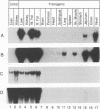
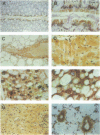
Abstract
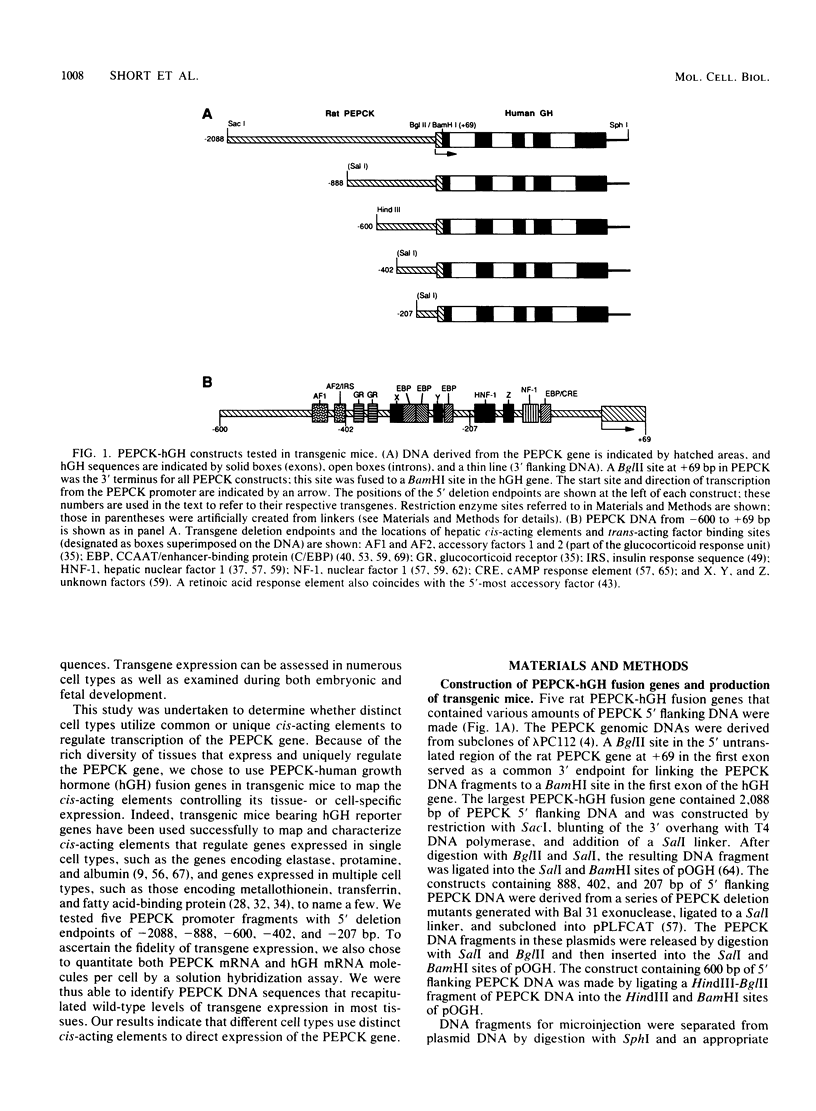
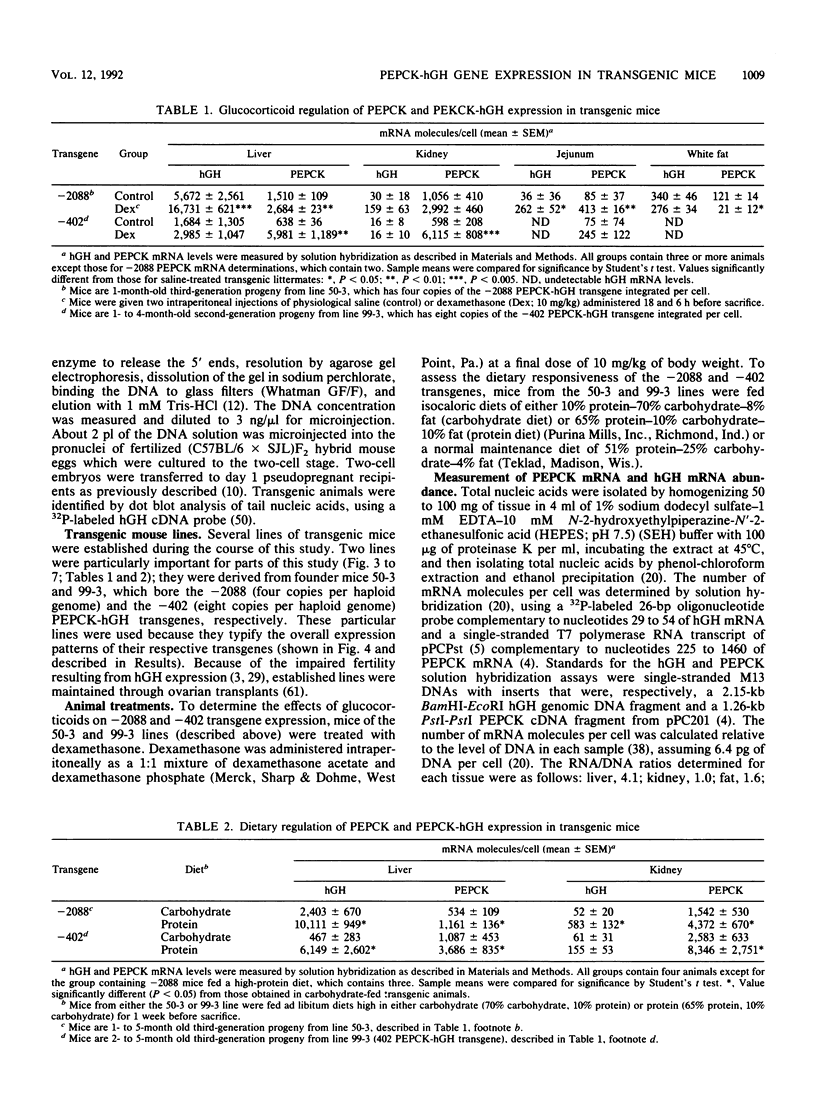
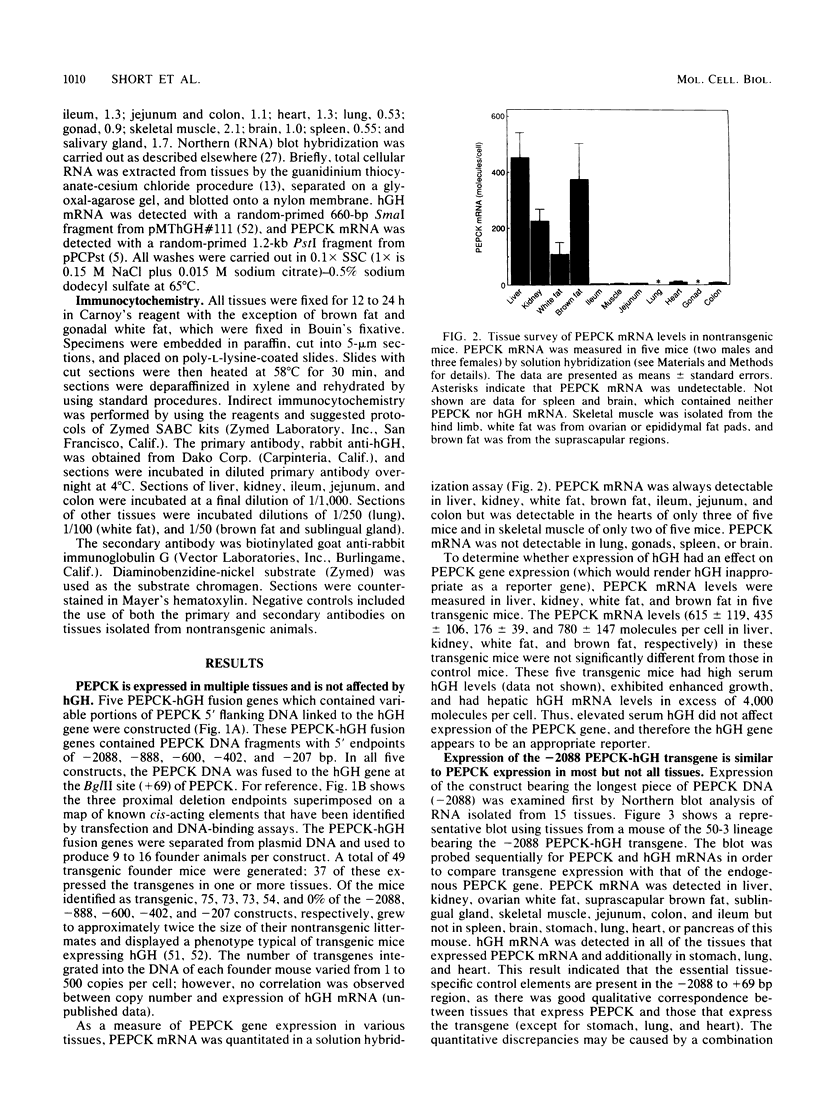
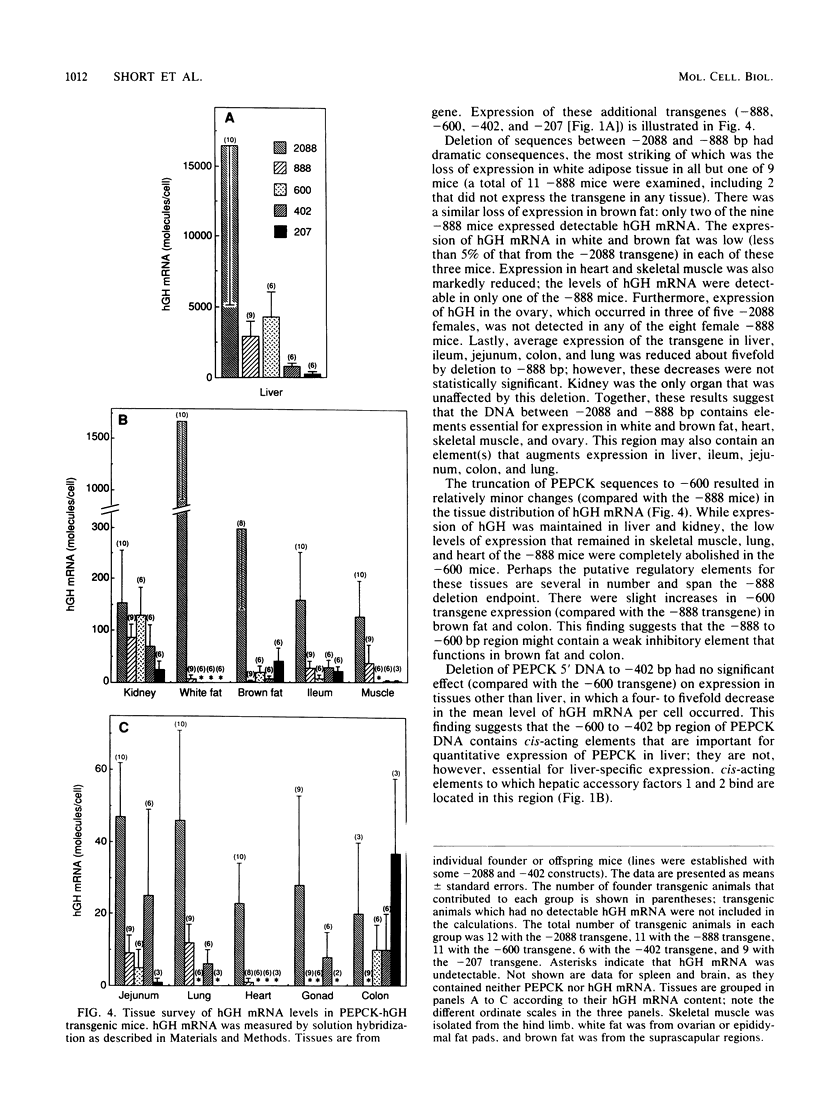
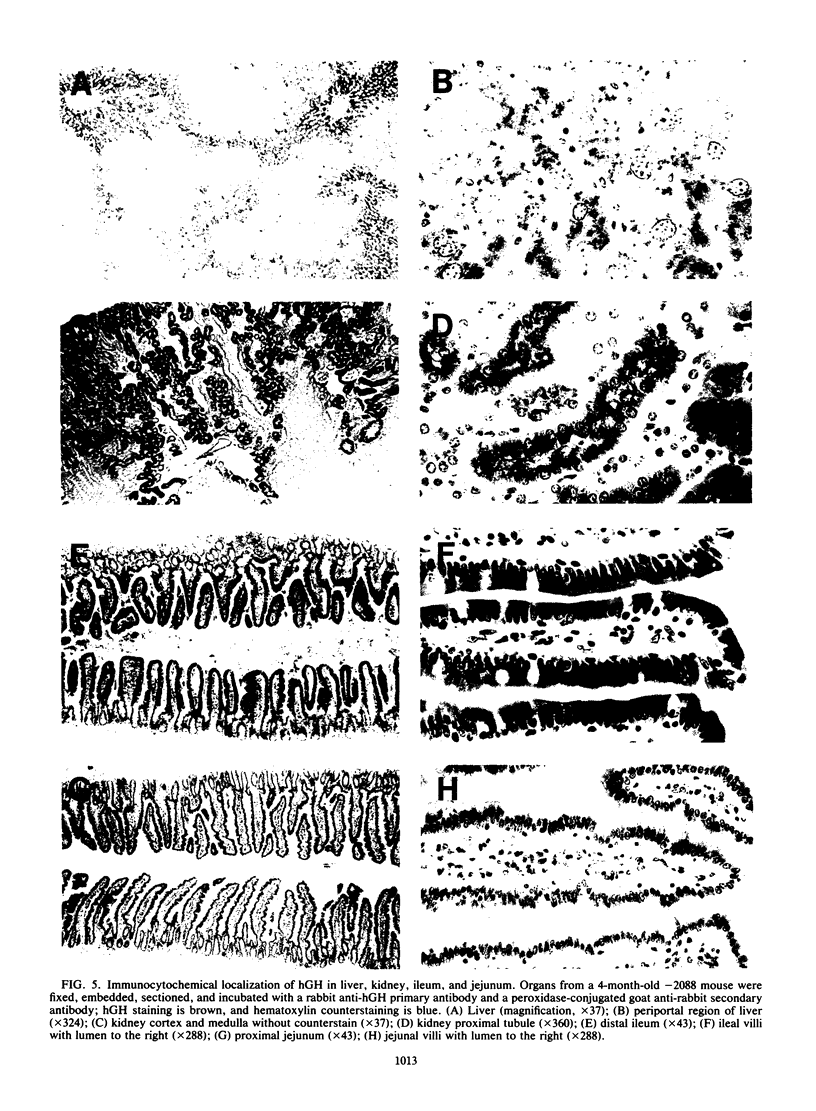
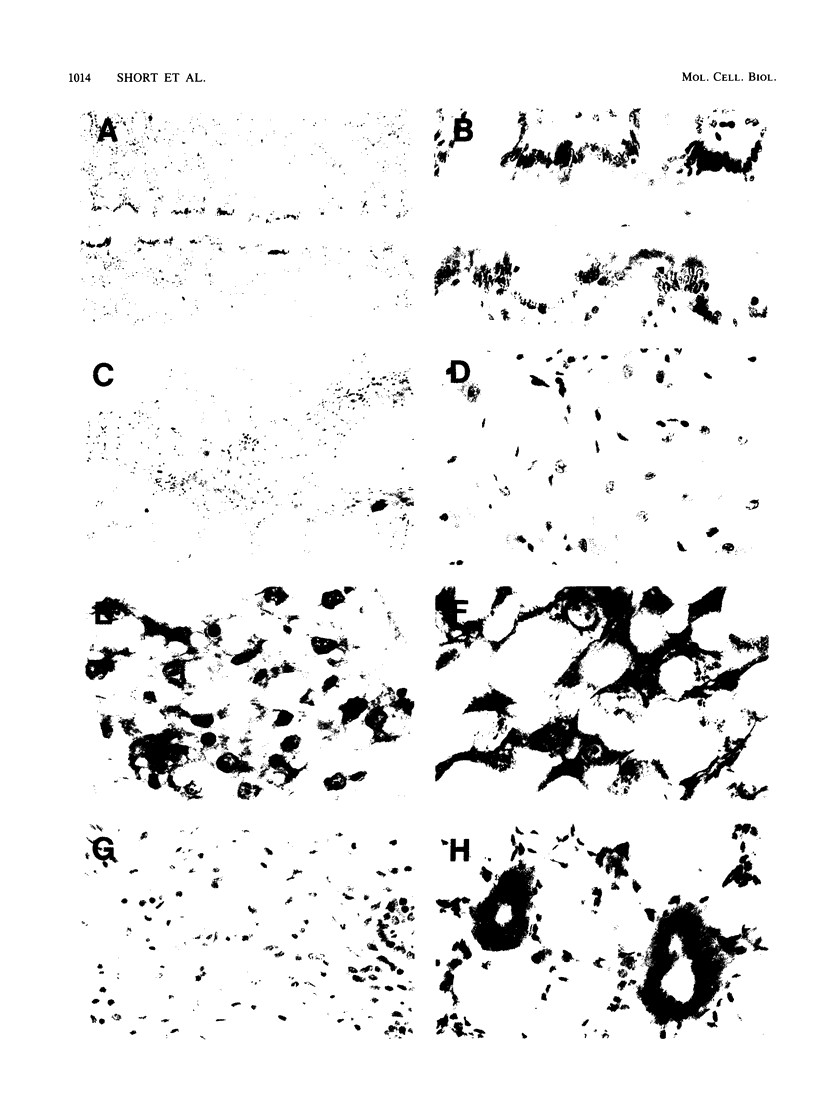
The cytosolic phosphoenolpyruvate carboxykinase (PEPCK) gene is expressed in multiple tissues and is regulated in a complex tissue-specific manner. To map the cis-acting DNA elements that direct this tissue-specific expression, we made transgenic mice containing truncated PEPCK-human growth hormone (hGH) fusion genes. The transgenes contained PEPCK promoter fragments with 5' endpoints at -2088, -888, -600, -402, and -207 bp, while the 3' endpoint was at +69 bp. Immunohistochemical analysis showed that the -2088 transgene was expressed in the correct cell types (hepatocytes, proximal tubular epithelium of the kidney, villar epithelium of the small intestine, epithelium of the colon, smooth muscle of the vagina and lungs, ductal epithelium of the sublingual gland, and white and brown adipocytes). Solution hybridization of hGH mRNA expressed from the transgenes indicated that white and brown fat-specific elements are located distally (-2088 to -888 bp) and that liver-, gut-, and kidney-specific elements are located proximally (-600 to +69 bp). However, elements outside of the region tested are necessary for the correct developmental pattern and level of PEPCK expression in kidney. Both the -2088 and -402 transgenes responded in a tissue-specific manner to dietary stimuli, and the -2088 transgene responded to glucocorticoid stimuli. Thus, different tissues utilize distinct cell-specific cis-acting elements to direct and regulate the PEPCK gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W. Pyruvate carboxylase and phosphoenolpyruvate carboxykinase in rat intestinal mucosa. Biochim Biophys Acta. 1970 Apr 14;208(1):165–167. doi: 10.1016/0304-4165(70)90066-8. [DOI] [PubMed] [Google Scholar]
