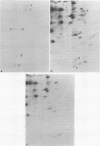
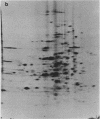
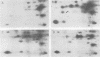
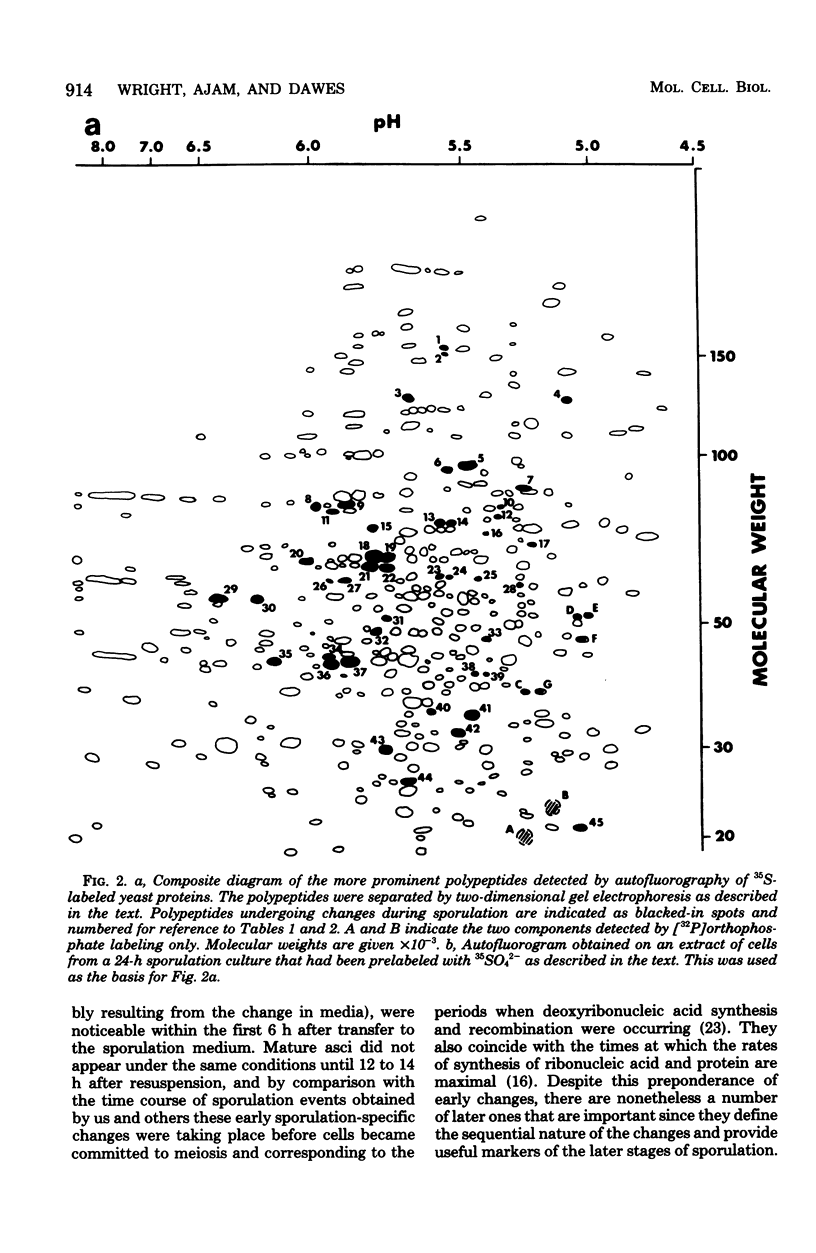
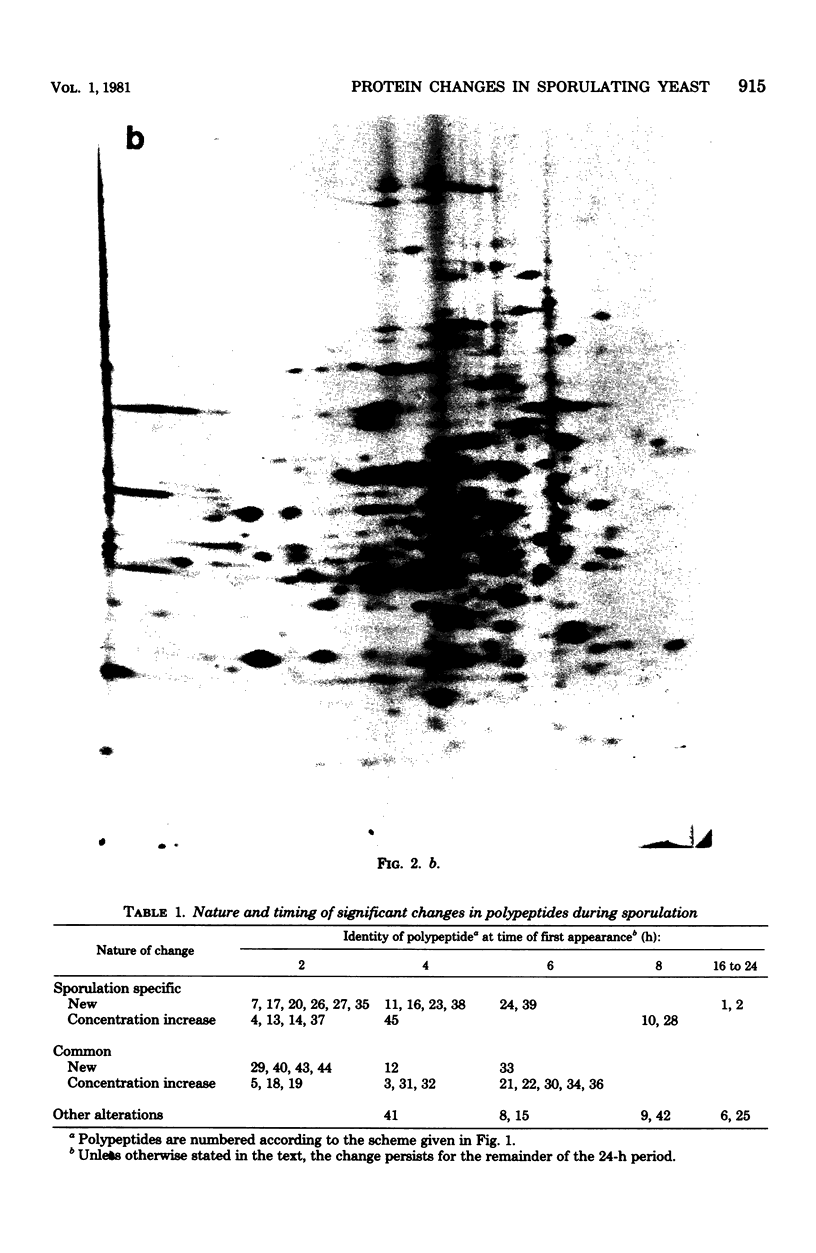
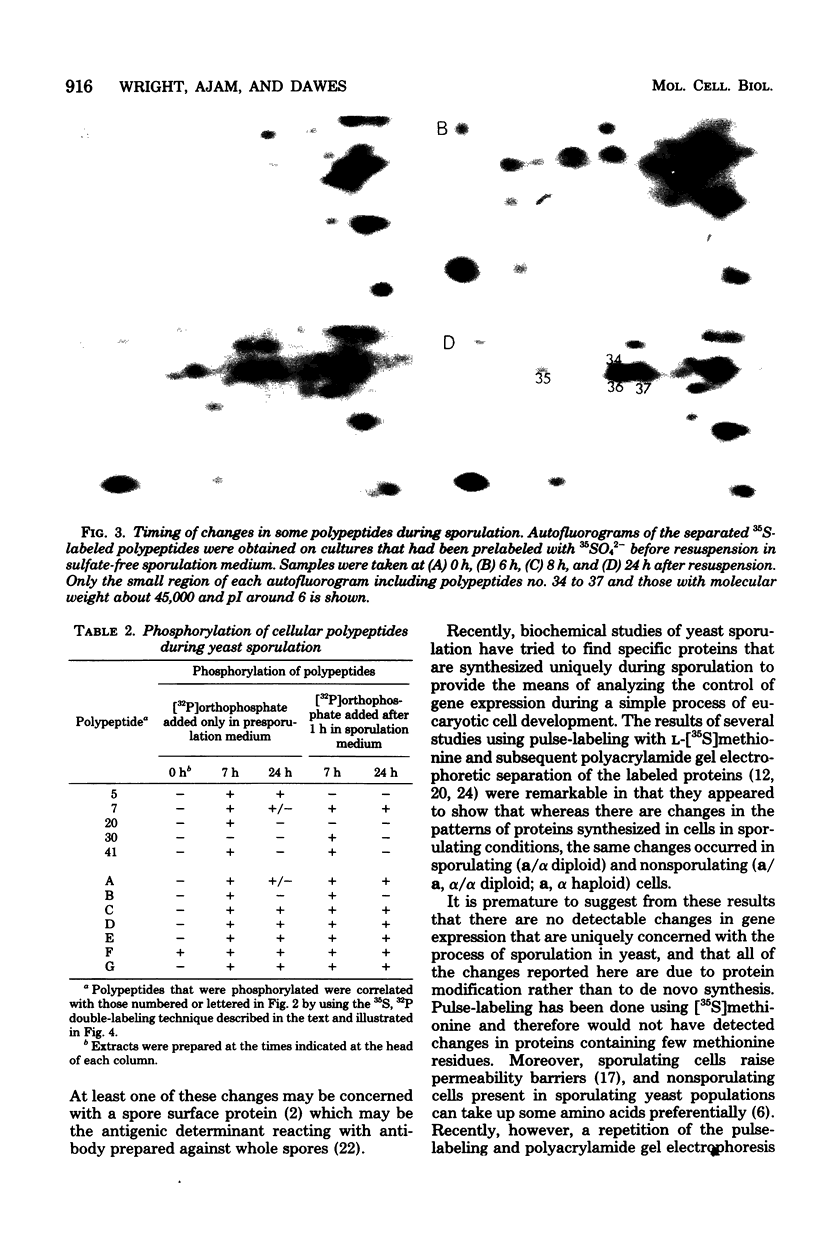
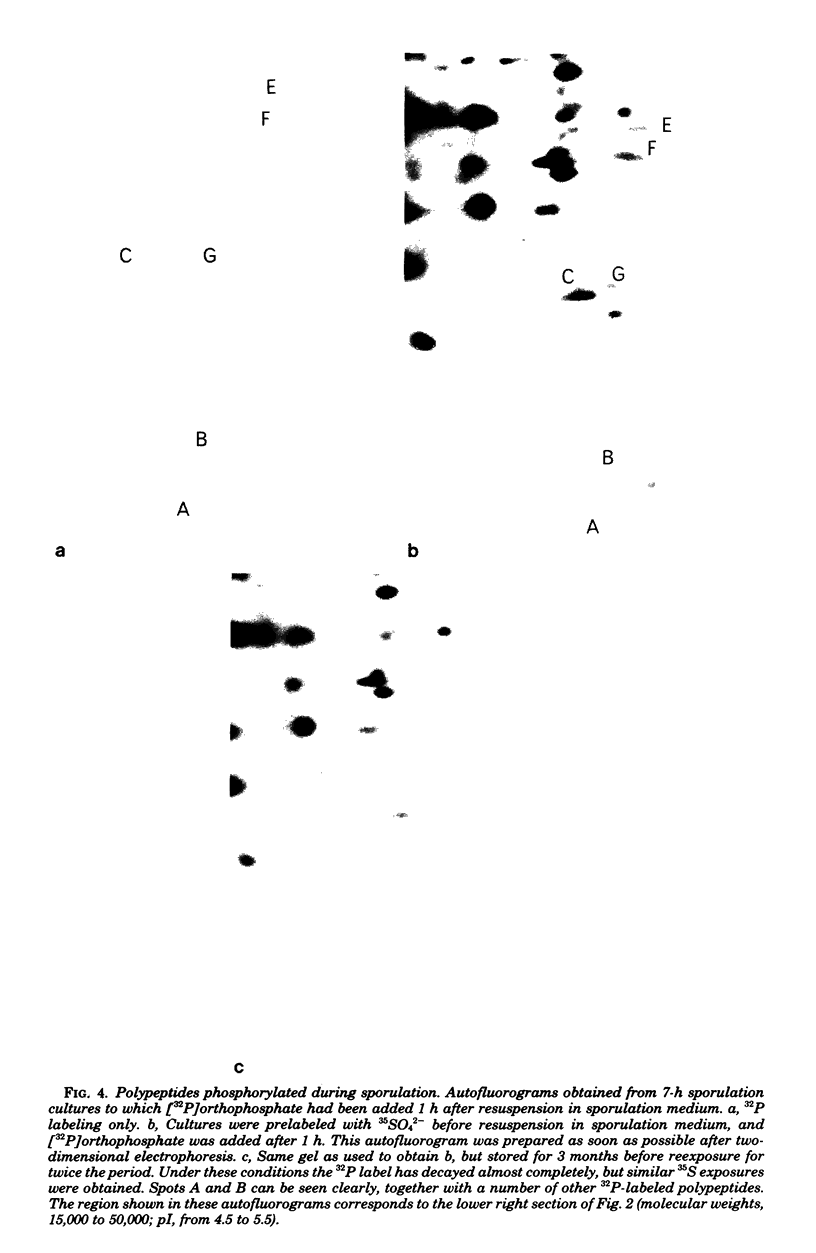
Abstract
During meiosis and spore formulation in Saccharomyces cerevisiae, changes that occur in a/alpha diploids, but not in isogenic nonsporulating a/a diploids, have been detected in cellular polypeptides. These were found by the technique of prelabeling growing cells with 35SO4(2-) and suspending them in sulfur-free sporulation medium. Under the conditions used, about 400 polypeptides were detected by two-dimensional gel electrophoresis, and 45 were altered during sporulation; of these, 21 changes were specific to a/alpha strains. These alterations were mainly due to the appearance of new polypeptides or to marked increases in the concentrations of a few polypeptides produced during vegetative growth. They could have been due either to modifications of existing polypeptides present in growing cells or to de novo synthesis of new gene products. They occurred at characteristic