Abstract
Latent membrane protein 2A (LMP2A) blocks B-cell receptor signal transduction in vitro by binding the Syk and Lyn protein tyrosine kinases. As well as blocking B-cell signal transduction, LMP2A has been shown to activate the phosphatidylinositol 3-kinase (PI3-K)/Akt pathway, which acts as a survival signal in both B cells and epithelial cells. Transforming growth factor β1 (TGF-β1) is a multifunctional cytokine that plays important roles in regulating cell growth and differentiation in many biological systems. The loss of the growth-inhibitory response to the TGF-β1 signal is found in many cancers and is widely thought to promote tumor development. In this study, we found that LMP2A induced the phosphorylation of Akt (serine 473) in Burkitt's lymphoma cell line Ramos and in gastric carcinoma cell line HSC-39 and partially enhanced cell viability following TGF-β1 treatment. In addition, LMP2A partially inhibited TGF-β1-induced DNA fragmentation and cleavage of poly(ADP-ribose) polymerase (PARP). In the presence of LY294002, an inhibitor of PI3-K, the LMP2A-mediated inhibitory effects on TGF-β1-induced DNA fragmentation and cleavage of PARP were alleviated. Furthermore, LMP2A did not alter the levels of expression of type I and type II TGF-β1 receptors. Taken together, these results suggest that LMP2A may inhibit TGF-β1-mediated apoptosis through activation of the PI3-K/Akt pathway.
Epstein-Barr virus (EBV) ubiquitously infects the majority of humans and is the causative agent of infectious mononucleosis. EBV infection has been closely linked to various lymphoid and epithelioid malignancies (22). Primary human B lymphocytes infected in vitro with EBV become immortalized, establishing lymphoblastoid cell lines. Within these cells, the EBV genome expresses nine EBV-encoded latent proteins, six in the nucleus (EBV nuclear antigens) and three in the membrane (latent membrane protein 1 [LMP1], LMP2A, and LMP2B), and two small viral RNAs (22, 30). The very strong association between EBV and nasopharyngeal carcinoma is well established (22), and there is a growing body of evidence showing an association of EBV with other epithelioid malignancies, such as gastric carcinoma (17, 46, 48). Analysis of EBV DNA, which shows clonality in gastric carcinoma cells, has shown that tumors arise from a single EBV-infected cell, suggesting that EBV infection occurs in the very early stages of tumor development (19). Among the 11 EBV genes expressed in lymphoblastoid cell lines, the LMP2A gene is expressed in vivo in humans with latent infections and most EBV-related malignancies, with the exception of Burkitt's lymphoma (BL) (22, 30, 51). Due to this persistent expression, LMP2A is thought to play a key role in ensuring EBV latency and may be an important risk factor in EBV-associated diseases.
In addition, while LMP2A is capable of blocking signaling through the B-cell receptor by binding the protein tyrosine kinases Syk and Lyn (32), LMP2A also activates the phosphatidylinositol 3-kinase (PI3-K)/Akt pathway, which acts as a cell survival signal through these same protein tyrosine kinases in B-cell lines (49). LMP2A appears to be a key determinant in the alteration of epithelial cell growth by activating Akt and c-Jun in epithelial cells and leading to an enhancement of cell growth (7, 44). In addition, LMP2A expression is important in epithelial cell clone outgrowth following infection of epithelial cells (35). Thus, the emerging importance of LMP2A in both B-cell and epithelial cell growth regulation led us to investigate the effect of LMP2A on transforming growth factor β1 (TGF-β1) signaling.
TGF-β is a multifunctional cytokine that plays important roles in regulating cell growth and differentiation in many biological systems (31). In the immune system, TGF-β acts as a potent immunosuppressive cytokine that inhibits the proliferation of activated B and T lymphocytes induced by various stimuli, including interleukins 2 and 4 (27). TGF-β can induce immunoglobulin A class switching and plays an important role in differentiation, growth, matrix formation, and the regulation of immune and inflammatory responses (28, 47). Recent studies revealed that the levels of TGF-β are significantly increased in the sera of patients with EBV-associated nasopharyngeal carcinoma, BL, and chronic active EBV infection and correlate positively with EBV-specific immunoglobulin A titers, suggesting a role for this cytokine in the pathogenesis of these diseases (52). Also, gastric carcinoma cells express a number of growth factors, gastrointestinal hormones, and cytokines that may enhance the growth of these tumor cells through potential autocrine and paracrine pathways (50). For example, elevated levels of TGF-β have been reported in patients with gastric cancer (33, 37). TGF-β1 is an inducer of apoptosis in BL and some gastric carcinoma cell lines (18, 20, 23, 55). In addition, recent studies have revealed that PI3-K and its downstream target Akt are responsible for the antiapoptotic activity of these factors against TGF-β (5, 6).
To determine the effect of LMP2A on the cellular response to apoptotic stimuli, LMP2A was expressed in BL cell line Ramos and in gastric carcinoma cell line HSC-39, and apoptosis following TGF-β1 treatment was monitored. LMP2A inhibited TGF-β1-induced DNA fragmentation and cleavage of poly(ADP-ribose) polymerase (PARP) in both cell lines tested, and the LMP2A-specific effect on TGF-β1 signaling was dependent on the activation of the PI3-K/Akt pathway by LMP2A. These studies are the first indication that LMP2A has an effect on the growth of BL cells in cultures and provide additional clues with regard to the role of LMP2A in EBV-associated proliferative disorders, such as gastric carcinoma and Hodgkin's disease.
MATERIALS AND METHODS
Cell culture and antibodies.
All cell lines were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1,000 U of penicillin/ml, and 1,000 μg of streptomycin/ml at 37°C in a humidified atmosphere of 5% CO2. Ramos (24) is an EBV-negative BL cell line. HSC-39 (54), a human signet ring cell gastric carcinoma cell line, was kindly provided by K. Yanagihara.
