Summary
The GroEL/ES chaperonin system is required for the assisted folding of many proteins. How these substrate proteins are encapsulated within the GroEL-GroES cavity is poorly understood. Using symmetry-free, single-particle electron cryo-microscopy, we have characterized a chemically modified mutant of GroEL (EL43Py) that is trapped at a normally transient stage of substrate protein encapsulation. We show that the symmetric pattern of the GroEL subunits is broken as the GroEL cis-ring apical domains reorient to accommodate the simultaneous binding of GroES and an incompletely folded substrate protein (RuBisCO). The collapsed RuBisCO folding intermediate binds to the lower segment of two apical domains, as well as the normally unstructured GroEL C-terminal tails. A comparative structural analysis suggests that the allosteric transitions leading to substrate protein release and folding involves concerted shifts of GroES and the GroEL apical domains and C-terminal tails.
Introduction
Many essential proteins fold only when assisted by ATP-powered machines known as molecular chaperones (Hartl et al., 2011). The GroEL/ES system of E. coli is a well-studied example of the chaperonin class of molecular chaperones (Horwich and Fenton, 2009; Lin and Rye, 2006). GroEL is a tetradecamer of 57 kDa subunits, arranged as two stacked, seven-membered rings, each containing a large, solvent-filled cavity (Braig et al., 1994). The cavity-facing surface of the apical domain of each subunit is lined with hydrophobic amino acids that tightly bind substrates that are neither random coil, nor natively folded proteins (so-called non-native proteins; Fenton et al., 1994). Efficient folding of proteins that strictly depend on GroEL (so-called stringent substrate proteins) requires encapsulation of the non-native substrate protein within a cavity formed by GroEL plus the smaller, ring-shaped co-chaperonin GroES (Mayhew et al., 1996; Rye et al., 1997; Weissman et al., 1995; 1996). Encapsulation seals the GroEL cavity and results in the release of the substrate protein into an enlarged GroEL-GroES chamber (a cis complex). Upon release, folding is initiated and continues for a brief period, until the cavity is disassembled and the protein, folded or not, is ejected back into free solution (Mayhew et al., 1996; Todd et al., 1994; Weissman et al., 1995; 1996; 1994).
Encapsulation and initiation of folding ultimately depend upon ATP-driven structural rearrangements (Chen et al., 1994; Roseman et al., 1996; Rye et al., 1999). ATP binding to GroEL equatorial domains results in large, cooperative rearrangements, which elevate and rotate the apical domains (Burston et al., 1995; Xu et al., 1997; Yifrach and Horovitz, 1995). Exposed sites bind GroES, which results in a switch of the apical-domain surfaces from hydrophobic to polar (Xu et al., 1997), a switch believed to be essential for releasing substrate and triggering folding. While protein folding is initiated inside the GroEL-GroES cavity, the relatively short lifetime of this complex limits the amount of time a protein has to fold (Burston et al., 1995; Rye et al., 1999; Todd et al., 1994; Weissman et al., 1994). The timer for complex disassembly is set by the rate of ATP hydrolysis within the cis cavity, ranging from 4–20 sec, depending on temperature and the concentration of non-native substrate protein (Burston et al., 1995; Grason et al., 2008; Rye et al., 1999).
Recent structural work provides insight into how a non-native substrate protein is bound to an open GroEL ring and a view of a fully folded protein inside the GroEL cavity (Clare et al., 2009; Elad et al., 2007; Falke et al., 2005, Kanno et al., 2009), but structural information about non-native proteins during and immediately following encapsulation, the point at which folding is initiated, remains limited. In fact, the cooperative structural transitions of the GroEL ring that occur in response to ATP binding appear to create a paradox. Given that non-native substrate proteins and GroES are thought to bind to overlapping sites on the GroEL apical domains, how is it possible for ATP binding to drive a GroEL ring into a state with high affinity for GroES without causing premature release of the folding intermediate outside the chaperonin?
Binding competition between GroES and a substrate protein to the apical domains could be avoided if the ATP-bound GroEL ring populates an intermediate conformation that transiently binds both substrate protein and GroES (Cliff et al., 2006). Entry into and exit from such a state would require an orderly allosteric cascade designed to enforce a specific ligand binding sequence (Clare et al., 2012; Cliff et al., 2006; Madan et al., 2008; Ueno et al., 2004). The sequence begins with the non-native substrate protein binding to the open trans ring of an asymmetric GroEL-GroES complex with high affinity for the substrate protein, but without significant affinity for GroES (Figure 1A; Lin et al., 2008). Cooperative binding of ATP to the same GroEL ring is then thought to initiate encapsulation through a series of conformational states, which sequentially weaken the interaction between GroEL and the substrate protein, while simultaneously strengthening the interaction with GroES (Figure 1B–1F). While functional and kinetic studies strongly suggested the existence of such an allosteric cascade, because they are only transiently populated, the structural nature of these key intermediate states has remained poorly understood. A recent electron cryo-microscopy (cryo-EM) study of GroEL in the presence of ATP has begun to fill in some of these missing details, by successfully classifying several intermediate conformations of the GroEL apical domains (Clare et al., 2012). This study provides structural evidence for a sequential allosteric cascade, as well as insight into the intermediates populated by an ATP-bound GroEL ring prior to encapsulation. However, the absence of GroES and non-native substrate protein in these studies leaves unresolved the key structural transitions that lead to substrate protein encapsulation, release and folding.
Figure 1. The GroEL protein folding cycle involves a series of allosteric transitions within the chaperonin complex.
Non-native substrate proteins enter the GroEL reaction cycle by binding to the open trans ring of an asymmetric GroEL-GroES complex, pulling the trans ring into the high-affinity “T” state (A; for cycle details, see Cliff et al., 2006; Horwich and Fenton, 2009; Lin and Rye, 2006). Protein encapsulation is initiated by highly cooperative binding of ATP to the trans ring, populating the R1 state (B), a conformational state of the GroEL ring with high affinity for the non-native protein but not yet for GroES. The R2 state (C) retains substantial, though weakened, affinity for the non-native protein, binds GroES and encapsulates the substrate protein (D). Transitions into or between the R1 and R2 states are also linked to disassembly of the GroEL-GroES complex on the opposite ring. The ATP-bound GroEL-GroES complex has a high affinity for GroES in the R3 state (E), which releases the non-native substrate protein into the enclosed cis cavity, to initiate folding. Hydrolysis of ATP within the cis ring triggers a transition of the complex to at least one additional conformational state (F).
Here we show a non-native substrate protein trapped inside the GroEL-GroES cavity during encapsulation. We used cryo-EM and single-particle three-dimenstional (3D) reconstruction to determine the structure of a chemically modified GroEL mutant, which stalls in an allosteric state just prior to substrate protein release (the R2 state; (Madan et al., 2008)). Population of this normally transient state requires a break in the 7-fold rotational symmetry of the GroEL ring. As the GroES heptamer engages the GroEL ring to seal the cavity, the non-native protein contacts the lower segment of the GroEL apical domains. Strikingly, the normally unstructured C-terminal tails of the GroEL subunits extend up from the base of the cavity to make extensive contact with the non-native protein. Removal of the C-terminal tails results in an increase in premature substrate protein release prior to GroES binding. Efficient encapsulation thus requires an intermediate conformation of the GroEL-GroES complex that simultaneously binds both the non-native protein and GroES.
