Abstract
Chronic and recurrent urinary tract infections pose a serious medical problem because there are few effective treatment options. Patients with chronic urinary tract infections are commonly treated with long-term prophylactic antibiotics that promote the development of antibiotic-resistant forms of uropathogenic Escherichia coli (UPEC), further complicating treatment. We developed small–molecular weight compounds termed mannosides that specifically inhibit the FimH type 1 pilus lectin of UPEC, which mediates bacterial colonization, invasion, and formation of recalcitrant intracellular bacterial communities in the bladder epithelium. Here, we optimized these compounds for oral bioavailability and demonstrated their fast-acting efficacy in treating chronic urinary tract infections in a preclinical murine model. These compounds also prevented infection in vivo when given prophylactically and strongly potentiated the activity of the current standard of care therapy, trimethoprim-sulfamethoxazole, against clinically resistant PBC-1 UPEC bacteria. These compounds have therapeutic efficacy after oral administration for the treatment of established urinary tract infections in vivo. Their unique mechanism of action—targeting the pilus tip adhesin FimH—circumvents the conventional requirement for drug penetration of the outer membrane, minimizing the potential for the development of resistance. The small–molecular weight compounds described herein promise to provide substantial benefit to women suffering from chronic and recurrent urinary tract infections.
INTRODUCTION
Antibiotics typically target essential metabolic pathways or factors required for cellular integrity and are broadly active against many different species of bacteria. Although these traditional antibiotics have led to significant improvements in human health and arguably have markedly increased the longevity of the human population, escalating bacterial resistance to traditional antibiotics and the lack of significant effort to develop new antibiotics threaten to reverse these pioneering advances. The latter has been described as an impending “public health crisis” (1). Exacerbating the situation, antibiotic therapy may perturb the normal beneficial gut microbiota, leading to a domination of opportunistic pathogens (2–4). The negative selection imposed on the normal microbiota by antibiotics may ultimately change the healthy state of the individual, resulting in an increased risk of opportunistic or recurrent infections. Thus, in this era when multidrug-resistant strains of uropathogens are spreading globally (5), there is a high and expanding need for new therapeutics that can treat and prevent infections or that can potentiate the efficacy of currently available antibiotics.
More than 15 million women suffer from urinary tract infections (UTIs) annually in the United States, with an estimated cost exceeding $2.5 billion (6). Uropathogenic Escherichia coli (UPEC) is the causative agent for more than 85% of all UTIs (7), which have become more difficult to treat as a result of increasing antimicrobial resistance to standard of care therapy (8) and high recurrence rates (9). Resistance of UPEC to the commonly prescribed antibiotic trimethoprim-sulfamethoxazole (TMP-SMZ) has risen in the past decade, and thus, therapy has increasingly required the use of last-line antibiotics such as fluoroquinolones (10), leading to increased treatment costs and an associated rise in multidrug resistance (11, 12). For example, 90% of bacteriuric E. coli strains from patients subjected to a 1-month prophylactic regimen of TMP-SMZ were TMP-SMZ–resistant compared to only 28% in a control group treated with cranberry juice (13). Elevated resistance rates were also observed for amoxicillin and ciprofloxacin. Thus, UTI is becoming one of the most visible manifestations of increasing Gram-negative antibiotic resistance (14).
UPEC are capable of colonizing all parts of the urinary tract including the urethra, ureters, kidney, and bladder (in both extracellular and intracellular niches) and urine. Further, UPEC can cause acute, chronic persistent, and recurrent infection (15, 16). Acute infections begin when UPEC introduced into the urinary tract use type 1 pili tipped with the FimH adhesin to bind specifically to mannosylated receptors on the luminal surface of mammalian bladder epithelial cells (17–19). This process facilitates both the colonization and the invasion of bacteria into uroepithelial cells (20–23). Bladder epithelial cells are known to expel UPEC out of the cell and back into the lumen of the bladder as part of a Toll-like receptor 4 (TLR4)–dependent innate defense (24). However, a single bacterium escaping into the cytoplasm can replicate rapidly into 104 to 105 bacteria that then aggregate in a type 1 pilus–dependent manner to found a clonal intracellular bacterial community (IBC) within the epithelial cell. This process allows UPEC to gain a foothold in the urinary tract protected from host defenses and antibiotics (17, 20, 22, 25–32). IBCs are transient in nature. After their maturation, bacteria disperse from the IBC, become filamentous, and spread to neighboring cells for additional rounds of IBC formation(28). In a 4-year clinical study, IBCs and bacterial filamentation were found in the urine of women with UPEC UTI (33). Host defense mechanisms eliminate most of the bacteria from the bladder, causing a population bottleneck (16). Mechanisms by which bacteria survive bottlenecks—in the case of UTI, the ability to escape into the cytoplasm of the uroepithelial cell and clonally expand to perpetuate the infection—are ideal targets for therapeutics (34). FimH is essential for invasion, IBC formation, and the ability of bacteria to colonize the bladder in chronic infection (15, 16). Indeed, FimH is under positive selection in clinical isolates of UPEC, consistent with its critical role in human UTI (35, 36). For these reasons, therapeutic agents targeting FimH have been developed.
The mannose-binding pocket of FimH is composed of amino acid residues that are invariant in all strains of E. coli. Mutations in these residues disrupt mannose binding and attenuate virulence (36, 37). The x-ray crystal structures of FimH bound to α-d-mannose, and mannose derivatives, called mannosides (37–39), were used to rationally design biphenyl FimH inhibitors with excellent cellular potency and low molecular weight (38). Despite these advances, there have been no reports on the ability of any such compounds to be able to treat an established UTI when delivered orally. Here, we first investigated the potency of compounds 1 to 6, reported in (38), for in vitro biofilm inhibition and then their bioavailability and efficacy in treating and preventing UTI in a preclinical murine model of chronic cystitis. We then set out to optimize these biphenyl compounds to have increased potency for the treatment and prevention of chronic UTI when delivered orally. We hypothesized that such compounds would potentiate the antimicrobial effects of TMP-SMZ even against a clinically resistant bacterial strain.
RESULTS
Compounds 1 to 6 block type 1 pilus–dependent biofilm formation in vitro
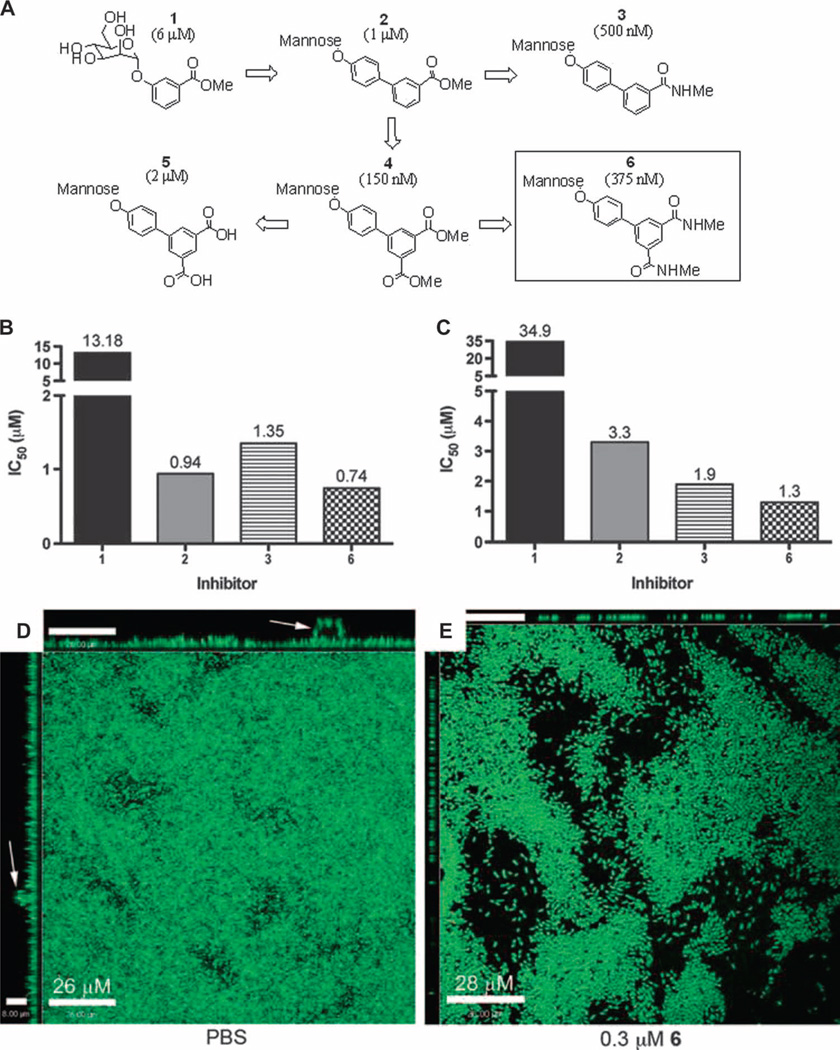
Functional activity of compounds 1 to 6 in vitro was previously assessed in hemagglutination inhibition (HAI) assays. Structures and EC>90 values (38), which represent the effective compound concentration where greater than 90% of hemagglutination is abrogated, are shown in Fig. 1A. We used biofilm inhibition assays (Fig. 1, B to E) to test the ability of 1 to 6 to block biofilm formation and disrupt preformed biofilms to prioritize and select compounds for further in vivo evaluation. E. coli biofilm formation in Luria broth (LB) at room temperature on polyvinyl chloride (PVC) is dependent on type 1 pili (40), and therefore, we used these conditions to determine the efficacy of compounds 1 to 6 to prevent type 1 pilus–dependent biofilms. The median inhibitory concentration (IC50) values for compounds 1 to 3 and 6 were all in the low micromolar range, but compound 6 showed the best activity with an IC50 of 0.74 µM (Fig. 1B). We could not test compounds 4 and 5 in the biofilm assay because of their insolubility. To evaluate the ability of the compounds to inhibit previously existing biofilms, we grew biofilms for 24 hours, added the compounds, and then evaluated the biofilm density 16 hours later. The compounds inhibited biofilm development under these conditions (Fig. 1C). Confocal microscopy revealed that compound 6 disrupted preformed biofilms (Fig. 1, D and E), likely explaining in part the results in Fig. 1C. Furthermore, preformed biofilms treated with compound 6 lacked continuity and the tall mushroom-like structures observed in untreated biofilms (Fig. 1, D and E). These data suggest that compounds 1 to 6 directly affect both initial cell adhesion and the interbacterial interactions necessary for biofilm persistence.