TGF-β1 was purchased from Pepro Tech EC Ltd. Rat monoclonal antibody against LMP2A (14B7-1-1) was described previously (16). Anti-phosphorylated Akt (serine 473) antibody and anti-Akt antibody were purchased from New England Biolabs. Anti-PARP antibody was purchased from Pharmingen, San Diego, Calif. Anti-TGF-β type I receptor (RI) (V-22) and anti-TGF-β type II receptor (RII) (H-567) antibodies were purchased from Santa Cruz Biotechnology, Inc. Anti-rat, anti-rabbit, and anti-mouse antibodies conjugated to horseradish peroxidase were purchased from Amersham, New England Biolabs, and Santa Cruz Biotechnology, respectively. Anti-rabbit antibody conjugated to fluorescein isothiocyanate (FITC) was purchased from Pharmingen. The pan-specific caspase inhibitor zVAD-fmk was purchased from Calbiochem. LY294002, a PI3-K-specific inhibitor, was obtained from Cell Signaling Technologies.
Production of recombinant retroviruses.
GP293 cells were transiently transfected with 2 μg of vector pBAMHYGRO (56) or pMP2LMP2A (29) and with 2 μg of vesicular stomatitis virus glycoprotein G envelope. At 48 h transfection, culture supernatant was harvested and filtered through a 0.45-μm-pore-size filter. Ramos and HSC-39 cells were transduced by incubation with retrovirus-containing GP293 supernatant and Polybrene at 4 μg/ml overnight. Following transduction, cells were split into 96-well plates containing selection medium with hygromycin at 0.4 mg/ml. Seven single clones were isolated after 2 to 3 weeks.
Cell viability.
Viability was assayed by the incorporation of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT) dye (Sigma). Cells were washed with phosphate-buffered saline (PBS) and used to seed 96-well plates at 3 × 104 cells/100 μl per well. Following 1 h of serum starvation, cells were preincubated for 1 h with or without LY294002 (50 μM) and then incubated with or without TGF-β1 (10 ng/ml). After 24 h or 48 h of incubation, 10 μl of MTT solution (5 mg/ml) was added to each well, and the plates were incubated for 4 h at 37°C. One hundred microliters of isopropanol with 0.04 N HCl was added to each well and mixed by repeated pipetting. The absorbance was measured at 570 nm with a microplate reader (SPECTRA MAX 190; Molecular Devices).
Western blotting.
Cells were lysed in buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 10% glycerol, 10 mM NaF, 1 mM Na3VO4, 2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 10 μg of pepstatin/ml, and 10 μg of leupeptin/ml. Protein levels were quantitated with a Dc protein assay (Bio-Rad, Hercules, Calif.). Equivalent amounts of proteins were subjected to heat denaturation at 70°C for 10 min. Proteins were resolved by SDS-polyacrylamide gel electrophoresis, transferred to Immobilon-P (Millipore, Bedford, Mass.), and blocked with 4% skim milk at room temperature for 1 h. Membranes were incubated with 10 mM Tris-HCl (pH 7.5)-150 mM NaCl-0.05% Tween 20 and primary antibodies for 2 h and then with appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h. Following incubation, the membranes were visualized by using an ECL detection kit (Amersham Pharmacia Biotech).
Flow cytometry.
Cells were incubated with primary antibodies and then incubated with FITC-labeled secondary antibodies on ice for 15 min. Cells were washed with cold staining buffer (PBS, 10 mM HEPES, 1% FBS, 0.1% sodium azide) and analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, Calif.) with CellQuest software (Becton Dickinson).
Fluorescence-activated cell sorting analyses for cell cycle and apoptosis.
Analyses for cell cycle and apoptosis were carried out with an Apo-Direct kit obtained from Pharmingen. Briefly, cells were washed with PBS, fixed in 1% paraformaldehyde for 15 min, and stored in 70% ethanol at −20°C until staining and analysis. Cells were labeled with FITC-dUTP and propidium iodide according to the manufacturer's instructions and analyzed by flow cytometry (FACSCalibur) with CellQuest software.
RESULTS
Phosphorylation of Akt in LMP2A-expressing Ramos and HSC-39 cells.
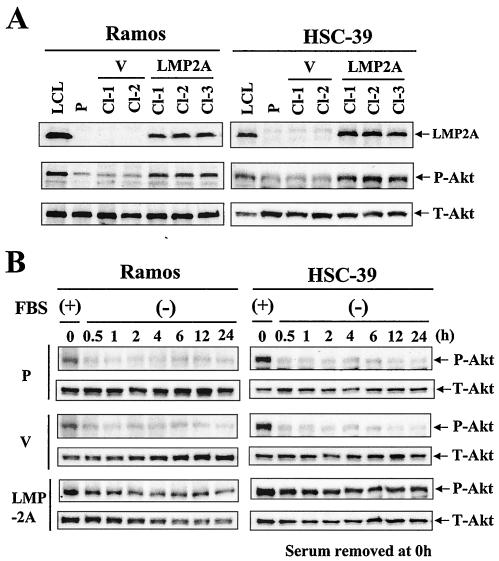
LMP2A activates the PI3-K/Akt pathway in B cells and epithelial cells (44, 49). To further investigate the importance of this activation, Ramos and HSC-39 cells were retrovirally transduced with an LMP2A expression vector and a vector control. Following transduction, hygromycin-resistant stable transfected clones were selected and verified for LMP2A expression and Akt phosphorylation by Western blot analysis (Fig. 1A). Parental Ramos and HSC-39 cells and cells transduced with the vector control (vector control cells) exhibited low levels of Akt phosphorylation under serum starvation conditions. Elevated Akt phosphorylation was detected in LMP2A-positive Ramos and HSC-39 clones (Cl-1, Cl-2, and Cl-3) (Fig. 1A).
