPZ (150 mg/kg). Western blot analysis of nuclear extracts shows an increase in CAR nuclear accumulation in both PB-and OPZ-treated animals (Fig. 6).
Fig. 6.
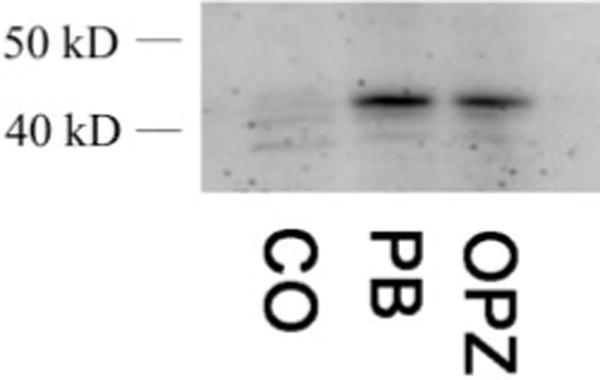
CAR nuclear accumulation. C57Bl/6 mice were treated with corn oil, PB (80 mg/kg), or OPZ (150 mg/kg). Nuclear extracts were immunoblotted to detect the presence of CAR.
Ability of OPZ to Overcome Androstanol Inhibition of CAR
Cotransfection of HepG2 cells with a mouse CAR expression construct and CYP2B6 reporter gene containing the PBREM resulted in a 5-fold increase in luciferase expression (Fig. 7). Known mCAR activity modulators and ligands androstanol and TCPOBOP were used to further investigate the mechanisms of CAR activation. As expected, treatment with the mCAR inhibitor androstanol repressed transcriptional activation of the CYP2B6 reporter. In contrast, cotreatment with the direct CAR activator TCPOBOP was sufficient to overcome this inhibition. Neither PB nor OPZ was able to overcome androstanol repression of CAR at the doses examined.
Fig. 7.
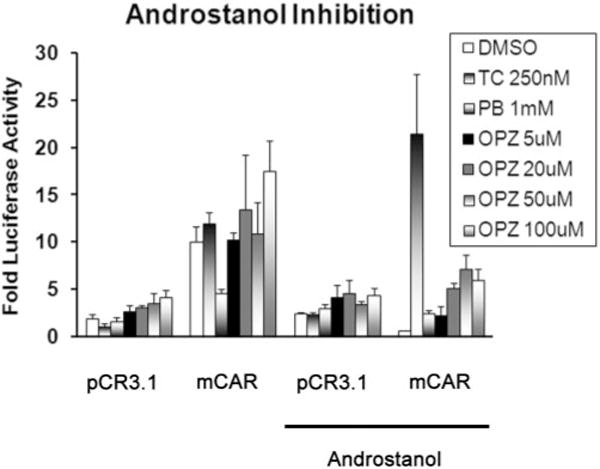
Activation of the CYP2B6-tk-luciferase reporter construct in HepG2 cells after treatment with TCPOBOP, PB, and OPZ. HepG2 cells were transiently transfected with the CYP2B6-tk-luciferase plasmid (0.1 μg) and pRL-TK (0.001 μg) into HepG2 cells, with or without a mouse CAR expression plasmid (0.25 μg). Twenty-four hours after transfection, the cells were treated with DMSO, TCPOBOP (TC, 250 nm), PB (1 mM), or OPZ (5, 20, 50, 100 μm) in the presence or absence of 10 μM 5α-androstan-3α-ol. Twenty-four hours after treatment the cell lysates were collected, and luciferase activity was determined by the Dual-Glo Luciferase Assay (Promega Corp.). The data are presented as the mean -fold activation + S.E.M. (n = 3 wells/treatment); *, significantly different from DMSO control (p ≤ 0.05).