Fig. 1.
Inhibition, prevention, and disruption of UTI89 biofilm by mannoside. (A) Biphenyl compounds 1 to 6 as described in (38). Cellular HAI titers (EC>90) from (38) are shown in parentheses. (B) IC50 for compounds 1 to 3 and 6 effects on UTI89 biofilm formation (n = 3). The test mannoside was added at the initiation of biofilm formation. (C) IC50 of 1 to 3 and 6 on UTI89 biofilm inhibition of established UTI89 biofilm. Mannoside was added 24 hours after biofilm growth was initiated, and percent biofilm was calculated 16 hours after addition of mannoside. Bars show the median value of the experiments (n = 3). (D and E) Effect of 6 on biofilm dispersal as measured by confocal microscopy of UTI89 biofilms grown for 24 hours (D), then incubated for an additional 16 hours in the presence of 0.3 µM compound 6 (E). Images along top and left are orthogonal views that show biofilm structures that protrude from the surface (arrow). Scale bars, 26 µm (D) and 28 µm (E).
Compound 6 displays oral bioavailability
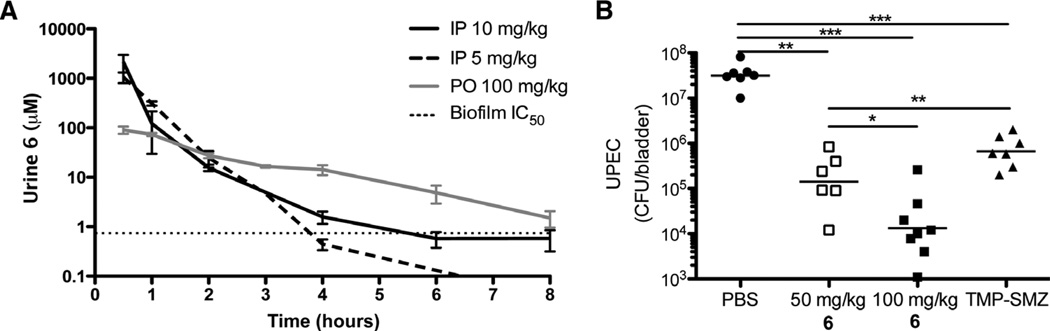
Compounds 4 and 6 were the most potent in the in vitro hemagglutination inhibition (HAI) (38), a measure of inhibition of FimH function. However, we reasoned that the methyl ester present in compound 4 would likely be unstable for oral administration as a result of hydrolysis to carboxylic acid 5. We found that the acid 5 was 13-fold less potent than the ester 4 in the HAI assay, suggesting that using esters would be a poor prodrug strategy (41). Compound 6, in which the ester is replaced with an amide, is not only equipotent to compound 4 in the HAI assay but is also stable to hydrolysis and has increased solubility. Therefore, compound 6 was selected as our lead compound for initial in vivo evaluation. Pharmacokinetic studies with compound 6 were performed in mice after intraperitoneal injection and oral gavage at several doses and time points.
After intraperitoneal administration, concentrations of compound 6 in the urine were quantified at several time points with high-performance liquid chromatography (HPLC) and mass spectrometry (MS). Doses of 5 and 10 mg/kg resulted in concentrations of 1 mM compound 6 in the urine 30 min after treatment (Fig. 2A). Eight hours after administration of compound 6 (10 mg/kg), levels remained near the biofilm IC50 (0.74 µM), which we used as a predictor of effective concentrations against our target FimH. Subsequently, we evaluated the mouse pharmacokinetics of compound 6 given orally at several concentrations up to 200 mg/kg. Compound 6 (100 mg/kg) yielded a three times higher concentration than the intraperitoneal dose (10 mg/kg) 8 hours after administration, demonstrating the oral bioavailability of compound 6. The only detectable metabolism of compound 6 was hydrolysis of the glycosidic bond (yielding d-mannose and the phenol) but >95% of drug was excreted in the urine unchanged (fig. S1); furthermore, no apparent toxicity was observed up to a dose of 200 mg/kg as measured by observable physiological changes and survival.
Fig. 2.
Compound 6 pharmacokinetic distribution and effect on chronic infection. (A) Pharmacokinetic analysis of 6 (n ≥ 3 mice) showing concentration in urine over time for each dose indicated. Horizontal dashed line is at IC50 (0.74 µM) determined by the biofilm inhibition assay. IP, intraperitoneally; PO, orally. (B) Compound 6 effect on UTI. Mice chronically infected with UTI89 were treated with PBS, 6 (orally, 100 and 50 mg/kg), or TMP-SMZ (54 and 270 µg/ml, respectively). Six hours after treatment, bacteria in the bladder were counted. In the mannoside- and TMP-SMZ–treated groups, there was a significant drop in bacterial load relative to that in PBS-treated mice. Horizontal lines indicate geometric mean. *P < 0.05; **P < 0.01; ***P < 0.0001, Mann-Whitney U test.
Compound 6 treats an established UTI
To determine the therapeutic potential of compound 6 for treating chronic UTIs, we adapted a preclinical murine model. Clinically, UTI ranges from acute resolving infection to chronic and/or recurrent UTI (42, 43). Similarly, the outcome of UTI in C3H/HeN mice ranges from self-limiting to long-lasting chronic cystitis. Chronic cystitis was characterized by persistent, high-titer bacteriuria [>104 colony-forming units (CFU)/ml], high-titer bacterial bladder burdens ≥2 weeks after infection, chronic inflammation, and urothelial necrosis (15). Bacteria present in the bladders of chronically infected mice primarily exist extracellularly and express type 1 pili (15). Thus, C3H/HeN mice were infected with 1 × 107 CFU of UTI89, and mice developing chronic cystitis were identified by the presence of persistent urine titers of >106 CFU of UTI89 for 2 weeks after infection. Mice with chronic UTI were treated orally 2 weeks after infection with compound 6 at a single dose of 100 or 50 mg/kg to evaluate the ability of compound 6 to treat chronic UTI. Within 6 hours of treatment, we observed a three-log drop in bacterial titers, suggesting that compound 6 is effective in treating a chronic, long-lasting infection (Fig. 2B). For comparison, mice with chronic UTI were treated with TMP-SMZ (54 and 270 µg/ml, respectively), and bacterial concentrations in the bladder were determined 6 hours later. Although there was a significant drop in the bacterial titers of the TMP-SMZ–treated mice compared to phosphate-buffered saline (PBS)–treated mice, the drop was significantly less than that seen in mice treated with compound 6 (50 mg/kg) (Fig. 2B). The higher potency of compound 6 than TMP-SMZ 6 hours after treatment is likely a result of the therapeutic action of the compound against FimH/type 1 pili to detach bacteria from tissue, causing their near instantaneous elimination by urine flow and innate defenses.