FIG. 1.
LMP2A induces the phosphorylation of Akt in Ramos and HSC-39 cells. (A) Ramos and HSC-39 cells were stably retrovirally transduced with LMP2A and vector control expression constructs. After 24 h of serum starvation, whole-cell extracts were separated by SDS-polyacrylamide gel electrophoresis. The levels of expression of LMP2A (54 kDa) and phosphorylated Akt (serine 473) (P-Akt) (60 kDa) in parental (P), vector control (V), and LMP2A-expressing Ramos and HSC-39 cells were determined by immunoblotting. LCL, lymphoblastoid cell line. LCL was used as the positive control for LMP2A and Akt phosphorylation. (B) Effect of serum starvation on the phosphorylation of Akt in LMP2A-expressing Ramos and HSC-39 cells. Parental (P), vector control (V), and LMP2A-expressing Ramos and HSC-39 cells were switched to RPMI 1640 medium without FBS (−), harvested at the indicated times, and analyzed by immunoblotting for P-Akt. The lower panels show equal loading of proteins and the expression of total Akt (T-Akt) (60 kDa).
We next addressed the serum dependence of Akt phosphorylation (Fig. 1B). The cells were serum starved for 0.5 to 24 h. A reduction in Akt phosphorylation was seen with serum starvation in parental and vector control Ramos cells. Significant attenuation of Akt phosphorylation with serum starvation also was observed in parental and vector control HSC-39 cells. The levels of Akt phosphorylation were reduced at 0.5 h and reached a minimum at 1 h in both parental and vector control Ramos and HSC-39 cells. In contrast, the levels of Akt phosphorylation in LMP2A-expressing cells appeared to be almost entirely unaffected by serum starvation.
LMP2A enhances cell viability in the presence of TGF-β1 treatment in Ramos and HSC-39 cells.
Several lines of evidence indicate that activation of the PI3-K/Akt pathway can block various stimuli, including TGF-β1 (1, 6, 57). To explore the role of activation of the PI3-K/Akt pathway by LMP2A in TGF-β1-induced cell death, we examined the effect of TGF-β1 on cell viability in parental, vector control, and LMP2A-expressing Ramos and HSC-39 cells by using an MTT assay (Fig. 2). To clarify the activation of Akt-mediated cell survival by LMP2A, tests were done under serum starvation conditions. TGF-β1-inhibited viability of parental and vector control Ramos and HSC-39 cells was explored either 24 or 48 h following TGF-β1 treatment. TGF-β1 treatment resulted in approximately 40 and 30% viability of parental and vector control Ramos cells relative to untreated cells (set at 100%) at 24 and 48 h posttreatment, respectively (Fig. 2A). TGF-β1 treatment resulted in approximately 60 and 40% viability of parental and vector control HSC-39 cells relative to untreated cells at 24 and 48 h posttreatment, respectively (Fig. 2B). For both cell lines, the expression of LMP2A resulted in an approximate 20% increase in cell viability at both times following TGF-β1 treatment (Fig. 2).
FIG. 2.
LMP2A enhances Ramos and HSC-39 cell viability in the presence of TGF-β1 treatment. Parental (P), vector control (V), and LMP2A-expressing Ramos (A) and HSC-39 (B) cells were seeded at 3 × 104 cells/100 μl per well with serum-free medium. Following 1 h of serum starvation, the cells were left untreated (control) or were treated with 10 ng of TGF-β1/ml for 24 or 48 h. Cell viability was measured with an MTT assay. Results are the means and standard deviations for five experiments.
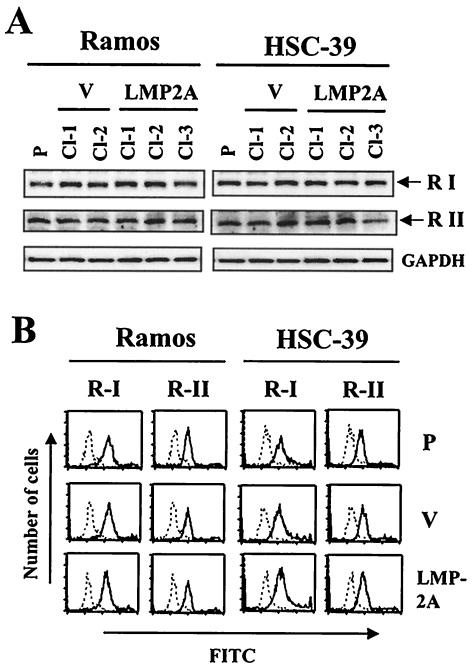
LMP2A does not affect the level of expression of TGF-β receptors.
Resistance to TGF-β1 can correlate with decreased expression or a lack of expression of TGF-β receptors in BL cell lines, EBV-transformed B-lymphoblastoid cell lines (21), and gastric carcinoma cell lines (39, 53). Western blot and flow cytometric analyses were performed to examine whether the increase in cell viability observed in Ramos and HSC-39 cells with LMP2A expression in the presence of TGF-β1 treatment was due to a loss of or a reduction in TGF-β receptors. Figure 3A shows that parental Ramos and HSC-39 cells as well as selected clones with or without LMP2A expression contained similar amounts of type I and II TGF-β receptors, as determined by Western blot analysis. The cell surface expression of type I and II TGF-β receptors in parental, vector control (Cl-2 or Cl-1), and LMP2A-expressing (Cl-2 or Cl-3) clones of Ramos or HSC-39 cells also was detected at similar levels by flow cytometric analysis (Fig. 3B).
FIG. 3.