Results
Cryo-EM of a functionally trapped GroEL (EL43Py)-GroES complex
Previous work showed that the GroEL variant EL43Py is a potent tool for examining the linkage between substrate protein encapsulation, release and folding (Madan et al., 2008). EL43Py was created through homogeneous N-1-pyrene maleimide alkylation of a surface-exposed Cys residue engineered into a stem-loop at the bottom of the GroEL cavity. EL43Py encapsulates non-native substrate proteins beneath GroES, but only very slowly releases them into the GroEL-GroES cavity to initiate folding. The EL43Py variant thus provides an excellent opportunity to trap and structurally characterize a key conformation of the GroEL-GroES complex that is essential for substrate protein encapsulation, but which is normally highly transient (the GroES-bound R2 state of the GroEL ring; Figure 1D). In order to facilitate this study, we incorporated one additional modification into the EL43Py background, introducing a well-established mutation (D398A) that prevents ATP hydrolysis by GroEL, without affecting ATP or GroES binding (Rye et al., 1997). EL43Py398A stalls at the same point in the allosteric cycle as EL43Py (Figure S1A), but cannot hydrolyze ATP (data not shown). Using EL43Py398A and limiting amounts of ATP and GroES, we were thus able to create a chaperonin sample enriched in asymmetric EL43Py398A-GroES-ATP complexes (a so-called ATP bullet complex) with the cis cavity trapped in the R2 configuration. Cryo-EM was used to image this sample (Figure S1B), which contains multiple molecular species even when using an optimized mixing protocol, because the assembly reaction can never be driven to completion to yield a single, unique EL43Py398A-GroES-ATP bullet complex.
We applied a consecutive multiple-model refinement strategy, previously used to successfully analyze images of chaperonins with mixed conformations and compositions (Chen et al., 2006; 2008; Cong et al., 2012). The first round of processing of 71,200 particle images yielded three sub-populations of images that resulted in free GroEL tetradecamer (no GroES bound), bullet-shaped GroEL-GroES complexes (with GroES bound to only one end of the GroEL tetradecamer) and football-shaped GroEL-GroES2 complexes (with GroES bound to both ends of the GroEL tetradecamer). Because we were only interested in the structure of the bullet-shaped complex in the present study, we did not pursue a structural determination of the other sub-populations. The subset of images corresponding to the bullet-shaped complex was subjected to several additional rounds of multiple-model refinement to yield a final homogeneous dataset of 8,372 bullet-shaped particle images. This final data set was split into two halves for a gold standard resolution assessment (Scheres and Chen, 2012). A 3D structure of the bullet complex at ~ 8.9 Å was reconstructed using C7 symmetry (Figure 2A and Figure S1C) from all 8,372 highly selected particle images. A symmetry-free reconstruction from the same set of 8,372 highly selected particle images was also obtained at 13.9 Å resolution. Without a symmetry imposition, the subunits are not perfectly symmetrically arranged, but do not deviate far from 7-fold symmetry (data not shown). Because the symmetry-free map is close to C7 symmetry, and the symmetry imposition generated a higher resolution map, we employed the symmetry-imposed map for subsequent structural analysis.
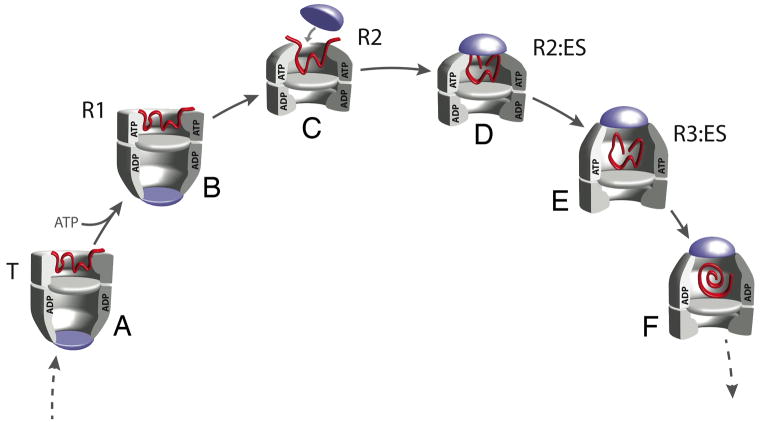
Figure 2. The structure of the EL43Py398A-GroES-ATP complex determined at 8.9 Å resolution by cryo-EM with C7 symmetry imposed.
(A) Side view of the EL43Py398A-GroES-ATP density map displayed at a contour level of 1.3 σ. Individual GroEL subunits are shown in different colors; GroES is magenta. All other density maps shown in this study are displayed at a contour level of 1.0 σ (unless otherwise noted). (B) Close-up view of a single EL43Py398A cis-ring subunit (contour level of 1.5 σ) overlapped with a rigid-body, flexibly refined fit of the GroEL-GroES-ADP crystal structure (PDB ID: 1AON; magenta) using the program DireX (Schröder et al., 2007). The stem loop containing Cys 43 and the GroEL C-terminus are labeled with arrows. (C) A medial slice of the density map shown in (A), with the density rendered transparent and superimposed on a rigid-body, flexibly refined fit of the GroEL-GroES-ADP crystal structure. In (C), extra density is visible at the tips of the equatorial stem loops of each GroEL subunit (amino acids 34–52; black dashed circles). The observed densities beyond amino acid 525 in the C-terminal tails are indicated by red arrows. (D) The additional stem-loop density for each subunit is shown (inside of black dashed circle), viewed from above, at a slice level indicated by the dashed blue line in (A). The seven stem loops are labeled 1–7, respectively. A single N-1-pyrene maleimide dye molecule (green) was rigid-body fit into the density at the tip of one stem loop in the EL43Py398A-GroES-ATP complex using Chimera. (E) View of the cis-ring equatorial domain near the subunit C-termini, viewed from above, at the slice level indicated by the black dashed line in (A). Substantial density (large red arrow) is visible in the region of the subunit C-termini, well beyond the last crystallographically resolved residue (small red arrow). The position of the GroEL subunit N-terminus is indicated by the blue arrow.
The structures of the wild type GroEL-GroES-ADP complex and the EL43Py398A-GroES-ATP complex bear substantial similarities at ~ 9 Å resolution, except in three locations. First, the position of the GroEL apical domains and the orientation of GroES are slightly shifted in the EL43Py398A-GroES-ATP complex (an R2-ES complex; see below). Secondly, substantial additional density protrudes into the chaperonin cavity from the end of a stem loop (amino acids 34 – 52) at the base of the EL43Py398A-GroES cis cavity (Figure 2B–2D and S1D). This density emanates in part from the expected attachment point of the pyrene dyes at position 43, which resides at the tip of the stem loop (Figure 2B). However, the density near this position is larger than can be accounted for by the dye molecule alone (Figure 2D). Thirdly, significant density rises up from the bottom of each GroEL subunit (large red arrow in Figures 2C, 2E and S1E), beyond the last crystallographically resolved residue at position 525, toward the dye attachment position. The location of this additional density is consistent with the normally flexible C-terminal tails of the GroEL subunits, which extend from residue 526 to the C-terminus (a total of 23 amino acids), rising from the bottom of the GroEL subunits and interacting with the pyrene dyes attached to the protruding stem loop (Figure 2C and 2D, Figure S1E). Additional density is also apparent in the trans ring of the complex (Figure S1E), though the density in this ring is somewhat more complex than that observed in the cis ring, and may suggest that the GroEL C-terminal tails in the trans ring make contact with both the pyrene dyes and the apical domains.
Visualizing an encapsulated non-native protein
We next examined the conformation of the EL43Py398A-GroES-ATP complex in the presence of a non-native substrate protein. To accomplish this goal, we modified our original preparation protocol to add the well characterized GroEL-dependent substrate protein RuBisCO (see Methods). In brief, EL43Py398A was first mixed with non-native RuBisCO to form a binary complex. The EL43Py398A-RuBisCO binary complex was then mixed with limiting ATP and GroES, which results in the formation of multiple species, including the bullet-shaped EL43Py398A-RuBisCO-GroES-ATP complex, both with and without non-native RuBisCO inside the cis chamber. After cryo-EM imaging (Figure S2A) and heterogeneity sorting of the particle images, we examined the first of two major sub-populations of bullet-shaped particle images, which were used to produce both symmetry-imposed and symmetry-free maps. The symmetry-imposed map for this RuBisCO-free subpopulation, which is devoid of density within either cis or trans cavities (Figures S2B–S2C) is very similar to that of the empty EL43Py398A-GroES-ATP complex (Figure 2A). Additionally, the unwrapped density in the symmetry-free reconstruction from the same set of RuBisCO-free particle images shows that the complex retains 7-fold symmetry (Figure S2D).