- Bartels H., Herbort H., Jungermann K. Predominant periportal expression of the phosphoenolpyruvate carboxykinase and tyrosine aminotransferase genes in rat liver. Dynamics during the daily feeding rhythm and starvation-refeeding cycle demonstrated by in situ hybridization. Histochemistry. 1990;94(6):637–644. doi: 10.1007/BF00271991. [DOI] [PubMed] [Google Scholar]
- Bartke A., Steger R. W., Hodges S. L., Parkening T. A., Collins T. J., Yun J. S., Wagner T. E. Infertility in transgenic female mice with human growth hormone expression: evidence for luteal failure. J Exp Zool. 1988 Oct;248(1):121–124. doi: 10.1002/jez.1402480116. [DOI] [PubMed] [Google Scholar]
- Beale E. G., Chrapkiewicz N. B., Scoble H. A., Metz R. J., Quick D. P., Noble R. L., Donelson J. E., Biemann K., Granner D. K. Rat hepatic cytosolic phosphoenolpyruvate carboxykinase (GTP). Structures of the protein, messenger RNA, and gene. J Biol Chem. 1985 Sep 5;260(19):10748–10760. [PubMed] [Google Scholar]
- Beale E. G., Schaefer I. M., Li Q. A. Culture at high density increases phosphoenolpyruvate carboxykinase messenger RNA in H4IIEC3 hepatoma cells. Mol Endocrinol. 1991 May;5(5):661–669. doi: 10.1210/mend-5-5-661. [DOI] [PubMed] [Google Scholar]
- Benvenisty N., Nechushtan H., Cohen H., Reshef L. Separate cis-regulatory elements confer expression of phosphoenolpyruvate carboxykinase (GTP) gene in different cell lines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1118–1122. doi: 10.1073/pnas.86.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenisty N., Reshef L. Regulation of tissue- and development-specific gene expression in the liver. Biol Neonate. 1991;59(4):181–189. doi: 10.1159/000243341. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Braun R. E., Peschon J. J., Behringer R. R., Brinster R. L., Palmiter R. D. Protamine 3'-untranslated sequences regulate temporal translational control and subcellular localization of growth hormone in spermatids of transgenic mice. Genes Dev. 1989 Jun;3(6):793–802. doi: 10.1101/gad.3.6.793. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajek-Shaul T., Barash V., Weidenfeld J., Friedman G., Ziv E., Shohami E., Shiloni E. Lethal hypoglycemia and hypothermia induced by administration of low doses of tumor necrosis factor to adrenalectomized rats. Metabolism. 1990 Mar;39(3):242–250. doi: 10.1016/0026-0495(90)90042-b. [DOI] [PubMed] [Google Scholar]