Determination of expression of TGF-β receptor type I and receptor type II in LMP2A-expressing Ramos and HSC-39 cells. (A) Immunoblot analysis. Parental (P), vector control (V), and LMP2A-expressing Ramos and HSC-39 cells were analyzed by immunoblotting. The positions of TGF-β receptor type I (53-kDa) (R I) and type II (70-kDa) (R II) bands are indicated at the right. The amount of protein loaded in each lane was assessed by rehybridization of the filter with a specific antibody for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B) Flow cytometric analysis. The broken line represents a negative control. The solid line represents cells stained with anti-TGF-β receptor type I or type II antibody and FITC-conjugated secondary antibody. The data are representative of three experiments.
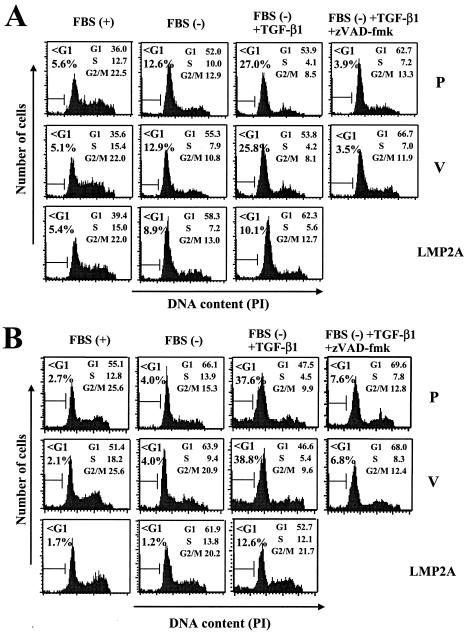
LMP2A inhibits TGF-β1-induced apoptosis and cleavage of PARP.
We next examined whether LMP2A inhibits TGF-β1-induced apoptosis under serum starvation conditions by flow cytometry with parental, vector control (Cl-2), and LMP2A-expressing (Cl-2) clones of Ramos cells and parental, vector control (Cl-1), and LMP2A-expressing (Cl-3) clones of HSC-39 cells (Fig. 4). First, we investigated the effect of serum starvation on cell cycle and apoptosis in these cells. For parental, vector control, and LMP2A-expressing Ramos cells, 24 h of serum starvation resulted in G1 arrest (G1 populations of 52.0, 55.3, and 58.3%, respectively) and partial induction of apoptosis, as evidenced by a significant increase in the percentage of cells in the sub-G1 fraction (sub-G1 populations of 12.6, 12.9, and 8.9%, respectively) (Fig. 4A). LMP2A slightly inhibited apoptosis (about 4%) induced by serum starvation in Ramos cells. For parental, vector control, and LMP2A-expressing HSC-39 cells, 24 h of serum starvation also induced G1 arrest (G1 populations of 66.1, 63.9, and 61.9%, respectively) and slight induction of apoptosis in parental and vector control cells (about 2%); however, there was no difference in LMP2A-expressing cells (Fig. 4B). Under serum starvation conditions, TGF-β1 induced apoptosis in parental and vector control Ramos cells (sub-G1 populations of 27.0 and 25.8%, respectively) and HSC-39 cells (sub-G1 populations of 37.6 and 38.8%, respectively) at 24 h (Fig. 4). LMP2A inhibited TGF-β1-mediated apoptosis in both Ramos cells (a >2-fold reduction in apoptotic cells; 25.8 to 10.1%) and HSC-39 cells (a >3-fold reduction in apoptotic cells; 38.8 to 12.6%).
FIG. 4.
LMP2A inhibits TGF-β1-induced DNA fragmentation in Ramos and HSC-39 cells. Parental (P), vector control (V), and LMP2A-expressing Ramos (A) and HSC-39 (B) cells (106/ml) were cultured for 1 h with or without serum. Cells were preincubated for 1 h without or with zVAD-fmk (50 μM) before incubation without or with TGF-β1 (10 ng/ml). After 24 h of incubation, cells were analyzed for DNA content by propidium iodide (PI) staining and flow cytometry. Gates used to ascertain cell cycle distribution and the percentage of cells with sub-G1 (<G1) and G2/M DNA contents are shown. The data are representative of three experiments.
Caspase activation is an important element in the apoptotic signaling pathway (14). Thus, experiments were performed to characterize the role of caspase activation by using a pan-specific inhibitor of caspase activity, zVAD-fmk. This compound effectively blocked TGF-β1-induced apoptosis in parental and vector control Ramos cells (sub-G1 populations of 3.9 and 3.5%, respectively) and HSC-39 cells (sub-G1 populations of 7.6 and 6.8%, respectively), although the cells were still arrested in G1 (G1 populations of 62.7 and 66.7% and of 69.6 and 68.0%, respectively) (Fig. 4). The activation of caspase 3 is increased markedly in many cells undergoing apoptosis, and the cleavage of one of its substrates, PARP, has been used as an indicator of its activity (26). We found a correlation between the appearance of the 89-kDa C-terminal PARP fragment and the appearance of the sub-G1 fraction in flow cytometry analyses (Fig. 4 and 5). TGF-β1 induced the cleavage of PARP in parental and vector control Ramos and HSC-39 cells (Fig. 5). LMP2A dramatically blocked the TGF-β1-induced cleavage of PARP in both Ramos and HSC-39 cells (Fig. 5). In the Ramos cell line, the expression of LMP2A had a modest effect on PARP cleavage, whereas PARP cleavage was blocked almost entirely in LMP2A-expressing HSC-39 cells.
FIG. 5.