Compound 6 prevents infection
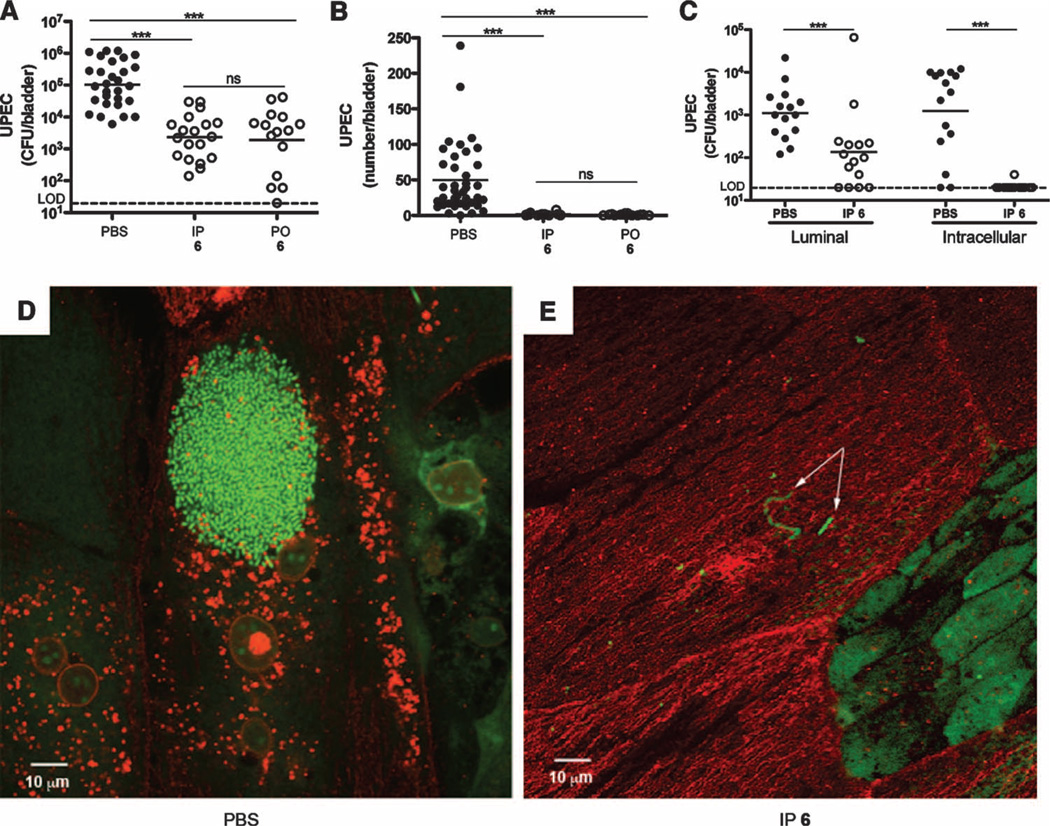
Because compound 6 successfully treated chronic cystitis in our mouse model, we tested whether it could also prevent a UTI when used as prophylactic therapy. Women suffering from chronic and recurrent UTIs are often given TMP-SMZ prophylactically to alleviate recurrence, but opposition to this TMP-SMZ regimen is rapidly expanding (8). To mimic the clinical scenario, we evaluated the preventative efficacy of compound 6 in vivo by administering it to mice either intraperitoneally or orally 30 min before infecting with UTI89. At 6 hours after infection, bladders were removed and total bacterial CFU were quantified. In both the intraperitoneally and the orally treated cohorts, we observed significantly fewer bacterial CFU, demonstrating the efficacy of compound 6 in reducing overall UPEC colonization of the bladder (Fig. 3A).
Fig. 3.
Compound 6 reduces UTI89 colonization by preventing invasion. (A) Total bacterial CFU at 6 hours after infection from mice treated with PBS or 6, either intraperitoneally (5 mg/kg) or orally (100 mg/kg) 30 min before inoculation with UTI89, revealing significantly less colonization in mannoside-treated mice. ns, not significant; LOD, limit of detection. (B) IBC quantification at 6 hours after infection from mice treated with PBS or 6, either intraperitoneally (5 mg/kg) or orally (100 mg/kg) 30 min before inoculation with UTI89, revealing significantly fewer IBCs in mannoside-treated mice. (C) Bacterial CFU at 6 hours after infection in the ex vivo gentamicin protection assay revealed that both luminal and intracellular bacteria were significantly reduced upon intraperitoneal (5 mg/kg) pretreatment of mice with 6. Horizontal lines indicate geometric mean. *P < 0.05; **P < 0.01; ***P < 0.0001, Mann-Whitney U test. (D and E) Confocal microscopy of bladders from PBS-treated (D) and 6-treated (E) mice. Bacteria were stained with SYTO9 (green; 1:1000 in PBS), and the bladder luminal surface was stained with WGA-594 (red; 1:1000 in PBS). The image in (D) shows a robust IBC; the arrows in (E) indicate luminal bacteria. Scale bars, 10 µm.
In the acute stages of infection, the ability of UPEC to invade the bladder tissue and rapidly replicate intracellularly into IBCs facilitates their quick expansion in numbers in the face of a robust immune response and micturition (28). Thus, elimination and/or prevention of this intracellular niche would likely prevent UPEC from persisting in the bladder. To test this, we evaluated the effect of compound 6 on IBC formation as before. Mice were given compound 6 either intraperitoneally or orally 30 min before infecting with UTI89, and 6 hours after infection, bladders were processed for IBC enumeration. The results showed that prophylactic therapy with compound 6 prevented IBC formation (Fig. 3B). We also treated the bladders ex vivo with gentamicin to determine whether compound 6 blocks UPEC invasion into the bladder tissue. Gentamicin kills extracellular UPEC but is unable to penetrate tissue, and thus, intracellular bacteria survive treatment (27). Consistent with the IBC data, we found that in the compound 6–treated mice, ex vivo gentamicin treatment of the bladders eliminated all CFU, suggesting that compound 6 prophylaxis prevented bacterial invasion into the bladder tissue (Fig. 3C). In bladders from untreated mice, 103 to 104 CFU remained after gentamicin treatment, likely a result of intracellular localization of the bacteria (Fig. 3C). Confocal microscopy of bladders from the untreated cohort showed robust IBC formation (Fig. 3D), whereas IBCs were absent or only rarely seen in the compound 6–treated mouse bladders. However, bacteria were observed in the bladder luminal compartment from the compound 6–treated mice, albeit significantly reduced in numbers (Fig. 3E), possibly reflecting the waning of compound 6 concentrations after the single dose. These results demonstrate that compound 6 prevents bacterial invasion into the bladder tissue and significantly reduces infection in the bladder.
Compound 6 potentiates antibiotic activity
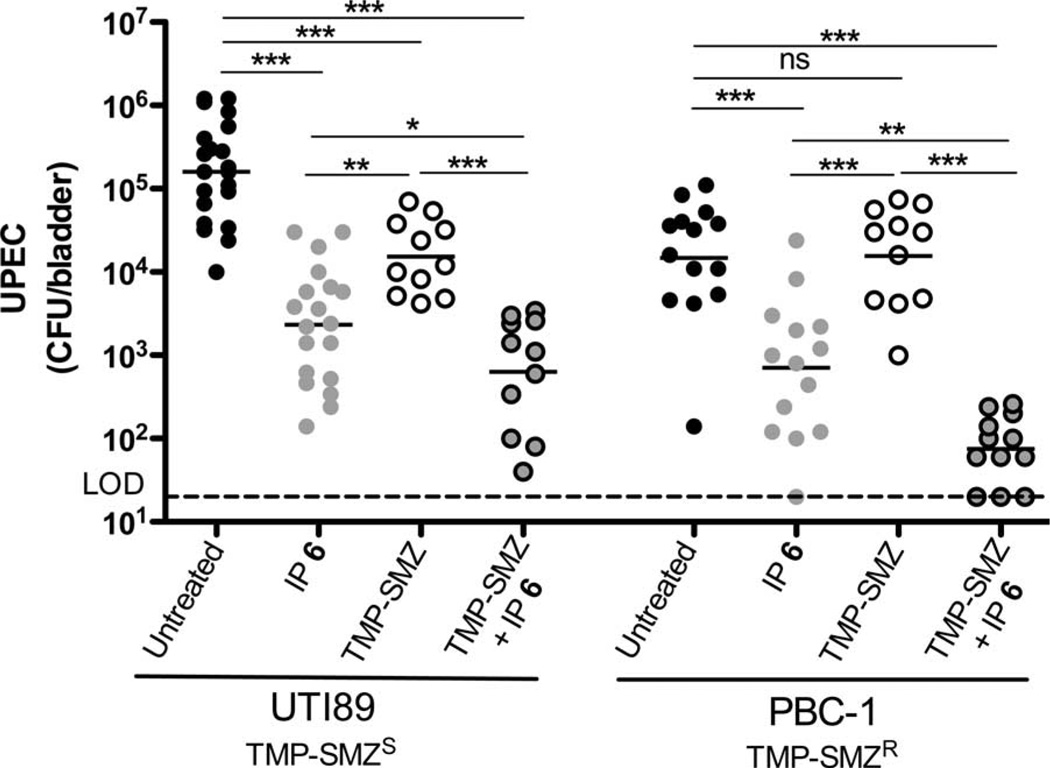
The first-line treatment of choice for UTI has been a 3-day course of TMP-SMZ (80 and 400 mg, respectively), which concentrates in the urine (44). Thus, we hypothesized that by preventing bacterial invasion into the bladder tissue, compound 6 may result in antivirulence synergism with TMP-SMZ and may curtail or circumvent the problem of TMP-SMZ resistance by causing UPEC exposure to TMP-SMZ concentrations sufficient for killing even strains that are clinically resistant to TMP-SMZ. Mice given TMP-SMZ (54 and 270 µg/ml, respectively) for 3 days were infected with either UTI89 or the TMP-SMZR strain PBC-1. Mice were intraperitoneally treated with compound 6 with a dose of 100 mg/kg 30 min before bacterial inoculation. A control group of untreated animals was used for comparison. After inoculating mice with UTI89 or PBC-1, bacterial CFU were quantified at 6 hours after infection. Treatment with TMP-SMZ alone resulted in a significantly reduced bacterial load in the UTI89-infected mice but had no effect on PBC-1 because it is resistant to TMP-SMZ (Fig. 4). Upon treatment with compound 6 alone, there was a significant drop in bacterial load of both strains in the bladder, showing that it is effective against different strains of UPEC that cause UTI. In the dual-treatment group, bacterial CFU were significantly reduced compared to compound 6 alone or TMP-SMZ alone for both strains, and the effect was most pronounced for PBC-1 (Fig. 4). The presence of compound 6 had no effect on growth or killing efficiency of either strain during growth in vitro in the presence or absence of TMP-SMZ. Therefore, the ability of compound 6 to potentiate TMP-SMZ’s activity in preventing infection by the TMP-SMZR strain PBC-1 indicates that the compound 6 effect is specific to the in vivo infection. On the basis of growth curves, we determined that the minimum inhibitory concentration (MIC) of TMP and SMZ was 256 and 1280 µg/ml, respectively, for PBC-1 and 0.05 and 0.25 µg/ml, respectively, for UTI89. Using quantitative HPLC-MS, we determined the concentration of TMP in the urine of mice to be 9.95 ± 4.36 mg/ml and SMZ to be 67.17 ± 32.51 µg/ml after 3 days of treatment with TMP (54 µg/ml) and SMZ (270 µg/ml) in the drinking water, respectively. These results indicate that by preventing bacterial invasion of bladder epithelial cells, compound 6 compartmentalizes the microbes to the bladder lumen, thus exposing them to TMP-SMZ concentrations well above the MIC of PBC-1, resulting in bacterial cell death. It is likely that during current standard treatment, TMP-SMZ reaches tissue concentrations above the MIC needed for UTI89 killing but fails to reach tissue concentrations needed for killing PBC-1. Thus, in the absence of compound 6, residence of PBC-1 in the intracellular niche likely protected it from antibiotic killing.