Discussion
OPZ was recognized as a microsomal enzyme inducer over 25 years ago, with apparent 4- to 6-fold increases in the activity of liver GSTs in mice treated with the drug (Ansher et al., 1983). The induction of GST and other cytoprotective enzymes has been termed the “antioxidant response” (Jaiswal, 1994) and is central to the proposed mechanism of action of OPZ in cancer prevention. The antioxidant response is an important mechanism for cellular defense and is mediated by the Nrf2/ARE pathway, resulting in the induction of several chemoprotective genes, including NQO1, HO-1, and GSTA1 and GSTA2 (Kensler et al., 1985). It is important to note that the induction of NQO1 by OPZ has been shown to be dependent on Nrf2 activation (Ramos-Gomez et al., 2001). Our current data showing the loss of Nqo1 induction in Nrf2−/− mice support that finding.
Although the induction of these genes has been shown to be ARE/EpRE-dependent, OPZ is also capable of inducing other genes in an Nrf2-independent manner (Le Ferrec et al., 2002; Ebert et al., 2005). For example, CYP2B is induced by OPZ yet is considered a hallmark gene of CAR activation. The mechanism of induction of CYP2B isoforms has been well characterized, and currently no ARE/ EpRE consensus sequences have been identified in their 5′-flanking regions. Consistent with these facts, our data show that OPZ activates gene transcription through activation of multiple and distinct transcription factors, including Nrf2 and CAR.
To analyze the role of both CAR and Nrf2 in OPZ gene induction, CAR- or Nrf2-deficient mice were treated with OPZ or the known CAR activator PB. Our data indicate that Nrf2 and CAR are required in the regulation of gene induction by OPZ. As expected, the loss of Nrf2 resulted in loss of Nqo1 induction, whereas induction of Cyp2b10 mRNA was unaffected. These results both confirm a role for Nrf2 in Nqo1 regulation and suggest an Nrf2-independent mechanism for the induction of Cyp2b10 mRNA by OPZ. The complete abrogation of Cyp2b10 mRNA induction in CAR-null mice treated with OPZ strongly implicates CAR as the regulatory mechanism. These data agree with our previously published data, which revealed that loss of the obligatory CAR-binding partner RXRα abrogates OPZ induction of Cyp2B (Cherrington et al., 2003).
Two additional lines of evidence in this study characterize OPZ as a CAR activator. First, OPZ treatment results in transcriptional activation of a human CYP2B6 promoter construct in transiently transgenic mice. The promoter region of this construct contains the PBREM, the well characterized CAR DNA-binding sequence. The evident induction of this reporter gene in OPZ-treated mice compared with corn oil controls shows the ability of OPZ to induce CAR-driven genes in vivo. Second, OPZ induces CAR nuclear accumulation in hepatocytes of OPZ-treated mice. Because the main component of CAR activation appears to be nuclear translocation (Kobayashi et al., 2003), these findings firmly establish OPZ as a CAR activator. Interestingly, the OPZ dose used in these in vivo studies did not cause maximal induction of the CAR target gene Cyp2b10 (Fig. 2). This indicates that CAR may have not been fully activated at that dose. The increase in CAR activity by OPZ treatment seen even at this lower dose helps to support OPZ as a CAR activator.
In vivo, CAR can be activated either directly by ligand binding (as in the case of TCPOBOP) or indirectly through the activation of currently unknown pathways (as with PB) (Stanley et al., 2006). In cultured cells lines, however, transfected CAR freely translocates to the nucleus without exposure to CAR activators (Kobayashi et al., 2003). This is apparent in our study by the increase in CYP2B6-promoter driven activity of the luciferase reporter gene on the addition of mCAR. The treatment of these cells with the inverse agonist androstanol repressed the activity of mCAR and the transcriptional activation of the luciferase reporter gene. Androstanol is a CAR ligand, and binding results in a conformational change in CAR that inhibits the recruitment of the transcriptional machinery (Wright et al., 2007). The ability of TCPOBOP to overcome this inhibition has been previously shown and results from the direct interaction of TCPOBOP with CAR, which both displaces androstanol and promotes the recruitment of the transcriptional machinery (Sueyoshi et al., 1999; Tzameli et al., 2000). In the current study, TCPOBOP treatment, but not PB or OPZ treatment, was able to reverse the inhibitory effects of androstanol. Although OPZ cotreatment resulted in a slight increase in luciferase activity, this increase never reached significance (p ≤ 0.05). Although these findings suggest that OPZ acts similarly to PB to indirectly activate CAR, further investigation of any OPZ-CAR interactions is necessary to definitively rule out OPZ as a CAR ligand.