LMP2A inhibits TGF-β1-induced cleavage of PARP. Parental (P), vector control (V), and LMP2A-expressing Ramos (A) and HSC-39 (B) cells (106/ml) were cultured for 1 h with or without serum. Cells were preincubated for 1 h without or with zVAD-fmk (50 μM) before incubation without or with TGF-β1 (10 ng/ml). After 24 h of incubation, PARP cleavage was analyzed by immunoblotting with a specific anti-PARP antibody. The full-length 113-kDa and cleaved 89-kDa (fragment) PARP proteins are indicated at the right. The amount of protein loaded in each lane was assessed by rehybridization of the filter with a specific antibody for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
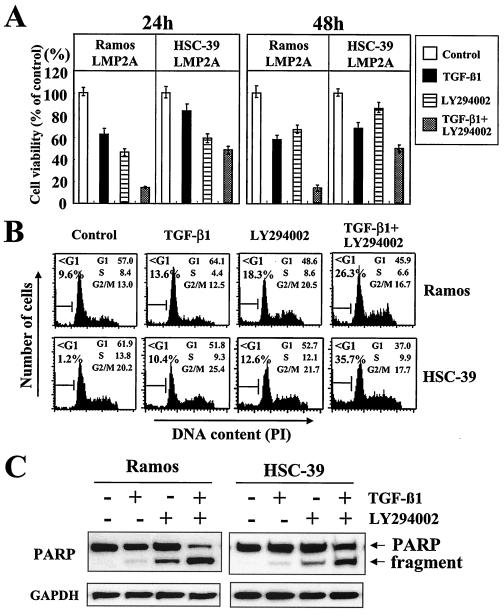
LY294002 inhibits LMP2A-mediated antiapoptotic effects.
Several studies have demonstrated that, under many circumstances, PI3-K transmits a survival signal to rescue cells from apoptosis (5, 6, 10, 25). LY294002, a specific inhibitor of PI3-K, was used to determine the involvement of PI3-K in the antiapoptotic effects of LMP2A. The specificity of Akt phosphorylation downstream of PI3-K activation was confirmed by the inhibition of serine 473 phosphorylation after treatment with LY294002 at 50 μM for 1 h. LY294002 almost completely inhibited Akt serine 473 phosphorylation in all LMP2A-expressing Ramos and HSC-39 cell clones (Fig. 6).
FIG. 6.
Effect of LY294002 on Akt phosphorylation in LMP2A-expressing Ramos and HSC-39 cells. LMP2A-expressing Ramos (A) and HSC-39 (B) cells were cultured for 1 h without serum. Cells were incubated for 1 h with LY294002 (50 μM). Levels of P-Akt (60 kDa) were determined by immunoblotting. The lower panels show equal loading of proteins and the expression of total Akt (T-Akt) (60 kDa).
LY294002 alone partially inhibited the viability of LMP2A-expressing Ramos and HSC-39 cell clones and increased the susceptibility to TGF-β1-induced toxicity in those cells at 24 and 48 h (about 50 and 40% and about 40 and 20%, respectively) (Fig. 7A). In agreement with the results of the cell viability assay, apoptosis was partially induced in LMP2A-expressing Ramos and HSC-39 cells (sub-G1 populations of 18.3 and 12.6%, respectively) (Fig. 7B). In addition, LY294002 increased the susceptibility to TGF-β1-induced apoptosis and the cleavage of PARP in LMP2A-expressing Ramos and HSC-39 cells (Fig. 7B and C).
FIG. 7.
LY294002, a specific inhibitor of PI3-K, alleviates LMP2A-mediated cell viability and antiapoptotic effects. (A) Cell viability. LMP2A-expressing Ramos and HSC-39 cells (3 × 104/100 μl) were switched to RPMI 1640 medium without FBS. Cells were preincubated for 1 h without or with LY294002 (50 μM) before incubation without or with TGF-β1 (10 ng/ml). After 24 or 48 h of incubation, cell viability was measured with an MTT assay. Results are the means and standard deviations for five experiments. (B) DNA fragmentation. Cells (106/ml) were switched to RPMI 1640 medium without FBS. Cells were preincubated for 1 h without or with LY294002 (50 μM) before incubation without or with TGF-β1 (10 ng/ml). After 24 h of incubation, cell cycle analyses were performed as described in the legend to Fig. 4. PI, propidium iodide. (C) Evaluation of cleavage of PARP. Cells (106/ml) were switched to RPMI 1640 medium without FBS. Cells were preincubated for 1 h without or with LY294002 (50 μM) before incubation without or with TGF-β1 (10 ng/ml). After 24 h of incubation, PARP cleavage was analyzed as described in the legend to Fig. 5. Control cells were not treated with various agents. The amount of protein loaded in each lane was assessed by rehybridization of the filter with a specific antibody for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
DISCUSSION
This study has demonstrated that LMP2A effectively suppresses TGF-β1-induced apoptotic death of B cells and gastric carcinoma cells. The LMP2A-mediated antiapoptotic effect was not mediated through decreased expression of TGF-β receptors. A specific inhibitor of PI3-K, LY294002, blocked the antiapoptotic effect of LMP2A, implicating that LMP2A utilizes the PI3-K signaling pathway in mediating this effect. In cells expressing LMP2A, phosphorylation of Akt at serine 473 increased, and LY294002 almost completely inhibited Akt serine 473 phosphorylation.
Akt is one of the PI3-K effectors that play an important role in mediating the transformation and antiapoptotic effects of PI3-K (3, 13, 15). Akt itself is a serine/threonine kinase that phosphorylates and regulates the activities of the cell cycle regulatory proteins glycogen synthase kinase 3 and cyclin D (9, 36) and of the cell death-related proteins Bad and pro-caspase 9 and Forkhead transcription factors (2, 4, 34). It appears that survival signaling may utilize different mechanisms in different cells. LMP2A induces the activation of the PI3-K/Akt pathway in both B cells and epithelial cells; however, LMP2A has not been shown to induce the transformation of B cells in cultures (49). An important functional finding from this study is that LMP2A can affect the regulation of apoptosis of B cells in cultures. This finding may provide an important tool for studying the effect of LMP2A on B cells in cultures. Previous studies were unable to discern any growth advantage in EBV LMP2A-expressing Akata cell clones compared to vector control cells (44, 49). Previous studies already had indicated an important role for LMP2A in the regulation of the growth of epithelial cells in cultures (35, 44).