- Chen C. W., Thomas C. A., Jr Recovery of DNA segments from agarose gels. Anal Biochem. 1980 Jan 15;101(2):339–341. doi: 10.1016/0003-2697(80)90197-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christ B., Nath A., Jungermann K. Mechanism of the inhibition by insulin of the glucagon-dependent activation of the phosphoenolpyruvate carboxykinase gene in rat hepatocyte cultures. Action on gene transcription, mRNA level and -stability as well as hysteresis effect. Biol Chem Hoppe Seyler. 1990 May;371(5):395–402. doi: 10.1515/bchm3.1990.371.1.395. [DOI] [PubMed] [Google Scholar]
- Chu D. T., Davis C. M., Chrapkiewicz N. B., Granner D. K. Reciprocal regulation of gene transcription by insulin. Inhibition of the phosphoenolpyruvate carboxykinase gene and stimulation of gene 33 in a single cell type. J Biol Chem. 1988 Sep 15;263(26):13007–13011. [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Weiss M., Darnell J. E., Jr Liver-specific RNA metabolism in hepatoma cells: variations in transcription rates and mRNA levels. Mol Cell Biol. 1985 Oct;5(10):2633–2641. doi: 10.1128/mcb.5.10.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Van Dyke T. A., Yan C., Kuo F., Darnell J. E., Jr Similarities in transthyretin gene expression and differences in transcription factors: liver and yolk sac compared to choroid plexus. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6589–6593. doi: 10.1073/pnas.87.17.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Flores H., Alleyne G. A. Phosphoenolpyruvate carboxykinase of kidney. Subcellular distribution and response to acid-base changes. Biochem J. 1971 Jun;123(1):35–39. doi: 10.1042/bj1230035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ruíz J. P., Lobato M. F., Ros M., Moreno F. J. Presence of cytosolic phosphoenolpyruvate carboxykinase activity in rat mammary gland. Enzyme. 1983;30(4):265–268. doi: 10.1159/000469587. [DOI] [PubMed] [Google Scholar]
- Girard J. Gluconeogenesis in late fetal and early neonatal life. Biol Neonate. 1986;50(5):237–258. doi: 10.1159/000242605. [DOI] [PubMed] [Google Scholar]