Fig. 4.
Compound 6 potentiates TMP-SMZ treatment. Total bacterial CFU were quantified 6 hours after infection. UTI89 colonization was reduced in mice treated with 6 (100 mg/kg), TMP-SMZ (54 and 270 µg/ml, respectively), and TMP-SMZ + 6. Horizontal lines indicate geometric mean. *P < 0.05; **P < 0.01; ***P < 0.0001, Mann-Whitney U test.
Compounds 7 to 10 show improved pharmacokinetics and efficacy in vivo
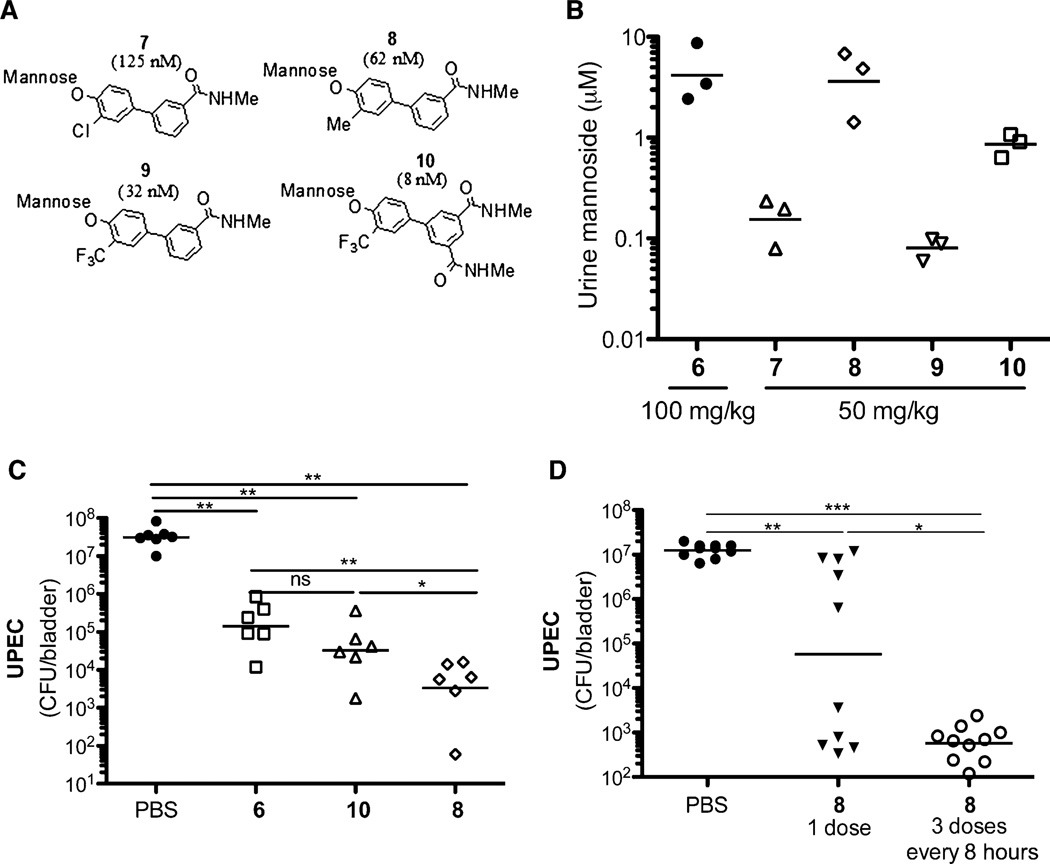
Although compound 6 was effective in vivo, we sought to develop compounds with improved pharmacokinetics, in particular those with increased cell permeability, better oral bioavailability, and better bladder tissue penetration. Although renal clearance is desirable for UTI therapeutics, the inherent polarity of mannose and the mannoside derivatives and other sugar-derived compounds often limits their cellular permeability. We therefore sought to increase their hydrophobicity (logD). In a rat plasma pharmacokinetic intravenous study (n = 3), compound 6 when given at 3 mg/kg had moderate clearance (Cl = 26.6 ml/min per kg) but a very short half-life (t1/2 = 0.4 hour) of less than an hour and low volume of distribution (Vdss = 0.17 liter). Mice given 6 intraperitoneally at 10 mg/kg or orally at 100 mg/kg showed high renal clearance to the urine, which could explain the short half-life of 6 seen in the rat. The low volume of distribution indicated poor tissue exposure with limited bioavailability, highlighting the need to improve the pharmacokinetics of these compounds. Computational modeling suggested that substitution at the biphenyl ring ortho position of compound 3 directly attached to mannose (38) could lead to improved binding to FimH. Therefore, we synthesized analogs of monoamide 3 with ortho-substituted methyl, trifluoromethyl, and chloro (41, 45, 46) groups to increase hydrophobicity and, at the same time, the metabolic stability of the glycosidic bond to acidic hydrolysis in the gut and by mannosidases. The compounds were evaluated for their activity in the HAI assay, and we discovered that all substitutions increased their potencies (Fig. 5A). ortho-Chloro compound 7 inhibited hemagglutination with a potency of 125 nM, which is 10 times better than matched pair 3, whereas the ortho-methyl analog 8 was even more active (HAI = 62 nM). Substitution with a trifluoromethyl group yielded the most potent analog 9 with an HAI = 32 nM. We also developed diamide 10, which is substantially more active than previously reported mannoside FimH inhibitors, with an HAI = 8 nM. This level of potency corresponds to a 50-fold improvement over lead compound 6. We next tested the optimized compounds for mouse oral pharmacokinetics (Fig. 5B) and found that at a dose of 50 mg/kg, compounds 8 and 10 yielded the highest concentrations in urine at 6 hours after administration, with 8 displaying levels equivalent to those produced by compound 6 (100 mg/kg), suggesting that it has increased oral bioavailability compared to 6.
Fig. 5.
Compounds 7 to 10 show enhanced pharmacokinetics and potency at treating infection. (A) Optimized ortho-substituted biphenyl compounds 7 to 10. Cellular HAI titers (EC>90) are shown in parentheses. (B) Compounds 7 to 10 show improved pharmacokinetics. Compounds 8 and 10 at 50 mg/kg yielded concentrations in the urine 6 hours after treatment equivalent to compound 6 at 100 mg/kg. (C) Chronically infected mice were treated with PBS or compound 6, 8, or 10 (orally, 50 mg/kg). Six hours after treatment, there was a significant drop in bacterial load in mannoside-treated mice relative to PBS-treated mice. The optimized compound 8 showed increased efficacy over 6. (D) Chronically infected mice were treated with PBS or compound 8 at one or three doses every 8 hours. Twenty-four hours after the initial treatment, both compound 8–treated groups showed a significant drop in bacterial counts over the PBS-treated animals. (C and D) Horizontal bars indicate geometric mean. *P < 0.05; **P < 0.01; ***P < 0.0001, Mann-Whitney U test.