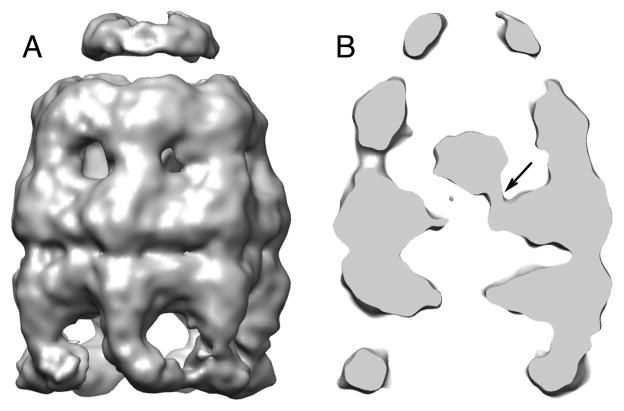
We examined the second major sub-population of bullet-shaped particle images to generate a 9.2 Å symmetry-free reconstruction of the bullet-shaped complex (Figure 3A and Figure S3A). Remarkably, this map displays strong density within the cis cavity, most likely from the non-native RuBisCO monomer trapped within the stalled R2 complex (gold in Figure 3A). The estimated mass of the visible RuBisCO monomer at a contour level of 1.0 σ is ~ 35 kDa, representing roughly 70% of the native RuBisCO monomer mass, assuming the central density comes from the RuBisCO alone. However, no regular secondary structural elements could be defined in the putative RuBisCO density either visually or quantitatively (based on SSEHunter; Baker et al., 2007), and no fragment of the RuBisCO crystal structure could be docked convincingly into the density.
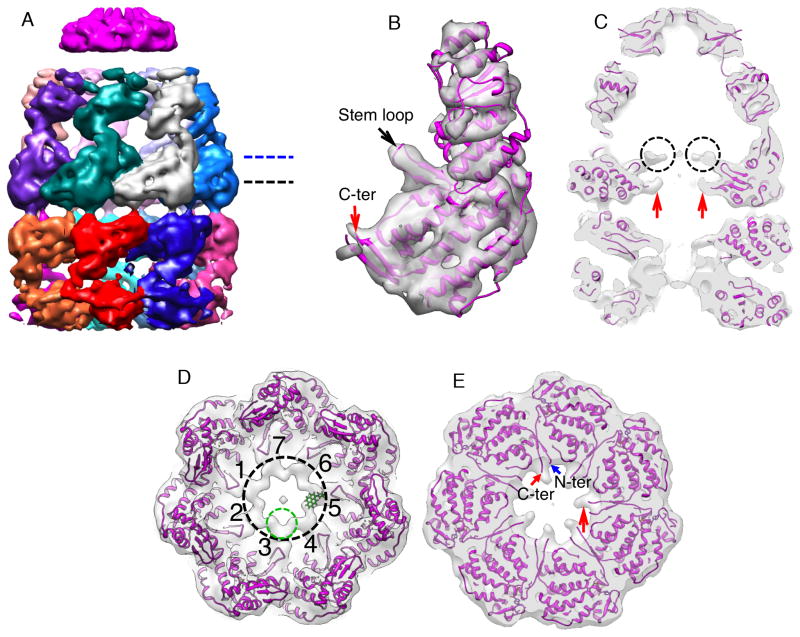
Figure 3. The structure of the EL43Py398A-GroES-ATP complex containing non-native RuBisCO within the cis cavity determined at 9.2 Å by cryo-EM without imposed symmetry.
(A) A side view of the density map of EL43Py398A-RuBisCO-GroES-ATP complex (contour level 1.23 σ) shown colored as in Figure 2, with density from the encapsulated, non-native RuBisCO monomer shown in gold. (B) A medial slice of the EL43Py398A-RuBisCO-GroES-ATP complex with the density rendered transparent and overlapped with a rigid-body, flexibly refined fit of the GroEL-ADP-GroES crystal structure (PDB ID: 1AON) to the cryo-EM map. Additional density around the GroEL equatorial domain stem loops makes direct contact with the non-native RuBisCO monomer (dashed black circles). The RuBisCO is also in contact with the lower region of the apical domain of one cis-ring GroEL subunit in the region of F281 (black arrow). (C) A close-up view of one cis-ring GroEL subunit in direct contact (long black arrow) with the non-native RuBisCO monomer (gold; contour level of 1.05 σ). The GroEL subunit stem loop (short black arrow) and C-terminus (red arrow) are indicated. (D) A medial slice of the variance map derived for the EL43Py398A-RuBisCO-GroES-ATP complex (red; see Experimental Procedures) is shown overlapped with the average map of the complex (gray; orientation as in panel B), calculated from 100 3D reconstructions of the complex computed during the variance calculations. The largest variations in the density map are from the non-native RuBisCO monomer and cavity-facing regions of the GroEL equatorial domains, most likely the C-termini of the cis and trans rings.
The presence of the non-native RuBisCO within the R2 cavity also alters the structure of the GroEL-GroES complex itself. The rotational symmetry of the EL43Py398A apical domains, on both the cis and trans rings, is broken in the presence of non-native RuBisCO (Figure 4A-4C and Figure S3B), with a gap appearing between the apical domains of two cis-ring neighboring subunits (Figure 4A and 4C). Interestingly, the point at which the cis ring appears to break 7-fold rotational symmetry coincides with direct physical interaction between the non-native RuBisCO monomer and the lower segment of the apical domains of two cis ring subunits (Figure 3B–3D; Figure 4C and 4D). The reliability of this connecting density is substantiated by its low variance in the 3D variance analysis from 100 reconstructed maps with different subsets of particle images (red in Figure 3D). The cis-ring equatorial domains also deviate slightly from C7 symmetry, which can be observed as differences in the separation of the equatorial domain helices between different subunits (Figure S3C).
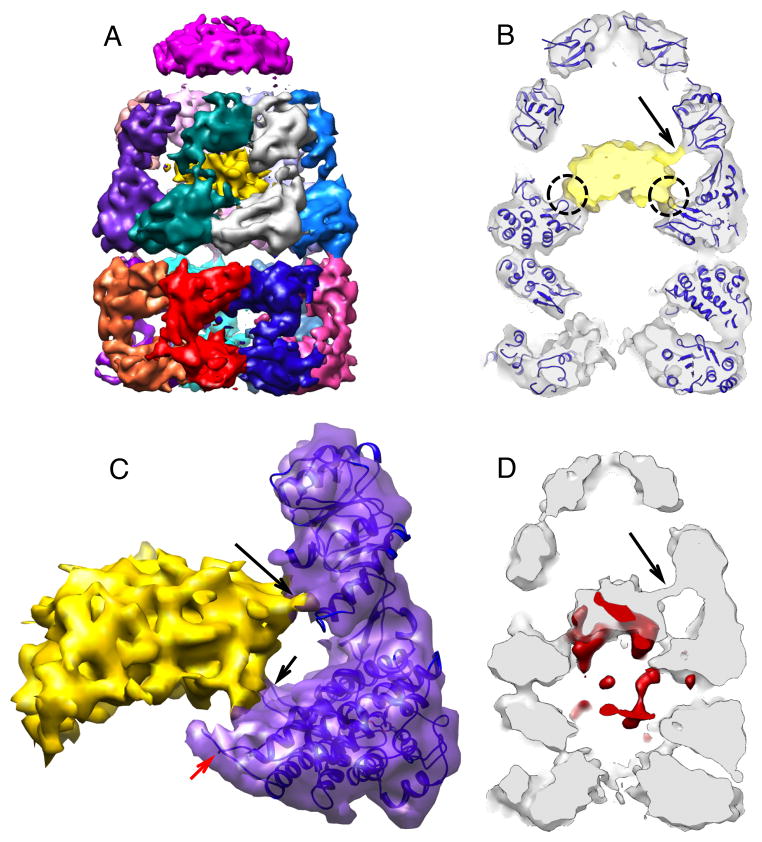
Figure 4. The C7 symmetry of the GroEL cis-ring is broken in the EL43Py398A-RuBisCO-GroES-ATP complex near points of contact between the non-native RuBisCO and the GroEL cavity wall.