- Granner D. K., Sasaki K., Chu D. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. Ann N Y Acad Sci. 1986;478:175–190. doi: 10.1111/j.1749-6632.1986.tb15530.x. [DOI] [PubMed] [Google Scholar]
- Habener J. F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990 Aug;4(8):1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Krumlauf R., Camper S. A., Brinster R. L., Tilghman S. M. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science. 1987 Jan 2;235(4784):53–58. doi: 10.1126/science.2432657. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Maika S. D., Richardson J. A., Tang J. P., Taurog J. D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990 Nov 30;63(5):1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Palmiter R. D., Brinster R. L. Partial correction of murine hereditary growth disorder by germ-line incorporation of a new gene. Nature. 1984 Sep 6;311(5981):65–67. doi: 10.1038/311065a0. [DOI] [PubMed] [Google Scholar]
- Hanson R. W., Garber A. J. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am J Clin Nutr. 1972 Oct;25(10):1010–1021. doi: 10.1093/ajcn/25.10.1010. [DOI] [PubMed] [Google Scholar]
- Hardon E. M., Frain M., Paonessa G., Cortese R. Two distinct factors interact with the promoter regions of several liver-specific genes. EMBO J. 1988 Jun;7(6):1711–1719. doi: 10.1002/j.1460-2075.1988.tb03000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauft S. M., Sweetser D. A., Rotwein P. S., Lajara R., Hoppe P. C., Birkenmeier E. H., Gordon J. I. A transgenic mouse model that is useful for analyzing cellular and geographic differentiation of the intestine during fetal development. J Biol Chem. 1989 May 15;264(14):8419–8429. [PubMed] [Google Scholar]
- Hod Y., Hanson R. W. Cyclic AMP stabilizes the mRNA for phosphoenolpyruvate carboxykinase (GTP) against degradation. J Biol Chem. 1988 Jun 5;263(16):7747–7752. [PubMed] [Google Scholar]