Because of their enhanced potency and improved pharmacokinetics, we tested compounds 8 and 10 in our chronic infection mouse model. C3H/HeN mice with chronic cystitis at 2 weeks after infection were treated with 6, 8, or 10. Treatment with 8 and 10 significantly reduced CFU by four logs in the bladder within 6 hours of treatment (Fig. 5C). Although 10 is eight times more potent in vitro than compound 8, when given orally at 50 mg/kg compound 8 showed better efficacy in vivo. Compound 8 reduced bacterial CFU in the bladder almost 100 times better than did compound 6 at the identical dose of 50 mg/kg and was more effective than compound 6 at the dose of 100 mg/kg. Thus, a combination of increased FimH inhibition and improved pharmacokinetics for the ortho-substituted compounds 8 and 10 (relative to 6) enables increased exposure in the bladder and urine, resulting in antibacterial efficacy with significant reduction of chronic cystitis within 6 hours of treatment. However, by 24 hours after treatment with compound 8, bacterial CFU began to increase, although they remained significantly lower than PBS-treated mice (Fig. 5D). Oral treatment of infected mice with three doses every 8 hours of compound 8 prevented the increase of CFU 24 hours after the initial treatment compared to PBS- or single dose–treated mice. These data suggest that continual treatment may achieve long-lasting effective treatment of chronic UTI. Although in most cases rapid renal drug clearance is undesired, this is an attractive feature of these compounds for UTI therapy where targeting of drug to the bladder is beneficial. These proof-of-principle experiments have identified optimized compound 8 as a promising lead preclinical candidate for the oral treatment and prevention of recurrent UTIs.
DISCUSSION
UTIs are often a major problem throughout the life span of women, particularly when the infection becomes chronic, recurrent, or recalcitrant to treatment because of pathogenic mechanisms or antibiotic resistance. Multidrug-resistant uropathogens are becoming more prevalent and globally distributed, making UTI an increasingly pressing public health concern (47). Thus, there is a need to design new therapeutic candidates that can be translated to the clinic to improve the lives of women suffering from this often-chronic disease.
Herein, we describe the synthesis and therapeutic efficacy in a mouse model of orally active small-molecule FimH antagonists and demonstrate their potential for treating established chronic UTIs and potentiating antibiotic treatment of a resistant bacterial infection. Our mannoside compounds block the FimH-mediated binding of UPEC to host epithelial mannosylated receptors and prevent UPEC adherence and invasion into the bladder epithelium, thus ultimately preventing the formation of IBCs, which has the potential to mitigate the severity and frequency of recurrence (22, 36, 48). Their mechanism of action is to inhibit the function of the extracellular FimH pilus tip adhesin, which does not require the compound to cross the bacterial outer membrane for efficacy, thus circumventing the development of resistance because of porin mutations or efflux.
The compounds described here are orally bioavailable and have potent, fast-acting efficacy in treating chronic cystitis. A single dose of compound 6 resulted in a significant reduction in bacterial colonization 6 hours after treatment in chronically infected mice. The optimized compound 8 was significantly more efficacious in treating chronic cystitis than compound 6, which reduced chronic cystitis significantly better than did the standard treatment of TMP-SMZ. By extension, the more potent compounds 8 and 10 are likely to be even more effective than 6 relative to TMP-SMZ. Further, enhanced efficacy is derived from three doses of compound 8 given in a 24-hour period, which reduces colonization of the bladder to near-undetectable levels. Currently, treatment of UTI typically requires a 3- to 10-day course of antibiotics or, in the case of chronic cystitis, daily prophylaxis. Also, addition of compound 6 to TMP-SMZ for treatment augmented TMP-SMZ activity as evidenced by a significant reduction in bacterial counts in the bladder over that produced by compound 6 alone or TMP-SMZ alone, even when the strain was clinically resistant to TMP-SMZ. Thus, if clinically translatable to humans, these mannoside compounds have the potential to shorten the standard course of treatment and/or increase the efficacy of TMP-SMZ, resulting in fewer treatment failures.
Prophylactic use of the compounds discussed here was also effective in vivo at preventing UTI in a mouse model. Compound 6 prevented UPEC from invading bladder epithelial cells and thus prevented IBC formation, which is thought to be important for prolonged colonization (36). If our results translate to clinical practice, prophylactic mannoside administration could benefit specific patient populations such as sexually active women with a propensity for UTI (49), the elderly, and possibly catheterized patients. Greater than 1 million catheter-associated UTIs are diagnosed annually with an estimated cost exceeding $600 million, of which UPEC cause about 30% (50–52). Whether prophylactic delivery of mannoside could reduce the incidence of catheter-associated UTIs is the subject of future research.
Treatment failures for chronic and recurrent UTIs, especially those related to antibiotic resistance, continue to impose a significant burden of morbidity and decreased quality of life. A recent study demonstrated that 17 antibiotics capable of killing the virulent cystitis isolate, UTI89, in vitro or in tissue culture, are ineffective in eliminating UTI89 from the bladder tissue during in vivo infection (9). UPEC were protected from even highly membrane-permeable antibiotics such as quinolones. Thus, by harboring antibiotic-tolerant bacteria, IBCs or quiescent intracellular reservoirs (53) could provide a reservoir of surviving pathogens responsible for relapsing or non–antibiotic-responsive infection (54). We reasoned that our mannoside compounds, which prevent these intracellular bacteria, and/or prevent their recurrence, would potentiate efficacy of existing antibiotics. We have demonstrated this potentiation, showing efficacy against two different clinical strains of UPEC: one that is broadly antibiotic-susceptible and one that carries a common but clinically troublesome form of antibiotic resistance. We demonstrated that administration of the mannoside compounds described here resulted in reduced intracellular bladder colonization of UPEC and sensitized a TMP-SMZ–resistant strain, presumably by prolonging exposure to antibiotic levels in the urine above its MIC. These results highlight the importance of the intra-cellular pathway in bacterial persistence. In addition to escaping the immune system in their intracellular niche, bacteria are also able to evade exposure to antibiotics as highlighted by the clinically TMP-SMZ–resistant strain. Translated to clinical practice, mannosides could be a cost-effective treatment that lowers the antibiotic resistance rate, which is currently as high as 30% in some studies (55). This could also have the benefit of reducing the use of fluoroquinolones and decreasing resistance. In our animal models, compounds 6 and 8 are effective as a treatment against UTI, and their oral availability represents a major step toward advancing these compounds into clinical trials and drug development. Further, preclinical lead optimization studies of these compounds are currently focused on improving their oral bioavailability in addition to increasing compound exposure in plasma and the bladder tissue to facilitate less frequent dosing.
MATERIALS AND METHODS
Bacterial strains
UTI89 is a prototypical cystitis isolate of serotype O18:K1:H7. PBC-1 is a TMP-SMZR strain of serotype OX13:K1:H10 isolated from a 59-year-old asymptomatic female with a history of recurrent UTI and diagnosis of primary biliary cirrhosis.
Synthesis of mannosides
General synthesis, purification, and analytical chemistry procedures
Starting materials, reagents, and solvents were purchased from commercial vendors unless otherwise noted. 1H NMR (nuclear magnetic resonance) spectra were measured on a Varian 300-MHz NMR instrument. The chemical shifts were reported as d ppm (parts per million) relative to TMS with residual solvent peak as the reference unless otherwise noted. The following abbreviations were used to express the multiplicities: s = singlet; d = doublet; t = triplet; q = quartet; m = multiplet; br = broad. HPLC purifications were carried out on a Gilson GX-281 with Waters C18 5 µM, 4.6 × 50 mm and Waters Prep C18 5 µM, 19 × 150 mm reverse-phase columns, eluted with a gradient system of 5:95 to 95:5 acetonitrile/water with a buffer consisting of 0.05% trifluoroacetic acid. Purity and structure identification studies were performed using liquid chromatography–mass spectrometry (LCMS) with electrospray ionization (ESI) for detection. All reactions were monitored by thin-layer chromatography (TLC) carried out on Merck silica gel plates (0.25 mm thick, 60F254), visualized with ultraviolet (254 nm) or dyes such as KMnO4, p-anisaldehyde, and ceric ammonium molybdate (CAM). Silica gel chromatography was carried out on a Teledyne ISCO CombiFlash purification system with prepacked silica gel columns (12- to 330-g sizes). All compounds used for biological assays are greater than 95% purity based on NMR and HPLC by absorbance at 220- and 254-nm wavelengths.