The mechanism by which PB indirectly activates CAR remains to be elucidated, although the involvement of a number of kinases and phosphatases has been proposed. The involvement of the extracellular signal-regulated kinase (ERK) in the repression of CAR nuclear translocation is of particular interest. Previous work has shown that epidermal growth factor is able to attenuate CAR-mediated induction of CYP2B by PB treatment (Bauer et al., 2004). Inhibitors of mitogen-activated protein kinase kinase 1/2 (a kinase downstream of epidermal growth factor signaling) have been shown to potentiate the induction CYP2B by PB (Joannard et al., 2006). Most recently, ERK1/2 de-phosphorylation (deactivation) was shown to be associated with CAR nuclear translocation (Koike et al., 2007). Our findings that OPZ activates CAR may provide additional support to the importance of ERK1/2 in the regulation of CAR. OPZ has been shown to inhibit the constitutive phosphorylation (activation) of ERK1/2 and has been shown to inhibit the activity of mitogen-activated protein kinase kinase 1 (Kang et al., 2003). Whether this inhibition of ERK signaling by OPZ is responsible for the activation of CAR is unclear and under investigation.
The data presented in this manuscript show that OPZ modulates gene expression through CAR activation, in addition to Nrf2. These results add to previous findings on the dual activation of Nrf2 and CAR. Trans-stilbene oxide and diallyl sulfide have both been identified as activators of both CAR and Nrf2 (Slitt et al., 2006; Fisher et al., 2007). The addition of yet another structurally diverse compound to this group raises the possibility of cross-talk between the two factors, although further studies are needed to determine the extent of any connections.
Acknowledgments
This work was supported by National Institutes of Health Grants ES011646 and ES006694 (to N.J.C.), ES09716 and ES09649 (to C.D.K.), and DK46546 (to D.D.M.).
ABBREVIATIONS
- OPZ
oltipraz
- GST
glutathione-S-transferase
- NQO1, NAD(P)H
quinone oxidoreductase
- Nrf2
nuclear factor E2-related factor 2
- ARE/EpRE
antioxidant response element/electrophile response element
- Keap1
Kelch-like ECH-associated protein 1
- CAR
constitutive androstane receptor
- TCPOBOP
1,4-bis-[2-(3, 5-dichloropyridyloxy)] benzene
- CITCO
6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbalde-hyde O-(3,4-dichlorobenzyl)oxime
- PB
phenobarbital
- RXR
retinoid X receptor
- bDNA
branched DNA
- mCAR
mouse constitutive androstane receptor
- PBREM
phenobarbital responsive element
- DMSO
dimethyl sulfoxide
- ERK
extracellular signal-regulated kinase
Footnotes
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
References
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci. 2005;83:44–52. doi: 10.1093/toxsci/kfi013. [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AL, Maher JM, Dieter MZ, Knight TR, Goedken M, Cherrington NJ, Chan JY, Klaassen CD, Manautou JE. Nuclear factor-E2-related factor 2 expression in liver is critical for induction of NAD(P)H:quinone oxidoreductase 1 during cholestasis. Cell Stress Chaperones. 2006;11:356–363. doi: 10.1379/CSC-217.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansher SS, Dolan P, Bueding E. Biochemical effects of dithiolthiones. Food Chem Toxicol. 1986;24:405–415. doi: 10.1016/0278-6915(86)90205-x. [DOI] [PubMed] [Google Scholar]
- Ansher SS, Dolan P, Bueding E. Chemoprotective effects of two dithiolthiones and of butylhydroxyanisole against carbon tetrachloride and acetaminophen toxicity. Hepatology. 1983;3:932–935. doi: 10.1002/hep.1840030608. [DOI] [PubMed] [Google Scholar]