- Idzerda R. L., Behringer R. R., Theisen M., Huggenvik J. I., McKnight G. S., Brinster R. L. Expression from the transferrin gene promoter in transgenic mice. Mol Cell Biol. 1989 Nov;9(11):5154–5162. doi: 10.1128/mcb.9.11.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai E., Stromstedt P. E., Quinn P. G., Carlstedt-Duke J., Gustafsson J. A., Granner D. K. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1990 Sep;10(9):4712–4719. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumara-Siri M. H., Surks M. I. Regulation of growth hormone mRNA synthesis by 3,5,3'-triiodo-L-thyronine in cultured growth hormone-producing rat pituitary tumor cells (GC cells). Dissociation between nuclear iodothyronine receptor concentration and growth hormone mRNA synthesis during the deoxyribonucleic acid synthesis phase of the cell cycle. J Biol Chem. 1985 Nov 25;260(27):14529–14537. [PubMed] [Google Scholar]
- Kuo C. J., Conley P. B., Hsieh C. L., Francke U., Crabtree G. R. Molecular cloning, functional expression, and chromosomal localization of mouse hepatocyte nuclear factor 1. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9838–9842. doi: 10.1073/pnas.87.24.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Leff T., Reue K., Melian A., Culver H., Breslow J. L. A regulatory element in the ApoCIII promoter that directs hepatic specific transcription binds to proteins in expressing and nonexpressing cell types. J Biol Chem. 1989 Sep 25;264(27):16132–16137. [PubMed] [Google Scholar]
- Loose D. S., Cameron D. K., Short H. P., Hanson R. W. Thyroid hormone regulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase (GTP) in rat liver. Biochemistry. 1985 Aug 13;24(17):4509–4512. doi: 10.1021/bi00338a004. [DOI] [PubMed] [Google Scholar]
- Lucas P. C., O'Brien R. M., Mitchell J. A., Davis C. M., Imai E., Forman B. M., Samuels H. H., Granner D. K. A retinoic acid response element is part of a pleiotropic domain in the phosphoenolpyruvate carboxykinase gene. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2184–2188. doi: 10.1073/pnas.88.6.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrane M. M., Yun J. S., Moorman A. F., Lamers W. H., Hendrick G. K., Arafah B. M., Park E. A., Wagner T. E., Hanson R. W. Metabolic effects of developmental, tissue-, and cell-specific expression of a chimeric phosphoenolpyruvate carboxykinase (GTP)/bovine growth hormone gene in transgenic mice. J Biol Chem. 1990 Dec 25;265(36):22371–22379. [PubMed] [Google Scholar]