(A) Cis-ring apical domains of the EL43Py398A-RuBisCO-GroES-ATP structure are shown as in Figure 3A, viewed from the top of the cis ring. A gap (black arrow) in the ring density is observed between subunit 2 (purple) and subunit 3 (dark cyan). (B) The cross correlation coefficient between the map of the cis-ring apical domains and a symmetric reference indicates subunit 4 is closer to subunit 3, which is approximately 9 degrees off its C7 symmetrical position, leaving a gap between subunits 3 and 2 (black arrow). (C) The gap (black arrow) between two neighboring GroEL subunits is shown in an unwrapped, planar display from the outside of the 9.2-Å density map of EL43Py398A-RuBisCO-GroES-ATP, as viewed from the side. (D) Top-view slice of the planar map, through the lower region of the cis ring apical domains (panel C, blue dashed line) shows interactions between the non-native RuBisCO monomer and the lower aspect of the GroEL apical domains of subunits 2 and 4 (blue circles). (E) Top-view slice of the planar map through the upper section of the equatorial domains (panel C, dashed black line) indicates contacts with the GroEL subunits near the stem-loop region of the equatorial domain. The isosurface threshold for (D and E) is 0.9σ.
A role for the GroEL C-terminal tails in protein encapsulation
A second striking feature of the R2 state revealed by the EL43Py398A-RuBisCO-GroES-ATP complex is a direct, physical contact between the non-native RuBisCO monomer and density from the base of the GroEL cavity wall (Figure 3B–3C; Figure 4E; Figure S3D). This interaction site is principally in the region of the dye-modified stem loops near amino acid 43, and some of this interaction is probably due to contact between the pyrene dyes and the non-native substrate protein (Figure S3D). However, in the absence of RuBisCO substantial density from the GroEL C-termini is also present in this region (Figure 2C and 2E; Figure S1E). Several studies have suggested that the C-terminal tails of the GroEL subunits play important, though poorly defined, roles in protein folding and regulation of the GroEL ATPase cycle (Farr et al., 2007; Machida et al., 2008; McLennan et al., 1993; Tang et al., 2006). Additionally, 3D variance analysis of the EL43Py398A-RuBisCO-GroES-ATP structure suggests a direct and heterogeneous interaction between the non-native RuBisCO and the C-terminal region of the GroEL subunits (Figure 3D). By far some of the largest 3D variance in the GroEL subunits in the EL43Py398A-RuBisCO-GroES-ATP complex appears around the equatorial domains, in regions near or containing the C-terminal tails of the GroEL subunits. While high variance regions (red in Figure 3D) are observed on both the cis and trans rings, in the cis ring these regions appear to be in intimate contact with the non-native substrate protein (Figure 3D). The putative RuBisCO density also displays high variance, suggesting that the non-native protein remains conformationally heterogeneous at this stage of the GroEL reaction cycle.
We next considered whether the observed contacts between the C-terminal tails and non-native RuBisCO require the presence of the pyrene dyes. Using a mixing protocol similar to that described above, we created a population of asymmetric complexes using the GroEL variant D398A (EL398A) that does not contain the pyrene dye. Following imaging (Figure S4A) and heterogeneity sorting, the bullet-shaped structure of the EL398A-RuBisCO-GroES-ATP complex with RuBisCO within the cis cavity was solved to 15.9 Å without imposing a symmetry constraint (Figure 5 and Figure S4B). Because the EL398A-GroES complex does not stall in the R2 state, but productively releases the substrate into the cis cavity and initiates folding, though it cannot hydrolyze its bound ATP (Rye et al., 1997), the EL398A-RuBisCO-GroES-ATP complex constitutes a substrate-occupied R3 state of the cis ring (Figure 1E).
Figure 5. The structure of the EL398A-GroES-ATP complex containing RuBisCO within the cis cavity determined at 15.9 Å by cryo-EM reconstruction without imposed symmetry.
(A) A side view of the EL398A-RuBisCO-GroES-ATP density map. (B) Medial slice of the EL398A-RuBisCO-GroES-ATP complex indicates direct contact between the folding RuBisCO monomer and the C-terminal and stem-loop region of one cis-ring GroEL subunit (black arrow).
Once again, a substantial amount of density from the RuBisCO monomer is visible within the cis cavity (Figure 5B). The apparent mass of the RuBisCO monomer in this complex appears to be less than that in the EL43Py398A-RuBisCO-GroES-ATP structure (Figure 3B and Figure S3D). This is likely due to the fact that the R3 cavity of the EL398A-GroES complex is fully folding active (Figure 1E), unlike the R2 cavity of the EL43Py398A-GroES complex (Figure 1D). Even though the EL398A complex was rapidly processed for cryo-EM freezing to prevent complete folding of the encapsulated RuBisCO, the initiation of folding in this complex could not be blocked at a specific step, as it is with EL43Py398A. The enclosed RuBisCO monomer will thus be a highly heterogeneous mix of both folded and non-native states, resulting in a lower resolution reconstruction. The C-terminal tails of the GroEL subunits are also not resolved, because the map represents a heterogeneous ensemble of interactions between the RuBisCO monomer and the C-terminal tails. Nonetheless, a significant contact between the base of the GroEL cavity wall, in the region of the C-terminal tails and stem loop and the RuBisCO monomer is apparent (Figure 5B). To further validate this structure, a completely independent reconstruction of this structure with a different initial model in which the cis cavity was empty (a low-passed X-ray structure of GroEL-GroES-ADP complex; PDB ID: 1AON) still converged well and displayed a very similar contact between the encapsulated RuBisCO and the GroEL C-terminal region and stem loops (Figure S4C). As expected for a released and folding competent RuBisCO monomer, this contact is less substantial than observed in the EL43Py398A complex (Figure 4E, Figure S3D and Figure 5B) and, more importantly, does not depend upon the presence of the pyrene dyes.
