Abstract
miRNAs are short regulatory RNAs that can regulate gene expression through interacting with the 3'UTR of target mRNAs. Although the role of miRNAs has been extensively studied in adult human and animal models of heart disease, nothing is known about their expression in pediatric heart failure patients. Different than adults with heart failure, pediatric patients respond well to phosphodiesterase inhibitor (PDEi) treatment, which is safe in the outpatient setting, results in fewer heart failure emergency department visits, fewer cardiac hospital admissions and improved NYHA classification. We have recently shown that the pediatric heart failure patients display a unique molecular profile that is different from adults with heart failure. In this study we show for the first time that pediatric heart failure patients display a unique miRNA profile, and that expression of some miRNAs correlate with response to PDEi treatment. Moreover, we show that expression of Smad4, a potential target for PDEi-regulated miRNAs, is normalized in PDEi-treated patients. Since miRNAs may be used as therapy for human heart failure, our results underscore the importance of defining the molecular characteristics of pediatric heart failure patients, so age-appropriate therapy can be designed for this population.
Keywords: microRNA, mRNA, gene regulation, idiopathic dilated cardiomyopathy, phosphodiesterase inhibition, pediatric heart failure
1. Introduction
Approximately 3 million adults have chronic heart failure (HF) resulting in an economic impact in the United States of over $38 billion dollars (in 1991), or 5.4% of total health care expenditures. Dilated cardiomyopathy (DCM) is the most common cause of HF and reason for cardiac transplantation in children [1]. The incidence of DCM in adults is about 5.5 per 100,000 people per year while the incidence in children is between 0.34 and 1.09 per 100,000 people per year [2]. As opposed to adults in whom the etiology of DCM is primarily ischemic heart disease, the DCM phenotype in children is much more heterogeneous. In the Pediatric Cardiomyopathy Registry (PCMR) an etiology could not be determined in two-thirds of DCM cases, thus making idiopathic DCM (IDC) the most common diagnosis [2].
Although the pathophysiology and treatment of adult HF is well studied, HF in children remains poorly understood with most clinical treatment paradigms extrapolated solely from experience in adults. Emerging experimental evidence and epidemiologic data suggest that the pediatric HF population is distinctly different from adult patients [1]. In 2007 Shaddy et al [3] reported the results from the first and only randomized controlled trial of medical therapy (carvedilol, a non-specific β-blocker) in children with heart failure. In the pediatric carvedilol trial, there was no significant clinical benefit of β-blocker therapy over placebo for children with symptomatic heart failure. In addition, a recent retrospective review from the University of Toronto Hospital for Sick Children by Kantor et. al. showed no survival benefit with the advancement of medical therapy from the digoxin plus diuretic era in the late 1970's to the β-blocker era in the early 2000's in children suffering from heart failure [4]. Moreover, inotropic therapy (phosphodiesterase inhibitors) carries a significant mortality risk in adults with chronic HF [5], while children are frequently placed on outpatient milrinone infusions [[6] and Dr. Miyamoto, personal communication] without an increase in mortality or defibrillator placement for primary prevention of sudden cardiac death [6]. Phosphodiesterase inhibitors (PDEi) are successfully and routinely used in children presenting with decompensated heart failure as a bridge to initiation of oral heart failure therapy or to recovery. In those children that fail to wean from PDEi, outpatient PDEi infusions are safely used as a palliative therapy or as a bridge to transplant without an increased risk of unexpected deaths [6]. The addition of PDEi treatment to standard oral medications for children with heart failure is safe in the outpatient setting, results in fewer heart failure emergency department visits, fewer cardiac hospital admissions and improved NYHA classification [6].
MicroRNAs (miRNA, miRs) are small noncoding ~22 nucleotide (nt) RNAs capable of modulating the expression of many genes with estimates suggesting that as much as 60% of the genome is subject to their regulation. At the most simplistic level, miRs bind to target mRNAs by recognition of reverse complementary 6 – 8 nucleotide “seed” sequences, most frequently located within 3' untranslated regions (3'UTR). miRs can cause translational repression or RNA destabilization [7].
Over the past few years, several array-based studies have been published detailing changes in miR expression in normal versus failing adult human heart. The results of these studies have been nicely summarized in a recent review by Topkara [7]. miRs that repeatedly surface as dynamically regulated (up or down) in adult heart failure include: several let-7s, miR-1, miR-133a/b, miR-100, miR-195, miR-199, miR-214, miR-222, miR-23a/b, miR-29a/b, miR-30 family, and miR-320.