Members of the caspase family of proteases have been shown to be key executioners in the apoptotic process in many cell types in response to diverse apoptotic stimuli (8, 14). TGF-β1 induces apoptosis in Ramos and HSC-39 cells through the activation of caspases (38, 41), and the caspase family of proteases is involved in TGF-β-induced apoptosis (20, 45). We demonstrated that DNA fragmentation was dependent on caspase activity in both Ramos and HSC-39 cells, since it was reduced greatly when cells were pretreated with zVAD-fmk, a broad caspase inhibitor. For the caspase-dependent apoptotic pathway, several studies have shown that caspase 3 may be activated by mitochondrion-dependent or -independent pathways (40). These different pathways leading to caspase 3 activation have been explored extensively with various stimuli (42, 43). Previous studies showed that TGF-β1 induces apoptosis in Ramos and HSC-39 cells through caspase 3-like activity (38, 41). In addition, the PI3-K/Akt pathway has been shown to suppress the apoptotic signaling of TGF-β1 by blocking its induction of caspase 3-like activity (6). For this reason, we tested whether LMP2A inhibits TGF-β1-induced cleavage of PARP as an indicator for caspase 3-like activity in Ramos and HSC-39 cells. Interestingly, PARP cleavage was blocked only modestly by LMP2A expression in Ramos cells, but LMP2A expression almost completely blocked PARP cleavage in HSC-39 cells. In the parental and vector control HSC-39 cells, the cleavage of PARP was complete. These results suggest an important role for LMP2A in gastric carcinoma. LMP2A may be particularly important in suppressing the effects of TGF-β on tumor cells.
The roles of LMP2A activation of the PI3-K/Akt pathway and the subsequent inhibition of caspase 3 remain to be elucidated further. Other caspases may be involved. For example, Akt was recently shown to be capable of phosphorylation of procaspase 9 (4) and Bad (11, 12), thereby preventing the caspase 9-dependent apoptotic pathway and Bad from associating with BcL-XL. BcL-XL is then free to resume its activity as a suppressor of apoptosis (58). It is likely that the PI3-K/Akt pathway protects Ramos and HSC-39 cells from apoptotic death induced by TGF-β1 through such mechanisms. In the presence of zVAD-fmk, TGF-β1-induced cleavage of PARP also was inhibited in Ramos and HSC-39 cells. LMP2A partially inhibited DNA fragmentation and cleavage of PARP in Ramos and HSC-39 cells, and these LMP2A-mediated antiapoptotic effects were alleviated by a PI3-K-specific inhibitor, LY294002. Taken together, these results suggest that LMP2A partially inhibits TGF-β1-induced caspase activity and apoptosis through activation of the PI3-K/Akt pathway in Ramos and HSC-39 cells.
LMP2A not only alters cell growth in epithelial cells (35, 44) but also inhibits TGF-β1-induced apoptosis through activation of the PI3-K/Akt pathway in the gastric carcinoma cell line HSC-39. In particular, EBV-associated gastric carcinoma cells express a limited number of EBV genomes, including EBV nuclear antigen 1, EBV-encoded small RNAs, BARF0, and LMP2A (19, 48). One can hypothesize that LMP2A-mediated cell transformation and resistance to TGF-β1-induced apoptosis through activation of the PI3-K/Akt pathway may be important for the development of EBV-associated gastric carcinoma. In addition, activation of the PI3-K/Akt pathway by LMP2A may maintain EBV persistence and latency rather than cell transformation in B cells, suggesting a possible dual role of LMP2A in EBV pathogenesis.
In summary, these results suggest that the inhibition of apoptosis by LMP2A disrupts the normal cellular surveillance mechanism for removing damaged cells, thereby providing a clonal selective advantage for B cells and epithelial cells expressing the LMP2A gene during the early stage of tumor development in EBV-associated lymphoid and epithelioid malignancies.
Acknowledgments
R.L. is supported by Public Health Service grants CA62234, CA73507, and CA93444 from the National Cancer Institute and DE13127 from the National Institute of Dental and Craniofacial Research and is a Stohlman Scholar of the Leukemia and Lymphoma Society of America. This work was supported in part by a Gramm Travel fellowship award from the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
We thank members of the laboratory of R.L. for help with these studies. In addition, we thank Masato Ikeda and Akiko Ikeda for helpful technical advice.