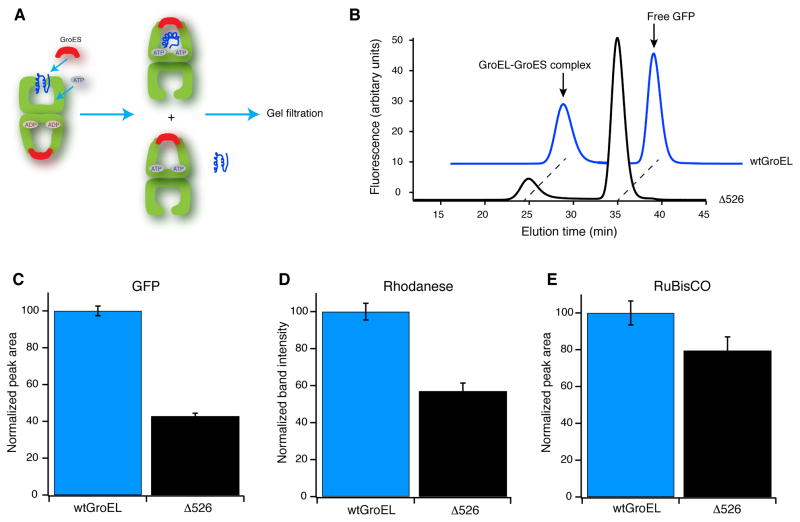
The contact between the GroEL C-terminal tails and non-native RuBisCO suggests that the C-termini play a direct and important role in ensuring efficient substrate protein encapsulation beneath GroES. In order to test this hypothesis, we generated a GroEL variant with a C-terminal truncation at the last crystallographically resolved residue (ELΔ526). A similar truncation has been previously observed to display both perturbed ATPase activity and a reduced ability to support folding of several model substrate proteins (Farr et al., 2007; Machida et al., 2008; Tang et al., 2006). We employed this GroEL variant in a gel filtration assay designed to score the efficiency of protein encapsulation (Figure 6A). When non-native GFP is bound to the trans ring of a wild-type GroEL-GroES-ADP bullet, approximately half of the initially bound protein is encapsulated inside a new cis cavity upon the addition of limiting ATP (Figure 6B). However, when the GroEL C-terminal tails are removed in the ELΔ526 variant, the efficiency of GFP encapsulation beneath GroES drops dramatically (Figure 6C). Similar results are obtained when the same experiment is conducted with both non-native rhodanese (Figure 6D) and RuBisCO (Figure 6E), though the drop in encapsulation efficiency is not as substantial.
Figure 6. Removal of the GroEL C-terminal tails results in premature substrate protein release and reduced encapsulation efficiency.
(A) Experimental schematic: non-native substrate protein (blue) is bound to the open trans ring of a GroEL ADP bullet complex in the presence of excess GroES. Encapsulation is initiated by the addition of ATP. ATP binding and turnover is limited to a single round by addition of hexokinase and glucose within 10 sec of ATP addition. Complexed and free substrate proteins are separated by gel filtration chromatography with an in-line fluorescence detector. (B) Example of an encapsulation experiment using GFP as the substrate protein. The positions of encapsulated GFP (GroEL-GroES complex) and released GFP (free GFP) are indicated with arrows, for both wild-type GroEL (wtGroEL) and the Δ526 truncation mutant (Δ526). Encapsulation is quantitated for three independent substrates: (C) GFP (normalized fluorescence peak area; n = 6), (D) rhodanese (normalized SDS-PAGE band intensity by densitometry; n = 4) and (E) RuBisCO (fluorescently labeled; n = 6). The reduction in encapsulation of non-native substrate protein by Δ526 GroEL relative to wtGroEL is robust: P = 6.5 ×10−9 for GFP, P = 0.0007 for rhodanese, and P = 0.0007 for RuBisCO (paired t-test; error bars are one standard deviation; see methods for additional details).
Structural changes in the GroEL-GroES complex between the R2 and R3 states
Following GroES binding and substrate protein encapsulation, the R2 state of the GroEL-GroES complex must then execute a shift to the R3 state, whereupon the substrate protein is released into the GroEL-GroES cavity and folding is triggered (Figure 1). In order to gain additional insight into this transition, we re-examined our cryo-EM data using strongly restrained flexible fitting of the atomic model of the GroEL-GroES-ADP complex (PDB ID: 1AON) into our cryo-EM maps (Figures 2A, 3A and 5A) with the program DireX (Schröder et al., 2007). The models generated from each flexible fitting analysis were then compared with each other in an attempt to isolate the conformational changes that lead from the R2 to the R3 state of the GroEL-GroES complex.
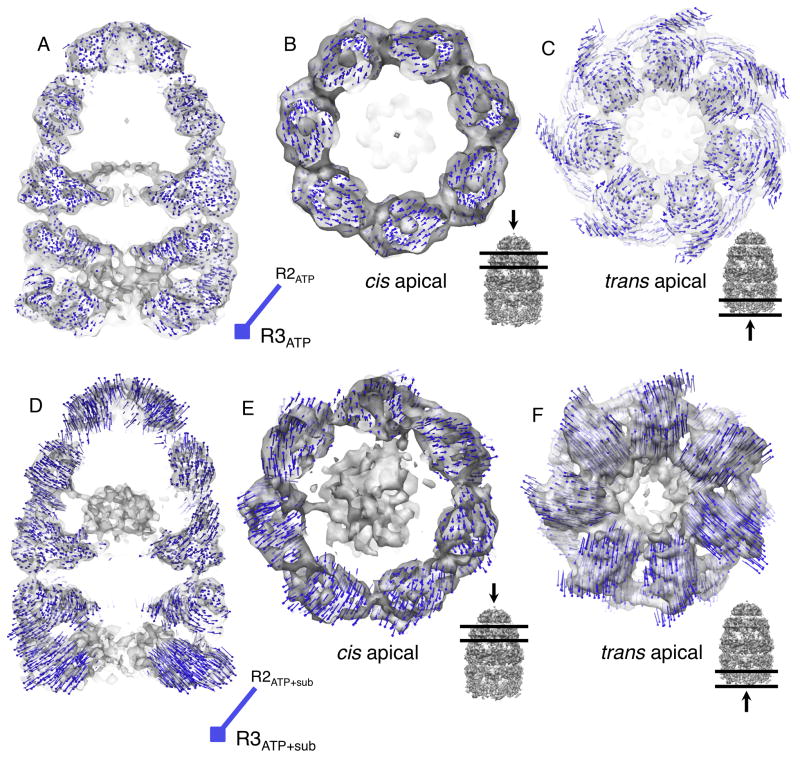
We first sought to identify structural differences between the R2 and R3 complexes that do not depend on the presence of the non-native substrate protein. This was accomplished by comparing the EL43Py398A-GroES-ATP complex, representing an empty R2 complex, to a previously described EL398A-GroES-ATP complex (Ranson et al., 2006), representing, in principle, an empty R3 complex (Figure 1E). Surprisingly, the conformational differences between these two complexes are relatively small. The cis ring apical domains appear to display slight counter-clockwise rotations (1–2 degrees) within the plane of the ring, but the overall position and elevation of the apical domains do not appear to change substantially (Figure 7A and 7B). Likewise, the equatorial domains show almost no movement at the current resolution (Figure 7A). The position and conformation of the GroES heptamer also appears mostly unchanged (Figure S5A). The lack of substantial conformational differences between these two complexes suggests that either (1) the conformation we observe for the EL43Py398A-GroES-ATP complex is further along the R2-to-R3 transition than expected or (2) that the detailed conformational properties of either the R2 or R3 state are not observable or stable in the absence of the non-native substrate protein.
Figure 7. The transition from the R2 to the R3 state in the presence RuBisCO involves large structural rearrangements of both the cis and trans rings.
(A–C)Atomic models of the GroEL-GroES complex (PDB ID: 1AON) were refined against density maps of the empty EL43Py398A-GroES-ATP (R2ATP; Figure 2A) and EL398A-GroES-ATP (R3ATP; (Ranson et al., 2006)) complexes. (D-F) Atomic models of the GroEL-GroES complex (PDB ID: 1AON) were refined against density maps of the EL43Py398A-RuBisCO-GroES-ATP complex (R2ATP + sub; Figure 3A) and the EL398A-RuBisCO-GroES-ATP (R3ATP + sub; Figure 5A). (A) Side view of the EL43Py398A-GroES-ATP density map: structural shifts associated with movement from an empty R2 complex to an empty R3 complex are illustrated with a field of difference vectors (blue lines and dots) to indicate the change in Cα-positions from R2 (start) to R3 (end; square). Vector lengths are scaled by a factor of 2 to improve visibility. (B) View of structural changes in the cis apical domains and (C) trans ring apical domains. (D) Side view of the EL43Py398A-RuBisCO-GroES-ATP density map: structural shifts indicating the differences between the substrate-occupied R2 and R3 complexes. (E) View of structural changes in the cis apical domains, and (F) the trans ring apical domains. For (B), (C), (E) and (F) the viewing direction and selected slice density are indicated by the black arrow and horizontal lines on the GroEL-GroES density map shown in the inset, to the lower right. In all cases, strongly restrained flexible model refinement was carried out with DireX. The designations R2 and R3 reference the functional allosteric states of the GroEL ring, illustrated in Figure 1.