- McGrane M. M., de Vente J., Yun J., Bloom J., Park E., Wynshaw-Boris A., Wagner T., Rottman F. M., Hanson R. W. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem. 1988 Aug 15;263(23):11443–11451. [PubMed] [Google Scholar]
- McKnight S. L., Lane M. D., Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989 Dec;3(12B):2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- Meisner H., Loose D. S., Hanson R. W. Effect of hormones on transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase (GTP) in rat kidney. Biochemistry. 1985 Jan 15;24(2):421–425. doi: 10.1021/bi00323a027. [DOI] [PubMed] [Google Scholar]
- Nechushtan H., Benvenisty N., Brandeis R., Reshef L. Glucocorticoids control phosphoenolpyruvate carboxykinase gene expression in a tissue specific manner. Nucleic Acids Res. 1987 Aug 25;15(16):6405–6417. doi: 10.1093/nar/15.16.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Lucas P. C., Forest C. D., Magnuson M. A., Granner D. K. Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription. Science. 1990 Aug 3;249(4968):533–537. doi: 10.1126/science.2166335. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L., Hammer R. E., Trumbauer M. E., Rosenfeld M. G., Birnberg N. C., Evans R. M. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982 Dec 16;300(5893):611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Norstedt G., Gelinas R. E., Hammer R. E., Brinster R. L. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983 Nov 18;222(4625):809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Park E. A., Roesler W. J., Liu J., Klemm D. J., Gurney A. L., Thatcher J. D., Shuman J., Friedman A., Hanson R. W. The role of the CCAAT/enhancer-binding protein in the transcriptional regulation of the gene for phosphoenolpyruvate carboxykinase (GTP). Mol Cell Biol. 1990 Dec;10(12):6264–6272. doi: 10.1128/mcb.10.12.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdereau D., Narkewicz M., Coupe C., Ferre P., Girard J. Hormonal control of specific gene expression in the rat liver during the suckling-weaning transition. Adv Enzyme Regul. 1990;30:91–108. doi: 10.1016/0065-2571(90)90011-p. [DOI] [PubMed] [Google Scholar]