We have previously analyzed expression of miRNAs in adult human IDC and ischemic cardiomyopathy [8]. Here we show that expression of a subset of miRNAs is differentially regulated in pediatric IDC patients. Importantly, pediatric IDC patients show a unique miRNA expression profile that is affected by PDEi treatment.
2. Materials and Methods
Tissue Procurement
Human subjects were males and females of all races and ethnic background under 14 years of age who donated their heart to the institutional review board-approved pediatric transplant tissue banks at the University of Colorado. Non-failing (NF) control hearts are obtained from donors whose heart could not be placed for technical reasons. A detailed description of all pediatric patients can be found in Table S1. Informed consent was obtained from all patients that donated their heart to the University of Colorado.
microRNA extraction and array analysis
miRNA was extracted from 5 non-failing and 5 IDC pediatric left ventricles. miRNA extraction was performed using the mirVana™ kit (Ambion) according to manufacturer's recommendation. miRNA expression analysis was performed by LC Science, LLC (Houston, TX) using arrays based on the Sanger miRBase 14.0 database, (http://www.sanger.ac.uk/Software/Rfam/mirna/), capable of detecting 894 miRNAs.
A transcript to be listed as detectable must meet at least two conditions: signal intensity higher than 3×(background standard deviation) and spot coefficient of variation (CV)< 0.5. CV is calculated by (standard deviation)/(signal intensity). t-Test was performed between “control” and “test” sample groups. 2 T-values were calculated for each miRNA, and p-values were computed from the theoretical t-distribution. All data processes were carried out using LC Science's developed computer programs.
miRNA RT-PCR
Reverse transcription of miRNAs was performed using the miScript Reverse Transcription Kit (Qiagen, Inc) according to manufacturer's recommendations. The miScript SYBR Green PCR Kit (Qiagen, Inc) 6 containing a QuantiTect SYBR Green PCR Master Mix and the miScript Universal Primer along with the miRNA-specific primer was used for the detection of mature miRNAs. miRNA expression was normalized to the small RNA U6, a standard endogenous control for miRNA expression. N= 12 non-failing, 8 PDEi untreated failing and 12 PDEi treated failing pediatric patients.
Western blot
Western blots were performed essentially as described [1]. GAPDH antibody was purchased from Santa Cruz and Smad4 antibody was purchased from Cell Signaling.
Statistical analysis
Statistical analyses were performed using Statview software (SAS Institute, Cary, NC). 2-way Analysis of Variance (ANOVA) was performed on all outcomes except for mRNA including all groups to evaluate for interactions. Statistical significance was set a priori at p<0.05 and all data are presented as mean + SEM in the figures.
3. Results and Discussion
Differential expression of miRNAs in pediatric IDC patients
Because miRNAs are important regulators of gene expression, and their expression profile has not been determined in pediatric IDC patients, we analyzed their expression in non-failing and IDC pediatric samples by miRNA array. As shown in Figure 1A, 17 miRNAs were significantly regulated in pediatric failing hearts; the majority of those are antithetically regulated or not regulated in adults. Specifically, miR-130b, miR-204, miR-331-3p, miR-188-5p, miR-1281, miR-572, miR-765, miR-223, miR-125a-3p and miR-1268 are not regulated in adults with HF. Three miRNAs (miR-638, miR-7, miR-132 and miR-146a) are antithetically regulated in pediatric and adult failing hearts [7]. Directionality of expression of miR-27a/b and miR-486 is the same in both populations although they are not detected in all studies [7]. Importantly, expression of several miRNAs known to change in adult cardiac disease (miR-133a/b, miR-1, miR-21, miR-30, miR-23, miR-29) [7] does not change in pediatric failing hearts.
Figure 1.