REFERENCES
- 1.Ahmed, N. N., H. L. Grimes, A. Bellacosa, T. O. Chan, and P. N. Tsichlis. 1997. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc. Natl. Acad. Sci. USA 94:3627-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 3.Cantley, L. C., and B. G. Neel. 1999. New insights into tumor suppression: PTE-N suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 96:4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 5.Chen, R. H., M. C. Chang, Y. H. Su, Y. T. Tsai, and M. L. Kuo. 1999. Interleukin-6 inhibits transforming growth factor-beta-induced apoptosis through the phosphatidylinositol 3-kinase / Akt and signal transducers and activators of transcription 3 pathways. J. Biol. Chem. 274:23013-23019. [DOI] [PubMed] [Google Scholar]
- 6.Chen, R. H., Y. H. Su, R. L. Chuang, and T. Y. Chang. 1998. Suppression of transforming growth factor-beta-induced apoptosis through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Oncogene 17:1959-1968. [DOI] [PubMed] [Google Scholar]
- 7.Chen, S. Y., J. Lu, Y. C. Shih, and C. H. Tsai. 2002. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J. Virol. 76:9556-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, G. M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 10.Dahl, J., A. Jurczak, L. A. Cheng, D. C. Baker, and T. L. Benjamin. 1998. Evidence of a role for phosphatidylinositol 3-kinase activation in the blocking of apoptosis by polyomavirus middle T antigen. J. Virol. 72:3221-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 12.del Peso, L., M. Gonzalez-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687-689. [DOI] [PubMed] [Google Scholar]
- 13.Dudek, H., S. R. Datta, T. F. Franke, M. J. Birnbaum, R. Yao, G. M. Cooper, R. A. Segal, D. R. Kaplan, and M. E. Greenberg. 1997. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661-665. [DOI] [PubMed] [Google Scholar]
- 14.Earnshaw, W. C., L. M. Martins, and S. H. Kaufmann. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68:383-424. [DOI] [PubMed] [Google Scholar]
- 15.Franke, T. F., D. R. Kaplan, and L. C. Cantley. 1997. PI3K: downstream AKT-ion blocks apoptosis. Cell 88:435-437. [DOI] [PubMed] [Google Scholar]
- 16.Fruehling, S., S. K. Lee, R. Herrold, B. Frech, G. Laux, E. Kremmer, F. A. Grasser, and R. Longnecker. 1996. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J. Virol. 70:6216-6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukayama, M., Y. Hayashi, Y. Iwasaki, J. Chong, T. Ooba, T. Takizawa, M. Koike, S. Mizutani, M. Miyaki, and K. Hirai. 1994. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab. Investig. 71:73-81. [PubMed] [Google Scholar]
- 18.Fukuda, M., K. Ikuta, K. Yanagihara, M. Tajima, H. Kuratsune, T. Kurata, and T. Sairenji. 2001. Effect of transforming growth factor-beta1 on the cell growth and Epstein-Barr virus reactivation in EBV-infected epithelial cell lines. Virology 288:109-118. [DOI] [PubMed] [Google Scholar]
- 19.Imai, S., S. Koizumi, M. Sugiura, M. Tokunaga, Y. Uemura, N. Yamamoto, S. Tanaka, E. Sato, and T. Osato. 1994. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA 91:9131-9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inman, G. J., and M. J. Allday. 2000. Apoptosis induced by TGF-beta 1 in Burkitt's lymphoma cells is caspase 8 dependent but is death receptor independent. J. Immunol. 165:2500-2510. [DOI] [PubMed] [Google Scholar]
- 21.Inman, G. J., and M. J. Allday. 2000. Resistance to TGF-beta1 correlates with a reduction of TGF-beta type II receptor expression in Burkitt's lymphoma and Epstein-Barr virus-transformed B lymphoblastoid cell lines. J. Gen. Virol. 81:1567-1578. [DOI] [PubMed] [Google Scholar]
- 22.Kieff, E., and A. Rickinson. 2001. Epstein Barr virus and its replication, p. 2511-2573. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 23.Kim, S. G., S. N. Kim, H. S. Jong, N. K. Kim, S. H. Hong, S. J. Kim, and Y. J. Bang. 2001. Caspase-mediated Cdk2 activation is a critical step to execute transforming growth factor-beta1-induced apoptosis in human gastric cancer cells. Oncogene 20:1254-1265. [DOI] [PubMed] [Google Scholar]
- 24.Klein, G., B. Giovanella, A. Westman, J. S. Stehlin, and D. Mumford. 1975. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology 5:319-334. [DOI] [PubMed] [Google Scholar]
- 25.Kulik, G., A. Klippel, and M. J. Weber. 1997. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol. Cell. Biol. 17:1595-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazebnik, Y. A., S. H. Kaufmann, S. Desnoyers, G. G. Poirier, and W. C. Earnshaw. 1994. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371:346-347. [DOI] [PubMed] [Google Scholar]
- 27.Letterio, J. J., and A. B. Roberts. 1998. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 16:137-161. [DOI] [PubMed] [Google Scholar]
- 28.Liang, C. L., J. L. Chen, Y. P. Hsu, J. T. Ou, and Y. S. Chang. 2002. Epstein-Barr virus BZLF1 gene is activated by transforming growth factor-beta through cooperativity of Smads and c-Jun/c-Fos proteins. J. Biol. Chem. 277:23345-23357. [DOI] [PubMed] [Google Scholar]
- 29.Longnecker, R., and E. Kieff. 1990. A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J. Virol. 64:2319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longnecker, R. 1998. Pathogenesis of Epstein-Barr virus, p. 133-174. In D. McCance (ed.), Human tumor viruses. ASM Press, Washington, D.C.