- Petersen D. D., Koch S. R., Granner D. K. 3' noncoding region of phosphoenolpyruvate carboxykinase mRNA contains a glucocorticoid-responsive mRNA-stabilizing element. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7800–7804. doi: 10.1073/pnas.86.20.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987 May;1(3):268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- Quinn P. G., Wong T. W., Magnuson M. A., Shabb J. B., Granner D. K. Identification of basal and cyclic AMP regulatory elements in the promoter of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1988 Aug;8(8):3467–3475. doi: 10.1128/mcb.8.8.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef L., Hanson R. W., Ballard F. J. A possible physiological role for glyceroneogenesis in rat adipose tissue. J Biol Chem. 1970 Nov 25;245(22):5979–5984. [PubMed] [Google Scholar]
- Ross S. R., Graves R. A., Greenstein A., Platt K. A., Shyu H. L., Mellovitz B., Spiegelman B. M. A fat-specific enhancer is the primary determinant of gene expression for adipocyte P2 in vivo. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9590–9594. doi: 10.1073/pnas.87.24.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. L., Russell L. B., Gower J. S. EXCEPTIONAL INHERITANCE OF A SEX-LINKED GENE IN THE MOUSE EXPLAINED ON THE BASIS THAT THE X/O SEX-CHROMOSOME CONSTITUTION IS FEMALE. Proc Natl Acad Sci U S A. 1959 Apr;45(4):554–560. doi: 10.1073/pnas.45.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Schubart U. K. Regulation of gene expression in rat hepatocytes and hepatoma cells by insulin: quantitation of messenger ribonucleic acid's coding for tyrosine aminotransferase, tryptophan oxygenase, and phosphoenolpyruvate carboxykinase. Endocrinology. 1986 Oct;119(4):1741–1749. doi: 10.1210/endo-119-4-1741. [DOI] [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Wynshaw-Boris A., Short H. P., Hanson R. W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. II. Identification of cAMP and glucocorticoid regulatory domains. J Biol Chem. 1986 Jul 25;261(21):9721–9726. [PubMed] [Google Scholar]