Experimental procedure for the preparation of compound 8
Step 1. [(2R,3S,4S,5R,6R)-4,5-diacetoxy-6-(acetoxymethyl)-2-(4-bromo-2-methyl-phenoxy)tetrahydropyran-3-yl] acetate
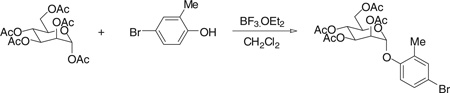
Under nitrogen atmosphere and at room temperature, boron tri-fluoride diethyl etherate (3.41 g, 24 mmol) was added dropwise into the solution of α-d-mannose pentaacetate (3.12 g, 8 mmol) and 4-bromo-2-methylphenol (2.99 g, 16 mmol) in 100 ml of anhydrous CH2Cl2. After a few minutes, the mixture was heated to reflux and stirring was kept for 45 hours. The reaction was then quenched with water and extracted with CH2Cl2. The CH2Cl2 layer was collected, dried with Na2SO4, and concentrated. The resulting residue was purified by silica gel chromatography with hexane/ethyl acetate combinations as eluent, giving the title compound (3.22 g) in 77% yield. 1H NMR (300 MHz, CHLOROFORM-d) δ 7.18 to 7.38 (m, 2H), 6.97 (d, J = 8.79 Hz, 1H), 5.50 to 5.59 (m, 1H), 5.43 to 5.50 (m, 2H), 5.32 to 5.42 (m, 1H), 4.28 (dd, J = 5.63, 12.50 Hz, 1H), 3.99 to 4.15 (m, 2H), 2.27 (s, 3H), 2.20 (s, 3H), 2.02 to 2.11 (three singlets, 9H). MS (ESI): found: [M + Na]+, 539.0.
Step 2. N-methyl-3-[3-methyl-4-[(2R,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-phenyl]benzamide (8)
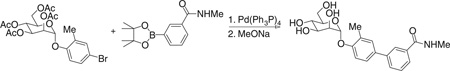
Under nitrogen atmosphere, the mixture of [(2R,3S,4S,5R,6R)-4,5-diacetoxy-6-(acetoxymethyl)-2-(4-bromo-2-methyl-phenoxy)tetrahydropyran-3-yl] acetate (0.517 g, 1 mmol), 3-(N-methylaminocarbonyl)phenylboronic acid pinacol ester (0.392 g, 1.5 mmol), cesium carbonate (0.977 g, 3 mmol), and tetrakis(triphenylphosphine)palladium (0.116 g, 0.1 mmol) in dioxane/ water (15 ml/3 ml) was heated at 80°C with stirring for 1 hour. After cooling to room temperature, the mixture was filtered through a silica gel column to remove the metal catalyst and salts with hexane/ethyl acetate combinations as eluent. The filtrate was concentrated and then dried in vacuo. The residue was diluted with 15 ml of methanol containing a catalytic amount of sodium methoxide (0.02 M), and the mixture was stirred at room temperature overnight. H+ exchange resin (DOWEX 50WX4–100) was added to neutralize the mixture. The resin was filtered off, and the filtrate was concentrated. The resulting residue was purified by silica gel chromatography with CH2Cl2/MeOH combinations as eluent, giving the title compound (0.260 g) in 64% yield for two steps. 1H NMR (300 MHz, METHANOL-d4) δ 7.94 (t, J = 1.65 Hz, 1H), 7.57 to 7.72 (m, 2H), 7.33 to 7.50 (m, 3H), 7.23 (d, J = 8.52 Hz, 1H), 5.48 (d, J = 1.92 Hz, 1H), 4.00 (dd, J = 1.79, 3.43 Hz, 1H), 3.83 to 3.94 (m, 1H), 3.60 to 3.76 (m, 3H), 3.46 to 3.58 (m, 1H), 2.87 (s, 3H), 2.24 (s, 3H). MS (ESI): found: [M + H]+, 404.2. Compounds 7 and 9 were prepared after a procedure similar to the synthesis of 8.
Experimental procedure for the preparation of compound 10
Step 1. [(2R,3S,4S,5R,6R)-4,5-diacetoxy-6-(acetoxymethyl)-2-(4-bromo-2-trifluoromethyl-phenoxy)tetrahydropyran-3-yl] acetate
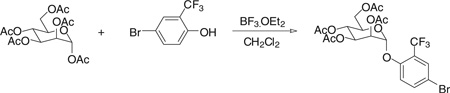
Using the procedure outlined in the [(2R,3S,4S,5R,6R)-4,5-diacetoxy-6-(acetoxymethyl)-2-(4-bromo-2-methyl-phenoxy)tetrahydropyran-3-yl] acetate section with 4-bromo-2-trifluoromethylphenol, we obtained the title compound (2.5 g) in 54% yield. 1H NMR (300 MHz, CHLOROFORM-d) d 7.75 (d, J = 2.20 Hz, 1H), 7.61 (dd, J = 2.47, 8.79 Hz, 1H), 7.15 (d, J = 8.79 Hz, 1H), 5.61 (d, J = 1.65 Hz, 1H), 5.32 to 5.58 (m, 3H), 4.28 (dd, J = 5.22, 12.36 Hz, 1H), 3.95 to 4.22 (m, 2H), 2.21 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 2.04 (s, 3H). MS (ESI): found: [M + Na]+, 593.0.
Step 2. N1,N3-dimethyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene-1,3-dicarboxamide
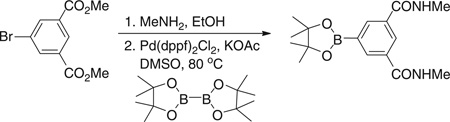
Dimethyl 5-bromobenzene-1,3-dicarboxylate (10.6 g, 36.8 mmol) was dissolved in a 33 wt % solution of methylamine in ethanol (30 ml) and stirred for 6 hours at room temperature. The precipitate that formed during the reaction was filtered to give 5.3 g (53%) of the intermediate 5-bromo-N1,N3-dimethyl-benzene-1,3-dicarboxamide as a white solid. Concentration of the remaining filtrate yielded an additional 4.6 g (46%) of product. 5-Bromo-N1,N3-dimethyl-benzene-1,3-dicarboxamide (5.3 g, 19.5 mmol), Pd(dppf)Cl2 (0.87 g, 1.2 mmol), di-pinacolborane (6.1 g, 24 mmol), and potassium acetate (7.8 g, 80 mmol) were dissolved in dimethyl sulfoxide (DMSO) (100 ml). The solution was stirred under vacuum and then repressurized with nitrogen. This process was repeated three times, and then the resultant mixture was stirred at 80°C for 5 hours under a nitrogen atmosphere. After removal of the solvent under high vacuum, the crude material was purified by silica gel chromatography to give the title compound as a light tan solid (2.2 g, 35%). 1H NMR (300 MHz, DMSO-d6) δ 8.63 (m, 2H), 8.41 (t, J = 1.51 Hz, 1H), 8.23 (d, J = 1.65 Hz, 2H), 2.80 (s, 3H), 2.78 (s, 3H), 1.33 (s, 12H). MS (ESI): found: [M + H]+, 319.2.
Step 3. N1,N3-dimethyl-5-[3-(trifluoromethyl)-4-[(2R,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-phenyl]benzene-1,3-dicarboxamide (10)
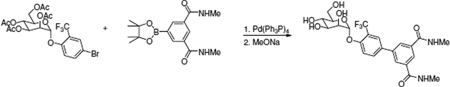
Using the procedure outlined in the N-methyl-3-[3-methyl-4-[(2R,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2- yl]oxy-phenyl]benzamide section with [(2R,3S,4S,5R,6R)-4,5-diacetoxy-6-(acetoxymethyl)-2-(4-bromo-2-trifluoromethyl-phenoxy)tetrahydropyran-3-yl] acetate (0.57 g) and N1,N3-dimethyl-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene-1,3-dicarboxamide (0.48 g), we obtained the title compound (0.340 g) in 69% yield for the two steps. 1H NMR (300 MHz, METHANOL-d4) d 8.17 to 8.24 (m, 1H), 8.14 (d, J = 1.65 Hz, 2H), 7.92 (d, J = 1.92 Hz, 1H), 7.87 (dd, J = 2.20, 8.79 Hz, 1H), 7.57 (d, J = 8.79 Hz, 1H), 5.64 (d, J = 1.65 Hz, 1H), 4.04 (dd, J = 1.65, 3.30 Hz, 1H), 3.87 to 3.96 (dd, 1H), 3.64 to 3.83 (m, 3H), 3.48 to 3.63 (m, 1H), 2.93 (s, 6H). MS (ESI): found: [M + H]+, 515.1.
Biofilm assay
UTI89 was grown in LB in wells of PVC microtiter plates at 23°C in the presence of individual compounds at varying concentrations. After 48 hours of growth, wells were rinsed with water and stained with crystal violet for quantification as described (56). For prevention of biofilm activity in PVC plates, UTI89 was grown in LB broth in wells of PVC microtiter plates at 23°C. After 24 hours of growth, mannoside was added and biofilms were grown for an additional 16 hours. Wells were then rinsed, stained with crystal violet, and quantified. For biofilm disruption activity on PVC coverslips, UTI89 was grown in LB broth in 50 ml of conicals containing PBC coverslips at 23°C. After 24 hours of growth, 0.3 µM compound 6 was added and the biofilm was grown for an additional 16 hours. Coverslips were then rinsed, fixed with 2% (v/v) paraformaldehyde, stained with SYTO9 (1:1000 in PBS; Molecular Probes), and observed with a Zeiss LSM410 confocal laser scanning microscope under a 63× objective.