times during sporulation; whereas the majority of changes took place early (within the first 6 h in sporulation conditions), there were several changes characterizing the later stages of sporulation. Ten of the 35SO4(2-)-labeled polypeptides were also labeled with 32P in the presence of [32P]orthophosphate; of these, three were previously found to be sporulation specific. One of these was phosphorylated at all stages of sporulation and was labeled when [32P]orthophosphate was added either during growth of the culture of 1 h after transfer to sporulation medium. Another was labeled in the same way by adding 32P at either time, so that by 7 h in sporulation medium it was phosphorylated, but was dephosphorylated by 24 h. The third sporulation-specific peptide was labeled in extracts prepared at 7 h in sporulation medium (but not at 24 h) when [32P]-orthophosphate was added during presporulation growth, but not when [32P]-orthophosphate was added 1 h after transfer of the culture to sporulation medium. This polypeptide appeared early during sporulation; it is probably phosphorylated as it appears and is dephosphorylated at some time between 7 h and 24 h of sporulation.
Full text
PDF

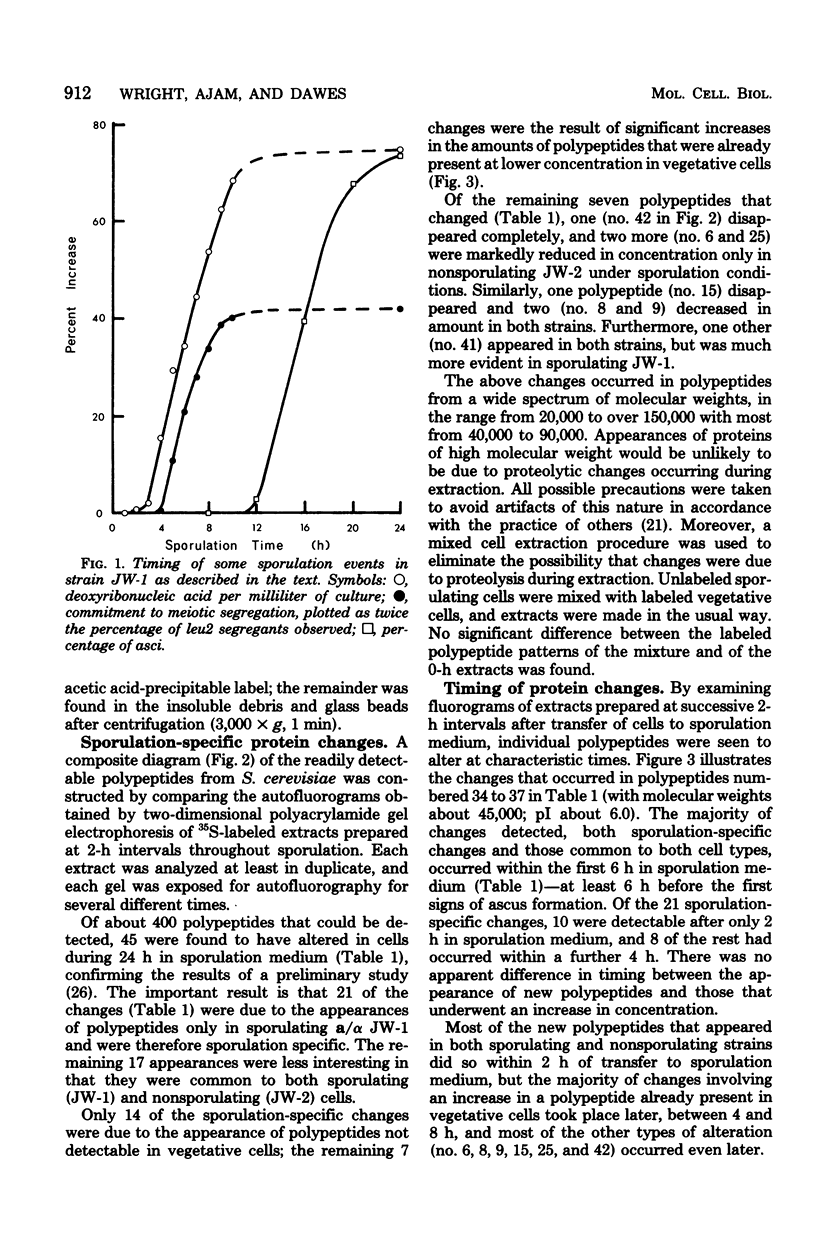


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Briley M. S., Illingworth R. F., Rose A. H., Fisher D. J. Evidence for a surface protein layer on the Saccharomyces cerevisiae ascospore. J Bacteriol. 1970 Oct;104(1):588–589. doi: 10.1128/jb.104.1.588-589.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna W. J., Magee P. T. Glycogenolytic enzymes in sporulating yeast. J Bacteriol. 1978 Jun;134(3):844–853. doi: 10.1128/jb.134.3.844-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes I. W. Study of cell development using depressed mutations. Nature. 1975 Jun 26;255(5511):707–708. doi: 10.1038/255707a0. [DOI] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R. E., Frink N., Bernstein P., Esposito M. S. The genetic control of sporulation in Saccharomyces. II. Dominance and complementation of mutants of meiosis and spore formation. Mol Gen Genet. 1972;114(3):241–248. doi: 10.1007/BF01788893. [DOI] [PubMed] [Google Scholar]