To address these questions, we compared the empty R2 complex (EL43Py398A-GroES-ATP from Figure 2A) with the substrate protein occupied R2 complex (EL43Py398A-RuBisCO-GroES-ATP from Figure 3A). As shown in Figure S6, only slight differences between the two complexes are apparent, with the notable exception of the disruption of rotational symmetry and local structural shifts in the apical domains that make direct contact with the non-native substrate protein. These observations suggest that the global conformation of the R2 state of the EL43Py398A cis ring is stable in the presence of the substrate protein.
We next examined whether conformational differences between an R2 and R3 complex can be detected when the cis cavity is occupied by a substrate protein. Strikingly, the substrate-occupied R3 complex (EL398A-RuBisCO-GroES-ATP from Figure 5) shows substantial rearrangements compared to the substrate-occupied R2 complex (EL43Py398A-RuBisCO-GroES-ATP from Figure 3; see Figure 7D–7F). The cis apical domains of the substrate-occupied R3 ring display sizable outward tilts and elevations (Figure 7D–7E), increasing the cavity volume compared to the R2 ring, as well as shifting the position of the bound GroES heptamer upward (Figure 7D). This tilt and elevation are also associated with a small clockwise rotation of the apical domains within the plane of the ring (Figure 7E). The conformation of the bound GroES heptamer also changes, with the average position of the GroES subunits shifting outward in concert with the apical domains, resulting in a somewhat larger opening in the GroES dome orifice (Figure S5B). The equatorial domains display only small movements, which are most notable as an outward shift in the region near the C-termini (Figure 7D). The collective movements of the R3 cis ring thus appear poised to peel away the remaining contacts between the non-native substrate protein and the apical domains and C-terminal tails. Notably, both release events occur once GroES is already bound. The final elevation and rotation of the apical domains in the R3 state are likely responsible for locking GroES into its highest affinity state for the ATP-bound GroEL ring (Cliff et al., 2006; Rye et al., 1997).
Discussion
Conformational properties of an encapsulated folding intermediate
The structure of the EL43Py398A-RuBisCO-GroES-ATP complex provides the first view of a protein folding intermediate inside the GroEL-GroES cavity (Figure 3). While earlier studies have visualized non-native proteins bound to an open GroEL ring (Clare et al., 2009; Elad et al., 2007; Falke et al., 2005), as well as fully folded proteins inside the GroEL-GroES cavity (Clare et al., 2009; Kanno et al., 2009), the RuBisCO folding intermediate we describe here exists in transition between the two. Earlier work demonstrated that the RuBisCO monomer upon which GroEL operates is likely a middle- to late-stage protein folding intermediate (Lin and Rye, 2004; Lin et al., 2008; van der Vies et al., 1992), where the polypeptide chain has collapsed but is not as compact as the native state, and which possesses significant secondary structure, but poorly organized and highly heterogeneous tertiary structure. We examined the RuBisCO density in the EL43Py398A-RuBisCO-GroES-ATP complex for recognizable structural elements of native RuBisCO. Secondary structural elements should theoretically be identifiable in this structure, given the sub-nanometer resolution of the entire reconstruction. For example, helices in the equatorial domain of the GroEL subunits can be readily assigned (Figure S3C). However, our analysis failed to objectively identify any native state secondary structural elements. The lack of identifiable secondary structural elements in the putative RuBisCO region is consistent with the RuBisCO monomer populating a heterogeneous ensemble of collapsed and partially organized states (Figure 3A).
Coordinated action of the GroEL apical domains and C-termini
Our results suggest that direct contact between non-native substrate proteins and the C-terminal tails of the GroEL subunits helps prevent premature substrate protein escape during encapsulation beneath GroES. However, this interaction alone cannot fully explain efficient encapsulation on an R2 ring. Indeed, most of the non-native RuBisCO and rhodanese are still correctly captured in the absence of the C-termini (Figure 6D–6E), suggesting that contacts between the GroEL apical domains and non-native RuBisCO must also play an important role. The structure of the R2 cavity in the EL43Py398A-RuBisCO-GroES-ATP complex provides strong evidence for this mechanism. As shown in Figure 3 and Figure 4, the non-native RuBisCO makes direct, physical contact with the lower section of two cis apical domains in the region of Phe 281, a segment of the inner apical domain previously identified as important for substrate protein encapsulation and folding (Fenton et al., 1994). Simultaneous binding of the non-native substrate protein by both the C-terminal tails and the lower segment of the cis apical domains could thus provide a mechanism for retaining the non-native substrate protein while the GroEL ring shifts into the R2 state to permit loading of GroES.
How GroES makes initial contact with a GroEL ring already occupied by a large and bulky non-native substrate protein remains unclear. The earliest stages of the encapsulation reaction undoubtedly follow an ATP-driven elevation and movement of the GroEL apical domains, structural shifts that are capable of mechanically unfolding the bound substrate protein (Clare et al., 2012; Lin et al., 2008). However, only a subset of the apical domains must maintain contact with the non-native protein during the encapsulation reaction (Farr et al., 2000). This observation suggests that, in the earliest stages of contact between GroES and a substrate-occupied GroEL ring, apical domains not in direct contact with the substrate protein are the ones employed to initially capture GroES. Such a loading mechanism would likely require that the cooperative interactions between the apical domains in the R2 cis ring be relaxed or partially uncoupled, in order for different apical domains to bind to two distinct ligands in different positions. In support of this idea, we find that the substrate-occupied EL43Py398A-GroES complex breaks C7 rotational symmetry (Figure 4A–4C).
Effects of non-native substrate protein on inter ring allostery
While changes in the cis ring complex are essential for the progression of the GroEL folding cycle, the trans ring also plays a central role. Substrate proteins first enter the GroEL reaction cycle on the open trans ring of the asymmetric GroEL-GroES complex, and the nucleotide state of each ring directly influences the functional state of the other ring (Horovitz, 2001; Lin et al., 2008; Rye et al., 1997; Sparrer and Buchner, 1997). For example, the presence of ATP on one ring inhibits ATP binding to the other ring (negative cooperativity) and the presence of ADP on one ring, while permitting ATP to bind to the second ring, nonetheless non-competitively inhibits ATP hydrolysis on the other ring (Burston et al., 1995; Kad et al., 1998; Yifrach and Horovitz, 1995). These trans ring effects are thought to be essential for imposing the ring-ring asymmetry needed for the GroEL-GroES machine to function as a two-stroke motor (Burston et al., 1995; Frank et al., 2010; Kad et al., 1998; Rye et al., 1999; Yifrach and Horovitz, 2000). While the structural nature of this ring-ring allostery remains incompletely understood, our flexible fitting analysis of different GroEL-GroES complexes provides insight into structural changes imposed on the trans ring by the ligand status of the cis ring.