Animal infections
Bacteria were grown under type 1 pilus–inducing conditions (2 × 24 hours at 37°C statically in LB). The bacteria were harvested and resuspended to an A600 (absorbance at 600 nm) of 0.5 in PBS. Eight-week-old C3H/HeN (Harlan) female mice were anesthetized by inhalation of isoflurane and infected via transurethral catheterization with 50 ml of the bacterial suspension, resulting in 1 × 107 to 2 × 107 inoculum. At 6 hours after infection, mice were killed by cervical dislocation under anesthesia and the bladders were immediately harvested and processed as described below. All animal studies using mice were approved by the Animal Studies Committee of Washington University (Animal Protocol Number 20100002).
Chronic infection
Mice were infected with UTI89, and the infection was allowed to continue for 2 weeks. At 12 days after infection, urine was collected and titered to determine which mice were chronically infected (urine titers >106). At 2 weeks after infection, chronically infected mice were treated orally with mannoside (50 or 100 mg/kg). Six hours after treatment, mice were killed and bladders were aseptically removed and homogenized to determine tissue titers.
Enumeration of bladder IBCs
For animal pretreatment experiments, compound 6 was administered either intraperitoneally (5 mg/kg) or orally (100 mg/kg) 30 min before inoculation with UTI89. To accurately count the number of IBCs, we killed the mice 6 hours after infection and aseptically removed, bisected, and splayed the bladders on silicone plates and fixed them in 2% (v/v) paraformaldehyde. IBCs, readily discernable as punctate violet spots, were quantified by LacZ staining of whole bladders (57).
Pharmacokinetic analysis
For intraperitoneal dosing, 50 µl of a solution of 6 in PBS [2 mg/ml (5 mg/kg) or 4 mg/ml (10 mg/kg)] was injected into the peritoneal cavity of the mouse. For oral dosing, 100 µl of a solution of mannoside in 8% DMSO [10 mg/ml (50 mg/kg) or 20 mg/ml (100 mg/kg)] was inoculated with a gavage needle into the mouse stomach. Urine was collected at 30 min and 1, 2, 3, 4, 6, and 8 hours after treatment. An equal volume of 10 mM internal standard (compound 3) was added to the urine. Mannosides were extracted from the urine by loading on C18 columns (100 mg, Waters), washing with 30% methanol, and eluting with 60% methanol. Vacuum-concentrated eluates were analyzed using an LC-MS system (58) with a lower heated capillary temperature of 190°C and a gradient as follows: Solvent B (80% acetonitrile in 0.1% formic acid) was held constant at 5% for 5 min, increased to 44% B by 45 min, and then to a 95% B by 65 min. Selected reaction monitoring (SRM) mode quantification was performed with collision gas energy of 30% for the following MS/MS transitions [precursor mass/charge ratio (m/z)/product m/z]: compound 6, 447/285; compound 3, 390/228. Absolute quantification was achieved by comparison to a calibration curve.
Bladder tissue bacterial titer determination
Compound 6 was administered either intraperitoneally (5 mg/kg) or orally (100 mg/kg) 30 min before inoculation with UTI89. To enumerate the bacteria present, we killed the mice at 6 hours after infection, and we aseptically removed and homogenized the bladders in 1 ml of PBS and serially diluted and plated them onto LB agar plates. CFU were enumerated after 16 hours of growth at 37°C.
Confocal microscopy
Compound 6 was administered intraperitoneally (5 mg/kg) 30 min before inoculation with UTI89. To visualize bacterial behavior within the bladder, we killed the mice at 6 hours after infection, and we aseptically removed, bisected, and splayed the bladders on silicone plates, revealing the luminal surface, and fixed them in 2% (v/v) paraformaldehyde. The splayed bladders were then incubated for 20 min at room temperature with (i) SYTO9 (1:1000 in PBS; Molecular Probes) to stain bacteria and (ii) Alexa Fluor 594–conjugated wheat germ agglutinin (WGA; 1:1000 in PBS; Molecular Probes) to stain the bladder luminal surface. Bladders were rinsed with PBS, mounted with ProLong Gold antifade reagent (Invitrogen), and examined with a Zeiss LSM510 confocal laser scanning microscope under a 63× objective. SYTO9 and WGA were excited at 488 and 594 nm, respectively.
Gentamicin protection assay
To enumerate bacteria present in the intracellular versus extracellular compartments, we aseptically harvested the bladders at 6 hours after infection. The bladders were then bisected twice and washed three times in 500 ml of PBS each. The wash fractions were pooled, lightly spun at 500 rpm for 5 min to pellet exfoliated bladder cells, serially diluted, and plated onto LB agar to obtain the luminal fraction. The bladders were treated with gentamicin (100 µg/ml) for 90 min at 37°C. After treatment, the bladders were washed twice with PBS to eliminate residual gentamicin, homogenized in 1 ml of PBS, serially diluted, and plated onto LB agar to enumerate the CFU in the intracellular fraction.
Antibiotic treatment
Mice were given TMP-SMZ oral suspension solution in the drinking water at a concentration of 54 and 270 µg/ml, respectively. Water was changed daily for 3 days before inoculation with UTI89. Mice remained on TMP-SMZ during the infection. To determine TMP-SMZ concentration in the urine, we collected urine after 3 days of TMP-SMZ treatment and quantified it by LC-MS after addition of sulfisoxazole as an internal standard.
Growth curve
An overnight culture of PBC-1 was diluted 1:1000 in LB in the presence or absence of TMP-SMZ and/or compound 6. The highest concentration of TMP-SMZ used was 512 and 2560 µg/ml, respectively. Twofold dilutions of TMP-SMZ were performed. Compound 6 was added at 100 µM. Growth curves were performed in a 96-well plate at 37°C with A600 readings taken every 30 min for 8 hours. The MIC was calculated as the lowest concentration of antibiotic that prevented growth of the bacterial strain.
Statistical analysis
Observed differences in bacterial titers and IBC numbers were analyzed for significance with the nonparametric Mann-Whitney U test (Prism; GraphPad Software). A P value of less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank K. Dodson for discussions and scrutiny of the manuscript and S. Chen for his experimental insight.
Funding: This work was supported by NIH National Institute of Allergy and Infectious Diseases grants AI48689 and AI49950, National Institute of Diabetes, Digestive and Kidney Diseases grants DK51406 and DK64540, and American Recovery and Reinvestment Act grant RC1DK086378-02 to S.J.H. MS analysis was supported by P41-RR00954, P60-DK20579, and P30-DK56341. J.P.H. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund and K12 HD001459-09. PBC-1 was isolated through funding from the American Liver Foundation, PBC Special Research Initiative.
Footnotes
SUPPLEMENTARY MATERIAL
www.sciencetranslationalmedicine.org/cgi/content/full/3/109/109ra115/DC1
Fig. S1. Minimal degradation of compound 6 occurs after oral gavage.
Author contributions: C.K.C., J.S.P., Z.H., B.A.F., J.W.J., and S.J.H. conceived and planned the study. C.K.C., J.S.P., and S.E.G. performed the experiments. Z.H. synthesized the mannosides. J.R.C. and J.P.H. performed MS analysis of the mannosides, and TMP-SMZ. C.K.C. and S.J.H. wrote the paper. All authors discussed the results and commented on the manuscript.
Competing interests: Washington University holds a patent (WO 2011/050323 A1) on the mannoside compounds reported in this paper.