- Sladek F. M., Zhong W. M., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990 Dec;4(12B):2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Swift G. H., Kruse F., MacDonald R. J., Hammer R. E. Differential requirements for cell-specific elastase I enhancer domains in transfected cells and transgenic mice. Genes Dev. 1989 May;3(5):687–696. doi: 10.1101/gad.3.5.687. [DOI] [PubMed] [Google Scholar]
- Trus M., Benvenisty N., Cohen H., Reshef L. Developmentally regulated interactions of liver nuclear factors with the rat phosphoenolpyruvate carboxykinase promoter. Mol Cell Biol. 1990 May;10(5):2418–2422. doi: 10.1128/mcb.10.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F., KURAHASHI K. Purification of oxalacetic carboxylase from chicken liver. J Biol Chem. 1954 Apr;207(2):787–802. [PubMed] [Google Scholar]
- Weber L. W., Lebofsky M., Greim H., Rozman K. Key enzymes of gluconeogenesis are dose-dependently reduced in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated rats. Arch Toxicol. 1991;65(2):119–123. doi: 10.1007/BF02034937. [DOI] [PubMed] [Google Scholar]
- Wynshaw-Boris A., Short J. M., Hanson R. W. Regulation of gene transcription by multiple hormones: organization of regulatory elements. Prog Nucleic Acid Res Mol Biol. 1987;34:59–87. doi: 10.1016/s0079-6603(08)60493-6. [DOI] [PubMed] [Google Scholar]
- Xanthopoulos K. G., Prezioso V. R., Chen W. S., Sladek F. M., Cortese R., Darnell J. E., Jr The different tissue transcription patterns of genes for HNF-1, C/EBP, HNF-3, and HNF-4, protein factors that govern liver-specific transcription. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3807–3811. doi: 10.1073/pnas.88.9.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala M. T., García-Ruíz J. P. Regulation of expression of the messenger ribonucleic acid encoding the cytosolic form of phosphoenolpyruvate carboxykinase in liver and small intestine of lactating rats. Endocrinology. 1989 Nov;125(5):2587–2593. doi: 10.1210/endo-125-5-2587. [DOI] [PubMed] [Google Scholar]
- Zimmer D. B., Magnuson M. A. Immunohistochemical localization of phosphoenolpyruvate carboxykinase in adult and developing mouse tissues. J Histochem Cytochem. 1990 Feb;38(2):171–178. doi: 10.1177/38.2.1688895. [DOI] [PubMed] [Google Scholar]