- Bauer D, Wolfram N, Kahl GF, Hirsch-Ernst KI. Transcriptional regulation of CYP2B1 induction in primary rat hepatocyte cultures: repression by epidermal growth factor is mediated via a distal enhancer region. Mol Pharmacol. 2004;65:172–180. doi: 10.1124/mol.65.1.172. [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Hartley DP, Li N, Johnson DR, Klaassen CD. Organ distribution of multidrug resistance proteins 1, 2, and 3 (Mrp1, 2, and 3) MRNA and hepatic induction of Mrp3 by constitutive androstane receptor activators in rats. J Pharmacol Exp Ther. 2002;300:97–104. doi: 10.1124/jpet.300.1.97. [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Slitt AL, Maher JM, Zhang XX, Zhang J, Huang W, Wan YJ, Moore DD, Klaassen CD. Induction of multidrug resistance protein 3 (Mrp3) in vivo is independent of constitutive androstane receptor. Drug Metab Dispos. 2003;31:1315–1319. doi: 10.1124/dmd.31.11.1315. [DOI] [PubMed] [Google Scholar]
- Cho IJ, Kim SG. Oltipraz inhibits 3-methylcholanthrene induction of CYP1A1 by CCAAT/enhancer-binding protein activation. J Biol Chem. 2003;278:44103–44112. doi: 10.1074/jbc.M307597200. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477– 8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson NE, Egner PA, Kensler TW. Transcriptional control of glutathione S-transferase gene expression by the chemoprotective agent 5-(2-pyrazinyl)-4-methyl-1,2-dithiole-3-thione (oltipraz) in rat liver. Cancer Res. 1990;50:2251–2255. [PubMed] [Google Scholar]
- Ebert B, Seidel A, Lampen A. Induction of phase-1 metabolizing enzymes by oltipraz, flavone and indole-3-carbinol enhance the formation and transport of benzo[a]pyrene sulfate conjugates in intestinal Caco-2 cells. Toxicol Lett. 2005;158:140–151. doi: 10.1016/j.toxlet.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35:995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Negishi M. Characterization of a phenobarbital-responsive enhancer module in mouse P450 Cyp2b10 gene. J Biol Chem. 1997;272:14943–14949. doi: 10.1074/jbc.272.23.14943. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor car-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Jackson JP, Ferguson SS, Moore R, Negishi M, Goldstein JA. The constitutive active/androstane receptor regulates phenytoin induction of Cyp2c29. Mol Pharmacol. 2004;65:1397–1404. doi: 10.1124/mol.65.6.1397. [DOI] [PubMed] [Google Scholar]
- Jackson JP, Ferguson SS, Negishi M, Goldstein JA. Phenytoin induction of the Cyp2c37 gene is mediated by the constitutive androstane receptor. Drug Metab Dispos. 2006;34:2003–2010. doi: 10.1124/dmd.106.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal AK. Antioxidant response element. Biochem Pharmacol. 1994;48:439–444. doi: 10.1016/0006-2952(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Joannard F, Rissel M, Gilot D, Anderson A, Orfila-Lefeuvre L, Guillouzo A, Atfi A, Lagadic-Gossmann D. Role for mitogen-activated protein kinases in phenobarbital-induced expression of cytochrome P450 2B in primary cultures of rat hepatocytes. Toxicol Lett. 2006;161:61–72. doi: 10.1016/j.toxlet.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kang KW, Cho IJ, Lee CH, Kim SG. Essential role of phosphatidylinositol 3-kinase-dependent CCAAT/enhancer binding protein beta activation in the induction of glutathione S-transferase by oltipraz. J Natl Cancer Inst. 2003;95:53– 66. doi: 10.1093/jnci/95.1.53. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Trush MA, Bueding E, Groopman JD. Modification of aflatoxin B1 binding to DNA in vivo in rats fed phenolic antioxidants, ethoxyquin and a dithiothione. Carcinogenesis. 1985;6:759–763. doi: 10.1093/carcin/6.5.759. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Sueyoshi T, Inoue K, Moore R, Negishi M. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol Pharmacol. 2003;64:1069–1075. doi: 10.1124/mol.64.5.1069. [DOI] [PubMed] [Google Scholar]