REFERENCES AND NOTES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: No ESKAPE! An update from the infectious diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, Pamer EG. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, Finlay BB. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 2008;76:4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. U.S.A. 2011;108(Suppl. 1):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho J, Tambyah PA, Paterson DL. Multiresistant Gram-negative infections: A global perspective. Curr. Opin. Infect. Dis. 2010;23:546–553. doi: 10.1097/QCO.0b013e32833f0d3e. [DOI] [PubMed] [Google Scholar]
- 6.Griebling TL. Urinary tract infection in women. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America. Washington, DC: U.S. Government Printing Office; 2007. pp. 587–620. [Google Scholar]
- 7.Ronald AR, Nicolle LE, Stamm E, Krieger J, Warren J, Schaeffer A, Naber KG, Hooton TM, Johnson J, Chambers S, Andriole V. Urinary tract infection in adults: Research priorities and strategies. Int. J. Antimicrob. Agents. 2001;17:343–348. doi: 10.1016/s0924-8579(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 8.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann. Intern. Med. 2001;135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 9.Blango MG, Mulvey MA. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010;54:1855–1863. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaeffer AJ. The expanding role of fluoroquinolones. Dis. Mon. 2003;49:129–147. doi: 10.1067/mda.2003.12. [DOI] [PubMed] [Google Scholar]
- 11.Aypak C, Altunsoy A, Düzgün N. Empiric antibiotic therapy in acute uncomplicated urinary tract infections and fluoroquinolone resistance: A prospective observational study. Ann. Clin. Microbiol. Antimicrob. 2009;8:27. doi: 10.1186/1476-0711-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlowsky JA, Hoban DJ, Decorby MR, Laing NM, Zhanel GG. Fluoroquinolone-resistant urinary isolates of Escherichia coli from outpatients are frequently multidrug resistant: Results from the North American Urinary Tract Infection Collaborative Alliance-Quinolone Resistance study. Antimicrob. Agents Chemother. 2006;50:2251–2254. doi: 10.1128/AAC.00123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beerepoot MA, ter Riet G, Nys S, van der Wal WM, de Borgie CA, de Reijke TM, Prins JM, Koeijers J, Verbon A, Stobberingh E, Geerlings SE. Cranberries vs antibiotics to prevent urinary tract infections: A randomized double-blind noninferiority trial in premenopausal women. Arch. Intern. Med. 2011;171:1270–1278. doi: 10.1001/archinternmed.2011.306. [DOI] [PubMed] [Google Scholar]
- 14.Dielubanza EJ, Schaeffer AJ. Urinary tract infections in women. Med. Clin. North Am. 2011;95:27–41. doi: 10.1016/j.mcna.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001042. e1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect. Immun. 2011;79:4250–4259. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 18.Wu XR, Sun TT, Medina JJ. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: Relation to urinary tract infections. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder JA, Lloyd AL, Lockatell CV, Johnson DE, Mobley HL. Role of phase variation of type 1 fimbriae in a uropathogenic Escherichia coli cystitis isolate during urinary tract infection. Infect. Immun. 2006;74:1387–1393. doi: 10.1128/IAI.74.2.1387-1393.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eto DS, Jones TA, Sundsbak JL, Mulvey MA. Integrin-mediated host cell invasion by type 1–piliated uropathogenic Escherichia coli . PLoS Pathog. 2007;3:e100. doi: 10.1371/journal.ppat.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J, Bishop BL, Li G, Grady R, Stapleton A, Abraham SN. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:14966–14971. doi: 10.1073/pnas.0900527106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thumbikat P, Berry RE, Zhou G, Billips BK, Yaggie RE, Zaichuk T, Sun TT, Schaeffer AJ, Klumpp DJ. Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog. 2009;5:e1000415. doi: 10.1371/journal.ppat.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exo-cytosis of Escherichia coli from infected bladder epithelial cells. Nat. Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 25.Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli . Infect. Immun. 2002;70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 27.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli . Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 28.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunther IV NW, Snyder JA, Lockatell V, Blomfield I, Johnson DE, Mobley HL. Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect. Immun. 2002;70:3344–3354. doi: 10.1128/IAI.70.7.3344-3354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song J, Bishop BL, Li G, Duncan MJ, Abraham SN. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 2007;1:287–298. doi: 10.1016/j.chom.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schembri MA, Klemm P. Biofilm formation in a hydrodynamic environment by novel FimH variants and ramifications for virulence. Infect. Immun. 2001;69:1322–1328. doi: 10.1128/IAI.69.3.1322-1328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connell H, Poulsen LK, Klemm P. Expression of type 1 and P fimbriae in situ and localisation of a uropathogenic Escherichia coli strain in the murine bladder and kidney. Int. J. Med. Microbiol. 2000;290:587–597. doi: 10.1016/S1438-4221(00)80006-5. [DOI] [PubMed] [Google Scholar]
- 33.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intra-cellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mecsas J. Use of signature-tagged mutagenesis in pathogenesis studies. Curr. Opin. Microbiol. 2002;5:33–37. doi: 10.1016/s1369-5274(02)00282-5. [DOI] [PubMed] [Google Scholar]
- 35.Ronald LS, Yakovenko O, Yazvenko N, Chattopadhyay S, Aprikian P, Thomas WE, Sokurenko EV. Adaptive mutations in the signal peptide of the type 1 fimbrial adhesin of uropathogenic Escherichia coli . Proc. Natl. Acad. Sci. U.S.A. 2008;105:10937–10942. doi: 10.1073/pnas.0803158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SL, Hung CS, Pinkner JS, Walker JN, Cusumano CK, Li Z, Bouckaert J, Gordon JI, Hultgren SJ. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl. Acad. Sci. U.S.A. 2009;106:22439–22444. doi: 10.1073/pnas.0902179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung CS, Bouckaert J, Hung D, Pinkner J, Widberg C, DeFusco A, Auguste CG, Strouse R, Langermann S, Waksman G, Hultgren SJ. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 2002;44:903–915. doi: 10.1046/j.1365-2958.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- 38.Han Z, Pinkner JS, Ford B, Obermann R, Nolan W, Wildman SA, Hobbs D, Ellenberger T, Cusumano CK, Hultgren SJ, Janetka JW. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J. Med. Chem. 2010;53:4779–4792. doi: 10.1021/jm100438s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wellens A, Garofalo C, Nguyen H, Van Gerven N, Slättegård R, Hernalsteens JP, Wyns L, Oscarson S, De Greve H, Hultgren S, Bouckaert J. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS One. 2008;3:e2040. doi: 10.1371/journal.pone.0002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cegelski L, Pinkner JS, Hammer ND, Cusumano CK, Hung CS, Chorell E, Aberg V, Walker JN, Seed PC, Almqvist F, Chapman MR, Hultgren SJ. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 2009;5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein T, Abgottspon D, Wittwer M, Rabbani S, Herold J, Jiang X, Kleeb S, Lüthi C, Scharenberg M, Bezençon J, Gubler E, Pang L, Smiesko M, Cutting B, Schwardt O, Ernst B. FimH antagonists for the oral treatment of urinary tract infections: From design and synthesis to in vitro and in vivo evaluation. J. Med. Chem. 2010;53:8627–8641. doi: 10.1021/jm101011y. [DOI] [PubMed] [Google Scholar]
- 42.Foxman B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002;113(Suppl. 1A) doi: 10.1016/s0002-9343(02)01054-9. 5S–13S. [DOI] [PubMed] [Google Scholar]
- 43.Foxman B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 44.Patel RB, Welling PG. Clinical pharmacokinetics of co-trimoxazole (trimethoprim-sulphamethoxazole) Clin. Pharmacokinet. 1980;5:405–423. doi: 10.2165/00003088-198005050-00001. [DOI] [PubMed] [Google Scholar]
- 45.Firon N, Ashkenazi S, Mirelman D, Ofek I, Sharon N. Aromatic alpha-glycosides of man-nose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect. Immun. 1987;55:472–476. doi: 10.1128/iai.55.2.472-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperling O, Fuchs A, Lindhorst TK. Evaluation of the carbohydrate recognition domain of the bacterial adhesin FimH: Design synthesis and binding properties of mannoside ligands. Org. Biomol. Chem. 2006;4:3913–3922. doi: 10.1039/b610745a. [DOI] [PubMed] [Google Scholar]
- 47.Hooton TM. Fluoroquinolones and resistance in the treatment of uncomplicated urinary tract infection. Int. J. Antimicrob. Agents. 2003;22(Suppl. 2):65–72. doi: 10.1016/s0924-8579(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 48.Berry RE, Klumpp DJ, Schaeffer AJ. Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli . Infect. Immun. 2009;77:2762–2772. doi: 10.1128/IAI.00323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. J. Infect. Dis. 2000;182:1177–1182. doi: 10.1086/315827. [DOI] [PubMed] [Google Scholar]
- 50.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities, NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 51.Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect. Control Hosp. Epidemiol. 2002;23:27–31. doi: 10.1086/501964. [DOI] [PubMed] [Google Scholar]
- 52.Parker D, Callan L, Harwood J, Thompson D, Webb ML, Wilde M, Willson M. Clinical Practice Continence Subcommittee, Catheter-associated urinary tract infections: Fact sheet. J. Wound Ostomy Continence Nurs. 2009;36:156–159. doi: 10.1097/01.WON.0000347656.94969.99. [DOI] [PubMed] [Google Scholar]
- 53.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 55.van der Starre WE, van Nieuwkoop C, Paltansing S, van′t Wout JW, Groeneveld GH, Becker MJ, Koster T, Wattel-Louis GH, Delfos NM, Ablij HC, Leyten EM, Blom JW, van Dissel JT. Risk factors for fluoroquinolone-resistant Escherichia coli in adults with community-onset febrile urinary tract infection. J. Antimicrob. Chemother. 2011;66:650–656. doi: 10.1093/jac/dkq465. [DOI] [PubMed] [Google Scholar]
- 56.O′Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 57.Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. Maturation of intracellular Escherichia coli communities requires SurA. Infect. Immun. 2006;74:4793–4800. doi: 10.1128/IAI.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, Stamm WE, Hooton TM, Hultgren SJ. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli . PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000305. e1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.