- 31.Massague, J. 1998. TGF-beta signal transduction. Annu. Rev. Biochem. 67:753-791. [DOI] [PubMed] [Google Scholar]
- 32.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant-negative effects on protein-tyrosine kinases. Immunity 2:155-166. [DOI] [PubMed] [Google Scholar]
- 33.Mizoi, T., H. Ohtani, K. Miyazono, M. Miyazawa, S. Matsuno, and H. Nagura. 1993. Immunoelectron microscopic localization of transforming growth factor beta 1 and latent transforming growth factor beta 1 binding protein in human gastrointestinal carcinomas: qualitative difference between cancer cells and stromal cells. Cancer Res. 53:183-190. [PubMed] [Google Scholar]
- 34.Mok, C. L., G. Gil-Gomez, O. Williams, M. Coles, S. Taga, M. Tolaini, T. Norton, D. Kioussis, and H. J. Brady. 1999. Bad can act as a key regulator of T cell apoptosis and T cell development. J. Exp. Med. 189:575-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moody, C. A., R. S. Scott, T. Su, and J. W. Sixbey. 2003. Length of Epstein-Barr virus termini as a determinant of epithelial cell clonal emergence. J. Virol. 77:8555-8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muise-Helmericks, R. C., H. L. Grimes, A. Bellacosa, S. E. Malstrom, P. N. Tsichlis, and N. Rosen. 1998. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 273:29864-29872. [DOI] [PubMed] [Google Scholar]
- 37.Naef, M., T. Ishiwata, H. Friess, M. W. Buchler, L. I. Gold, and M. Korc. 1997. Differential localization of transforming growth factor-beta isoforms in human gastric mucosa and overexpression in gastric carcinoma. Int. J. Cancer 71:131-137. [DOI] [PubMed] [Google Scholar]
- 38.Ohta, S., K. Yanagihara, and K. Nagata. 1997. Mechanism of apoptotic cell death of human gastric carcinoma cells mediated by transforming growth factor beta. Biochem. J. 324:777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park, K., S. J. Kim, Y. J. Bang, J. G. Park, N. K. Kim, A. B. Roberts, and M. B. Sporn. 1994. Genetic changes in the transforming growth factor beta (TGF-beta) type II receptor gene in human gastric cancer cells: correlation with sensitivity to growth inhibition by TGF-beta. Proc. Natl. Acad. Sci. USA 91:8772-8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter, A. G., and R. U. Janicke. 1999. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6:99-104. [DOI] [PubMed] [Google Scholar]
- 41.Saltzman, A., R. Munro, G. Searfoss, C. Franks, M. Jaye, and Y. Ivashchenko. 1998. Transforming growth factor-beta-mediated apoptosis in the Ramos B-lymphoma cell line is accompanied by caspase activation and Bcl-XL downregulation. Exp. Cell Res. 242:244-254. [DOI] [PubMed] [Google Scholar]
- 42.Scaffidi, C., I. Schmitz, J. Zha, S. J. Korsmeyer, P. H. Krammer, and M. E. Peter. 1999. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 274:22532-22538. [DOI] [PubMed] [Google Scholar]
- 43.Schendel, S. L., R. Azimov, K. Pawlowski, A. Godzik, B. L. Kagan, and J. C. Reed. 1999. Ion channel activity of the BH3 only Bcl-2 family member, BID. J. Biol. Chem. 274:21932-21936. [DOI] [PubMed] [Google Scholar]
- 44.Scholle, F., K. M. Bendt, and N. Raab-Traub. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrantz, N., D. A. Blanchard, M. T. Auffredou, S. Sharma, G. Leca, and A. Vazquez. 1999. Role of caspases and possible involvement of retinoblastoma protein during TGFbeta-mediated apoptosis of human B lymphocytes. Oncogene 18:3511-3519. [DOI] [PubMed] [Google Scholar]
- 46.Shibata, D., and L. M. Weiss. 1992. Epstein-Barr virus-associated gastric adenocarcinoma. Am. J. Pathol. 140:769-774. [PMC free article] [PubMed] [Google Scholar]
- 47.Stavnezer, J. 1995. Regulation of antibody production and class switching by TGF-beta. J. Immunol. 155:1647-1651. [PubMed] [Google Scholar]
- 48.Sugiura, M., S. Imai, M. Tokunaga, S. Koizumi, M. Uchizawa, K. Okamoto, and T. Osato. 1996. Transcriptional analysis of Epstein-Barr virus gene expression in EBV-positive gastric carcinoma: unique viral latency in the tumour cells. Br. J. Cancer 74:625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swart, R., I. K. Ruf, J. Sample, and R. Longnecker. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tahara, E. 1993. Molecular mechanism of stomach carcinogenesis. J. Cancer Res. Clin. Oncol. 119:265-272. [DOI] [PubMed] [Google Scholar]
- 51.Thorley-Lawson, D. A. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1:75-82. [DOI] [PubMed] [Google Scholar]
- 52.Xu, J., A. Ahmad, J. F. Jones, R. Dolcetti, E. Vaccher, U. Prasad, and J. Menezes. 2000. Elevated serum transforming growth factor β1 levels in Epstein-Barr virus-associated diseases and their correlation with virus-specific immunoglobulin A (IgA) and IgM. J. Virol. 74:2443-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto, M., Y. Maehara, Y. Sakaguchi, T. Kusumoto, Y. Ichiyoshi, and K. Sugimachi. 1996. Transforming growth factor-beta 1 induces apoptosis in gastric cancer cells through a p53-independent pathway. Cancer 77:1628-1633. [DOI] [PubMed] [Google Scholar]
- 54.Yanagihara, K., T. Seyama, M. Tsumuraya, N. Kamada, and K. Yokoro. 1991. Establishment and characterization of human signet ring cell gastric carcinoma cell lines with amplification of the c-myc oncogene. Cancer Res. 51:381-386. [PubMed] [Google Scholar]
- 55.Yanagihara, K., and M. Tsumuraya. 1992. Transforming growth factor beta 1 induces apoptotic cell death in cultured human gastric carcinoma cells. Cancer Res. 52:4042-4045. [PubMed] [Google Scholar]
- 56.Yang, Z., A. J. Korman, J. Cooper, D. Pious, R. S. Accolla, R. C. Mulligan, and J. L. Strominger. 1987. Expression of HLA-DR antigen in human class II mutant B-cell lines by double infection with retrovirus vectors. Mol. Cell. Biol. 7:3923-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao, R., and G. M. Cooper. 1995. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267:2003-2006. [DOI] [PubMed] [Google Scholar]
- 58.Zha, J., H. Harada, E. Yang, J. Jockel, and S. J. Korsmeyer. 1996. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87:619-628. [DOI] [PubMed] [Google Scholar]