- Koike C, Moore R, Negishi M. Extracellular signal-regulated kinase is an endogenous signal retaining the nuclear constitutive active/androstane receptor (CAR) in the cytoplasm of mouse primary hepatocytes. Mol Pharmacol. 2007;71:1217–1221. doi: 10.1124/mol.107.034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol Med. 2001;7:135–145. [PMC free article] [PubMed] [Google Scholar]
- Le Ferrec E, Lagadic-Gossmann D, Rauch C, Bardiau C, Maheo K, Massiere F, Le Vee M, Guillouzo A, Morel F. Transcriptional induction of CYP1A1 by oltipraz in human Caco-2 cells is aryl hydrocarbon receptor- and calcium-dependent. J Biol Chem. 2002;277:24780–24787. doi: 10.1074/jbc.M111319200. [DOI] [PubMed] [Google Scholar]
- Maheo K, Morel F, Antras-Ferry J, Langouet S, Desmots F, Corcos L, Guillouzo A. Endotoxin suppresses the oltipraz-mediated induction of major hepatic glutathione transferases and cytochromes P450 in the rat. Hepatology. 1998;28:1655–1662. doi: 10.1002/hep.510280627. [DOI] [PubMed] [Google Scholar]
- Merrell MD, Augustine LM, Slitt AL, Cherrington NJ. Induction of drug metabolism enzymes and transporters by oltipraz in rats. J Biochem Mol Toxicol. 2008;22:128–135. doi: 10.1002/jbt.20225. [DOI] [PubMed] [Google Scholar]
- Morel F, Fardel O, Meyer DJ, Langouet S, Gilmore KS, Meunier B, Tu CP, Kensler TW, Ketterer B, Guillouzo A. Preferential increase of glutathione S-transferase class alpha transcripts in cultured human hepatocytes by phenobarbital, 3-methylcholanthrene, and dithiolethiones. Cancer Res. 1993;53:231–234. [PubMed] [Google Scholar]
- Primiano T, Gastel JA, Kensler TW, Sutter TR. Isolation of CDNAs representing dithiolethione-responsive genes. Carcinogenesis. 1996;17:2297–2303. doi: 10.1093/carcin/17.11.2297. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in Nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slitt AL, Cherrington NJ, Dieter MZ, Aleksunes LM, Scheffer GL, Huang W, Moore DD, Klaassen CD. Trans-stilbene oxide induces expression of genes involved in metabolism and transport in mouse liver via CAR and Nrf2 transcription factors. Mol Pharmacol. 2006;69:1554–1563. doi: 10.1124/mol.105.014571. [DOI] [PubMed] [Google Scholar]
- Stanley LA, Horsburgh BC, Ross J, Scheer N, Wolf CR. PXR and CAR: nuclear receptors which play a pivotal role in drug disposition and chemical toxicity. Drug Metab Rev. 2006;38:515–597. doi: 10.1080/03602530600786232. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Kobayashi R, Nishio K, Aida K, Moore R, Wada T, Handa H, Negishi M. A nuclear factor (NF2d9) that binds to the male-specific P450 (Cyp 2d-9) gene in mouse liver. Mol Cell Biol. 1995;15:4158–4166. doi: 10.1128/mcb.15.8.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi T, Negishi M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol. 2001;41:123–143. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)] benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Wright E, Vincent J, Fernandez EJ. Thermodynamic characterization of the interaction between CAR-RXR and SRC-1 peptide by isothermal titration calorimetry. Biochemistry. 2007;46:862–870. doi: 10.1021/bi061627i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