The occupancy of a cis ring R2 cavity by non-native RuBisCO appears to be communicated to the trans ring through substantial and asymmetric displacements of the trans ring apical domains (Figure 7C, 7F and Figure S3B). The apical domains of the R2 complex trans ring appear to be drawn inward, resulting in a smaller ring opening (Figure 7C and 7F). This change involves both counter-clockwise rotations and outward tilting of the trans ring apical domains (Figure 7C and 7F), resulting in a reordering the cavity-facing apical surface. Interestingly, the conformational shift of the trans ring is different in detail when RuBisCO is present in the cis cavity, with the magnitude of the apical domain movement in the trans ring being considerably larger, and the extent of domain rotation being much smaller (Figure 7C and 7F). The observed closing down of the trans ring opening in the R2 complex, both with and without non-native protein in the cis cavity, could provide a mechanism to prevent non-native substrate proteins from binding to the trans ring until the substrate protein inside the cis complex is committed to release and folding.
A model for substrate protein encapsulation, release and folding
The observations described here thus suggest a multi-step model for substrate protein encapsulation, release and folding. Initial capture of a non-native substrate protein on the apical face of a GroEL ring is accompanied by additional binding contacts between the substrate protein and the C-terminal tails of the GroEL subunits. Subsequent binding of ATP to the GroEL ring initiates the movement of the GroEL apical domains, weakening the interaction between the non-native substrate protein and the apical domains (Badcoe et al., 1991; Martin et al., 1991; Viitanen et al., 1991). Binding contacts between the non-native substrate protein and the C-terminal tails at the base of the cavity serve to reduce the probability of premature substrate protein escape as the apical domains move to accommodate GroES. Our structural analysis of the EL43Py398A complexes further suggests that population of the GroES acceptor state (the R2 state) requires an intermediate arrangement of the GroEL apical domains (Figure 7). The consequence of this altered apical position involves a shift in the binding position of the GroES heptamer and the simultaneous exposure of a partial binding surface for the non-native substrate protein at the bottom of the apical domains (Figure 7). A subsequent allosteric transition of the GroEL-GroES cavity to the R3 state of the ring then results in a shift of the apical domains to their high-affinity state for GroES, fully occluding the apical binding surface and ejecting the non-native substrate protein from the apical face (Cliff et al., 2006; Madan et al., 2008; Ueno et al., 2004). Coordinated movements in the C-terminal regions of the equatorial domains serve to draw the C-terminal tails away from the substrate protein, resulting in full release of the non-native substrate protein and the initiation of folding. However, this release from the C-termini does not appear to be total, as the C-terminal tails continue to make ongoing, though reduced, physical contacts with the substrate protein following release and the initiation of folding (Figure 5). Whether these ongoing contacts directly influence the folding of a substrate protein remains to be determined.
Experimental Procedures
Full details of the Experimental Procedures can be found in the Extended Experimental Procedures section of the Supplemental Information.
Proteins
Wild-type GroEL, EL398A, EL43C and EL43C398A, were expressed and purified as previously described (Lin et al., 2008). ELΔ526 was purified in the same manner as wild-type GroEL. GroES, RuBisCO and GFP were also expressed and purified as previously described (Lin and Rye, 2006; Lin et al., 2008; Rye et al., 1997; 1999). Bovine rhodanese was purchased from Sigma and purified as previously described (Madan et al., 2008; Weissman et al., 1994).
Labeling of proteins with fluorescent dyes
EL43C and EL43C398A were specifically labeled at Cys43 with the thiol-reactive dye N-1-pyrene maleimide (PM) to generate EL43Py and EL43Py398A, as previously described (Madan et al., 2008). Wild-type RuBisCO was fluorescently labeled at Cys58 with the thiol-reactive dye 5-iodoacetamidofluorescein (5IAF) to create 58F-RuBisCO as previously described (Lin and Rye, 2006; Lin et al., 2008; Rye et al., 1999). Both PM and 5IAF were obtained from Invitrogen (Molecular Probes; Eugene, OR) and were prepared fresh from dry powder in anhydrous DMF immediately prior to use. The extent and specificity of dye conjugation was confirmed as previously described (Lin and Rye, 2004; Lin et al., 2008; Rye, 2001). For 58F-RuBisCO, EL43Py and EL43Py398A, the labeling efficiency was confirmed to be 98–100% by at least two different methods of analysis (Madan et al., 2008; Rye, 2001).
Refolding, enzymatic and encapsulation assays
Encapsulation experiments were conducted in sample buffer: 50mM HEPES, pH 7.6, 15 mM Mg(OAc)2, 100 mM KOAc and 2 mM DTT. Substrate protein (58F-RuBisCO, GFP or rhodanese) was denatured in acid urea buffer for 30–60 minutes at room temperature (in the dark) to yield working stocks of dRub, dGFP, dRho. ADP bullet complexes (Lin et al., 2008) with either non-native GFP, rhodanese or RuBisCO bound to the trans ring were formed by adding dGFP, dRho or dRub to ADP bullets at a mixing stoichiometry of 1:1 in cold buffer. After 5 min at room temperature, ATP was added to permit GroES binding and protein encapsulation, then quenched by addition of hexokinase and glucose. The complex mixture was separated on a Superose 6 gel filtration column, equilibrated in sample buffer and supplemented with 50 μM ADP, connected to an inline fluorescence detector. For experiments with GFP and RuBisCO, captured protein was quantified using in-line fluorescence detection. For experiments with rhodanese, the GroEL-GroES peak was collected, and encapsulated rhodanese was quantified by SDS-PAGE and densitometry (see Extended Experimental Procedures).
Cryo-EM and data processing
The freezing of chaperonin samples for cryo-EM was done according to the standard method (see Extended Experimental Procedures). Electron images of frozen, hydrated specimens were recorded at 300 keV in either JEM3000SFF or JEM3200FSC electron cryo-microscopes on photographic films or CCD camera (see Extended Experimental Procedures). The digital images were pre-processed by boxing particle images and determining contrast transfer function parameters for each micrograph using EMAN1 program (Ludtke et al., 1999).
The methodology of EMAN1 multiple-model refinement (multirefine) for compositionally and conformationally heterogeneous complex analysis, which has been previously described (Chen et al., 2006), was used to sort different particle sub-populations from all the datasets (Figure S7A). Here several consecutive multiple-model refinements were applied to “purify” the relatively homogeneous bullet-shaped particle images of greatest interest for each of three complexes described in this study (See Extended Experimental Procedures). Each sub-population with “purified” particle images was subject to a single-model refinement (refine) to obtain a final converged bullet-shaped 3D reconstruction. The Euler angular distributions of particle images corresponding to each of the reconstructions (Figure 2A, 3A and 5A) are shown in Figures S7B-D, respectively.
The final resolutions for the refined structures were assessed using the gold-standard criterion of Fourier Shell Correlation (FSC) cut-off at 0.143 from two independent half-sets of data (Scheres and Chen, 2012) after the particle images were highly purified from our consecutive multiple-model refinement procedures. Chimera (Pettersen et al., 2004) was used for the surface representations of all the cryo-EM density maps. The 3D variance map of the EL43Py398A-RuBisCO-GroES-ATP complex was calculated using the EMAN1 program calculateMapVariance.py with the bootstrap technique implemented (Chen et al., 2008; Penczek et al., 2006).
Fitting of X-ray structure into cryo-EM density maps
To standardize the pixel size of the substrate occupied and empty density maps of the GroEL-GroES complexes, a number of density maps were generated with different pixel sizes between 2.0 and 2.2 Å because different electron microscopes and recording media were used. The X-ray structure of the GroEL-GroES-ADP complex (PDB ID: 1AON) was then refined against each of these density maps using DireX (Schröder et al., 2007). As the orientation of the equatorial domains in the density maps was very similar to the X-ray structure, we used those domains as reference regions for magnification calibration for each map. The RMSD between all equatorial domains of the fitted models and the X-ray structure of the GroEL-GroES-ADP complex was calculated and the optimal pixel size was chosen as the one that leads to the smallest RMSD value. The optimized pixel sizes for the three density maps of the EL43Py398A-GroES-ATP, EL43Py398A-RuBisCO-GroES-ATP and EL398A-RuBisCO-GroES-ATP complexes were 2.08, 2.04 and 2.12 Å, respectively.