- Fast D. Sporulation synchrony of Saccharomyces cerevisiae grown in various carbon sources. J Bacteriol. 1973 Nov;116(2):925–930. doi: 10.1128/jb.116.2.925-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig E., Haber J. E. Messenger ribonucleic acid and protein metabolism during sporulation of Saccharomyces cerevisiae. J Bacteriol. 1980 Dec;144(3):1098–1112. doi: 10.1128/jb.144.3.1098-1112.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Magee P. T. Changes in DNA-dependent RNA polymerase in sporulating yeast. Mol Biol Rep. 1974 Feb;1(5):275–281. doi: 10.1007/BF00417583. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Hopper A. K. Protein synthesis in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Sep;119(3):952–960. doi: 10.1128/jb.119.3.952-960.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. Effect of pH on adenine and amino acid uptake during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Oct;112(1):519–526. doi: 10.1128/jb.112.1.519-526.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosanchuk J. S., Dawes P., Kelly A., Heckler C. An automated blood smear analysis system. Clinical experience and performance. Am J Clin Pathol. 1980 Feb;73(2):165–171. doi: 10.1093/ajcp/73.2.165. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pringle J. R. Methods for avoiding proteolytic artefacts in studies of enzymes and other proteins from yeasts. Methods Cell Biol. 1975;12:149–184. doi: 10.1016/s0091-679x(08)60956-5. [DOI] [PubMed] [Google Scholar]
- Snider I. J., Miller J. J. A serological comparison of vegetative cell and ascus walls and the spore coat of Saccharomyces cerevisiae. Can J Microbiol. 1966 Jun;12(3):485–488. doi: 10.1139/m66-070. [DOI] [PubMed] [Google Scholar]
- Trew B. J., Friesen J. D., Moens P. B. Two-dimensional protein patterns during growth and sporulation in Saccharomyces cerevisiae. J Bacteriol. 1979 Apr;138(1):60–69. doi: 10.1128/jb.138.1.60-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejksnora P. J., Haber J. E. Ribonucleoprotein particle appearing during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):246–260. doi: 10.1128/jb.134.1.246-260.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey F., Santos T., García-Acha I., Nombela C. Synthesis of beta-glucanases during sporulation in Saccharomyces cerevisiae: formation of a new, sporulation-specific 1,3-beta-glucanase. J Bacteriol. 1980 Aug;143(2):621–627. doi: 10.1128/jb.143.2.621-627.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]