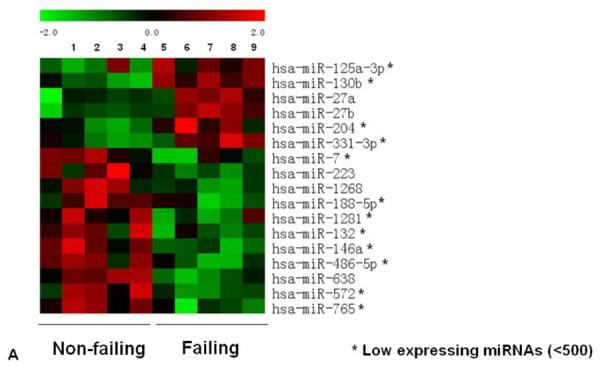
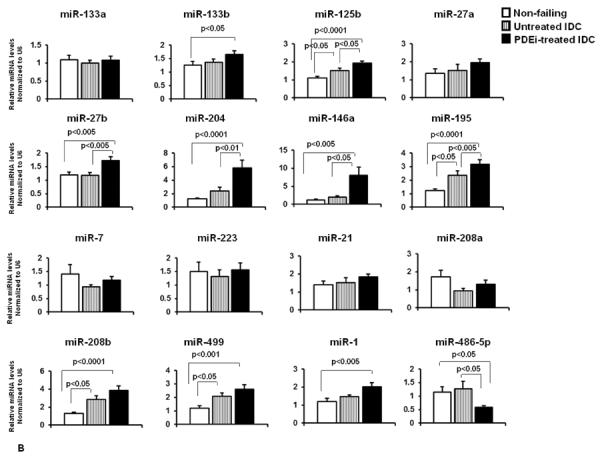
miRNA expression in pediatric heart failure patients. (A) Expression profile of miRNAs in non-failing and failing (IDC) pediatric subjects by miRNA array. Only miRNAs with a pvalue of <0.05 are shown. (B) Relative expression of a subset of miRNAs in non-failing, untreated IDC and PDEi-treated IDC patients was confirmed by RT-PCR, as described in the Methods section. Statistically significant expression of miRNAs is shown in the Figure.
To confirm the array results, expression of a subset of miRNAs was determined by RT-PCR. We also tested expression of miRNAs known to change in adults with DCM (miR-133a/b, miR-21, miR-1 and miR-195) and known to be important for myocyte contraction (miR-208a/b, miR-499) [9]. Because PDEi is an efficacious therapy for pediatric HF, miRNA expression of PDEi-treated (milrinone) and untreated patients was performed. As shown in Figure 1B several differences in miRNA expression in response to treatment were observed. A thorough discussion of regulated miRNAs and potential targets can be found in the supplementary material.
As shown in Figure 1B, expression of six miRNAs changed only in PDEi-treated patients. To determine the possible role of these miRNAs in cardiovascular diseases, an analysis of possible targets was performed using the Ingenuity Pathways Systems. Pathway analysis of all possible targets for miR-133b, miR-204, miR-1, miR-146a, mir-27b and miR-486-5p that are involved in cardiovascular disease was performed. As shown in Figure S1, mRNAs in the cardiac hypertrophy, PKA signaling and role of NFAT in cardiac hypertrophy pathways are consistent targets of these miRNAs. Interestingly, the pathway related to factors promoting cardiogenesis in vertebrates is also a target for miR-1, miR-27b and miR-486-5p, suggesting that expression of mRNAs involved in cardiogenesis may be affected by PDEi treatment.
To determine a functional role for miRNAs regulated by PDEi treatment we analyzed expression of potential common targets for miRNAs that are up-regulated in response to PDEi treatment. As shown in Table S2, there are 5 potential cardiovascular targets that are associated with at least 3 of the 5 PDEi-regulated miRNAs. Of those, SMAD4, Tgfβ receptor and calmodulin are genes involved directly or indirectly in the transition from hypertrophy to heart failure [10–12]. Since these miRNAs are up-regulated in response to PDEi it is possible that this is a compensatory response to prevent up-regulation of these targets, blunting the transition from hypertrophy to heart failure. The only exception is adenylyl cyclase 6, expression of which is thought to be protective [13].
Smad4 is an important mediator of the transition between cardiac hypertrophy and heart failure, and it is activated by Tgfβ [10]. Since Tgfβ 1, 3 and Smad4 are potential targets for PDEi-regulated miRNAs, we analyzed expression of Smad4 in non-failing, PDE untreated and PDEi-treated IDC patients. As shown in Figure 2, Smad4 expression is increased in untreated IDC patients, and treatment with PDEi prevents up-regulation of Smad4. These results suggest that PDEi treatment may be involved in preventing/blunting the transition from hypertrophy to heart failure through regulation of the Tgfβ pathway.
Figure 2.
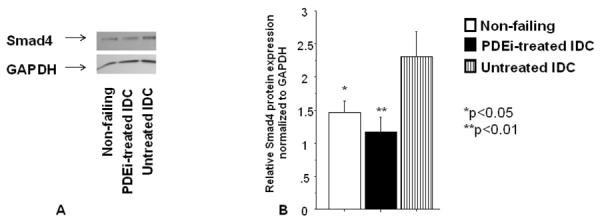
PDEi affects Smad4 expression in pediatric IDC patients. Smad4 protein levels were measured by Western blot using a specific antibody and were normalized to GAPDH. (A) Representative Western blot. (B) Western blot quantification. N=11 non-failing, 10 PDEi-treated and 10 untreated IDC patients.
In summary, we have shown that the pediatric IDC population has a unique miRNA profile, and that expression of several miRNAs change in response to PDEi. We have recently shown that several molecular pathways are differentially regulated in adult and pediatric IDC patients [1]. It is interesting that some miRNAs and pathways known to be altered in end-stage adult HF are not changed in pediatric IDC patients with end-stage HF. Although speculative, it is possible that this may be related to disease progression and duration. Dilated cardiomyopathy in adults often develops and persists over several years, whereas the disease process has a more rapid progression in the pediatric population with one-third of children dying or requiring a heart transplant within one year of diagnosis [14].
Importantly, our results show that children with heart failure have a unique miRNA profile, and expression of miRNAs is altered by PDEi, a treatment that is efficacious and safe in the pediatric but not adult heart failure population. Moreover, we show that changes in miRNA expression due to PDEi treatment results in increased expression of a subset of miRNAs. Importantly, our results suggest that increased expression of these miRNAs may be involved in preventing the transition from cardiac hypertrophy to heart failure due to blunting of Smad4 expression. As shown in Figure 2, Smad4 expression is increased in untreated patients. We speculated that this up-regulation is miRNA-independent, and is most likely due to an increase in Tgfβ receptor signaling. However, up-regulation of miRNAs that may target Smad4 is specific for treated patients and may reflect a protective effect in response to increased stimulation of the Tgfβ receptor pathway. Collectively, our results suggest that the pediatric IDC population is unique and that adult treatment paradigms may not be perfectly extrapolated to the pediatric population.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by the National Institutes of Health grants: [K01 HL088708 (to CCS), AHA [K08 HL080212 (to BLS), R21 HL097123 (to SDM, BLS, CCS), R01 HL107715 (to BLS)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Carmen Sucharov: Equity in miRagen, Inc., Brian Stauffer: Research support from Forest Laboratories, Inc.
References
- [1].Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. Jama. 2006;296:1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- [3].Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. Jama. 2007;298:1171–9. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- [4].Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55:1377–84. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- [5].Hauptman PJ, Mikolajczak P, George A, Mohr CJ, Hoover R, Swindle J, et al. Chronic inotropic therapy in end-stage heart failure. Am Heart J. 2006;152:1096, e1–8. doi: 10.1016/j.ahj.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Price JF, Towbin JA, Dreyer WJ, Moffett BS, Kertesz NJ, Clunie SK, et al. Outpatient continuous parenteral inotropic therapy as bridge to transplantation in children with advanced heart failure. J Card Fail. 2006;12:139–43. doi: 10.1016/j.cardfail.2005.11.001. [DOI] [PubMed] [Google Scholar]
- [7].Topkara VK, Mann DL. Role of microRNAs in cardiac remodeling and heart failure. Cardiovasc Drugs Ther. 2011;25:171–82. doi: 10.1007/s10557-011-6289-5. [DOI] [PubMed] [Google Scholar]
- [8].Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: functional correlates. J Mol Cell Cardiol. 2008;45:185–92. doi: 10.1016/j.yjmcc.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abdellatif M. Differential expression of microRNAs in different disease states. Circ Res. 2012;110:638–50. doi: 10.1161/CIRCRESAHA.111.247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heger J, Peters SC, Piper HM, Euler G. SMAD-proteins as a molecular switch from hypertrophy to apoptosis induction in adult ventricular cardiomyocytes. J Cell Physiol. 2009;220:515–23. doi: 10.1002/jcp.21805. [DOI] [PubMed] [Google Scholar]
- [11].Heger J, Warga B, Meyering B, Abdallah Y, Schluter KD, Piper HM, et al. TGFbeta receptor activation enhances cardiac apoptosis via SMAD activation and concomitant NO release. J Cell Physiol. 2011;226:2683–90. doi: 10.1002/jcp.22619. [DOI] [PubMed] [Google Scholar]
- [12].Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–40. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov. 2009;8:321–35. doi: 10.1038/nrd2827. [DOI] [PubMed] [Google Scholar]
- [14].Pietra BA, Kantor PF, Bartlett HL, Chin C, Canter CE, Larsen RL, et al. Early Predictors of Survival to and After Heart Transplantation in Children with Dilated Cardiomyopathy. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.110.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