The GroEL-GroES-ADP crystal structure (PDB ID: 1AON) was fitted as a rigid body into the density maps of the EL43Py398A-GroES-ATP, EL43Py398A-RuBisCO-GroES-ATP, EL398A-RuBisCO-GroES-ATP and EL398A-GroES-ATP complexes. This rigidly docked structure served as the starting point for our strongly restrained flexible fitting using the program DireX (Schröder et al., 2007).
Supplementary Material
Highlights.
Cryo-EM shows structure of GroEL-ES encapsulating a protein folding intermediate
GroEL C-terminal tails and apical domains hold substrate protein while GroES binds
The GroEL ring loses its 7-fold symmetry as substrate protein is encapsulated
Protein folding is initiated by a conformational shift within the GroEL-ES complex
Acknowledgments
This research has been supported by the National Institutes of Health grants (W.C. P41GM103832 and PN2EY016525; H.R. GM065421). We would like to thank Dr. Chavela Carr for comments on the manuscript and Dr. Steven J. Ludtke and Dr. Michael F. Schmid for helpful discussions.
Footnotes
Accession Numbers
The cryo-EM density maps for EL43Py398A-GroES-ATP complex, EL43Py398A-RuBisCO-GroES-ATP complex and EL398A-RuBisCO-GroES-ATP complex are deposited to the EMDB with accession numbers EMD-2325, EMD-2326 and EMD-2327, respectively. The fitted models are deposited in the PDB with accession numbers 3zpz, 3zq0 and 3zq1, respectively.
Supplemental Information includes Extended Experimental Procedures and seven figures, and can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badcoe IG, Smith CJ, Wood S, Halsall DJ, Holbrook JJ, Lund P, Clarke AR. Binding of a chaperonin to the folding intermediates of lactate dehydrogenase. Biochemistry. 1991;30:9195–9200. doi: 10.1021/bi00102a010. [DOI] [PubMed] [Google Scholar]
- Baker ML, Ju T, Chiu W. Identification of secondary structure elements in intermediate-resolution density maps. Structure. 2007;15:7–19. doi: 10.1016/j.str.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig K, Otwinowski Z, Hegde R, Boisvert D, Joachimiak A, Horwich A, Sigler P. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Burston SG, Ranson NA, Clarke AR. The origins and consequences of asymmetry in the chaperonin reaction cycle. J Mol Biol. 1995;249:138–152. doi: 10.1006/jmbi.1995.0285. [DOI] [PubMed] [Google Scholar]
- Chen D, Song J, Chuang D, Chiu W, Ludtke S. An expanded conformation of single-ring GroEL-GroES complex encapsulates an 86 kDa substrate. Structure. 2006;14:1711–1722. doi: 10.1016/j.str.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Chen DH, Luke K, Zhang J, Chiu W, Wittung-Stafshede P. Location and flexibility of the unique C-terminal tail of Aquifex aeolicus co-chaperonin protein 10 as derived by cryo-electron microscopy and biophysical techniques. J Mol Biol. 2008;381:707–717. doi: 10.1016/j.jmb.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Roseman A, Hunter A, Wood S, Burston S, Ranson N, Clarke A, Saibil H. Location of a folding protein and shape changes in GroEL-GroES complexes imaged by cryo-electron microscopy. Nature. 1994;371:261–264. doi: 10.1038/371261a0. [DOI] [PubMed] [Google Scholar]
- Clare D, Bakkes P, van Heerikhuizen H, van der Vies S, Saibil H. Chaperonin complex with a newly folded protein encapsulated in the folding chamber. Nature. 2009;457:107–110. doi: 10.1038/nature07479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare DK, Vasishtan D, Stagg S, Quispe J, Farr GW, Topf M, Horwich AL, Saibil HR. ATP-Triggered Conformational Changes Delineate Substrate-Binding and -Folding Mechanics of the GroEL Chaperonin. Cell. 2012 doi: 10.1016/j.cell.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff M, Limpkin C, Cameron A, Burston S, Clarke A. The GroE encapsulation mechanism: elucidation of steps in the capture of a protein substrate. J Biol Chem. 2006;281:21266–21275. doi: 10.1074/jbc.M601605200. [DOI] [PubMed] [Google Scholar]
- Cong Y, Schröder GF, Meyer AS, Jakana J, Ma B, Dougherty MT, Schmid MF, Reissmann S, Levitt M, Ludtke SL, et al. Symmetry-free cryo-EM structures of the chaperonin TRiC along its ATPase-driven conformational cycle. Embo J. 2012;31:720–730. doi: 10.1038/emboj.2011.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elad N, Farr G, Clare D, Orlova E, Horwich A, Saibil H. Topologies of a Substrate Protein Bound to the Chaperonin GroEL. Mol Cell. 2007;26:415–426. doi: 10.1016/j.molcel.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke S, Tama F, Brooks CL, III, Gogol EP, Fisher MT. The 13Å Structure of a Chaperonin GroEL Protein Substrate Complex by Cryo-electron Microscopy. Journal of Molecular Biology. 2005;348:219–230. doi: 10.1016/j.jmb.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Farr G, Fenton W, Horwich A. Perturbed ATPase activity and not “close confinement” of substrate in the cis cavity affects rates of folding by tail-multiplied GroEL. Proc Natl Acad Sci U S A. 2007;104:5342–5347. doi: 10.1073/pnas.0700820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr G, Furtak K, Rowland M, Ranson N, Saibil H, Kirchhausen T, Horwich A. Multivalent binding of nonnative substrate proteins by the chaperonin GroEL. Cell. 2000;100:561–73. doi: 10.1016/s0092-8674(00)80692-3. [DOI] [PubMed] [Google Scholar]
- Fenton W, Kashi Y, Furtak K, Horwich A. Residues in chaperonin GroEL required for polypeptide binding and release [see comments] Nature. 1994;371:614–619. doi: 10.1038/371614a0. [DOI] [PubMed] [Google Scholar]
- Frank G, Goomanovsky M, Davidi A, Ziv G, Horovitz A, Haran G. Out-of-equilibrium conformational cycling of GroEL under saturating ATP concentrations. Proc Natl Acad Sci U S A. 2010;107:6270–6274. doi: 10.1073/pnas.0910246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grason J, Gresham J, Lorimer G. Setting the chaperonin timer: A two-stroke, two-speed, protein machine. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0807418105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Horovitz A. Review: Allostery in Chaperonins. Journal of Structural Biology. 2001;135:104–114. doi: 10.1006/jsbi.2001.4377. [DOI] [PubMed] [Google Scholar]
- Horwich AL, Fenton WA. Chaperonin-mediated protein folding: using a central cavity to kinetically assist polypeptide chain folding. Q Rev Biophys. 2009;42:83–116. doi: 10.1017/S0033583509004764. [DOI] [PubMed] [Google Scholar]
- Kad NM, Ranson NA, Cliff MJ, Clarke AR. Asymmetry, commitment and inhibition in the GroE ATPase cycle impose alternating functions on the two GroEL rings. J Mol Biol. 1998;278:267–278. doi: 10.1006/jmbi.1998.1704. [DOI] [PubMed] [Google Scholar]
- Kanno R, Koike-Takeshita A, Yokoyama K, Taguchi H, Mitsuoka K. Cryo-EM structure of the native GroEL-GroES complex from thermus thermophilus encapsulating substrate inside the cavity. Structure. 2009;17:287–293. doi: 10.1016/j.str.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Lin Z, Rye H. GroEL-mediated protein folding: making the impossible, possible. Crit Rev Biochem Mol Bio. 2006;41:211–239. doi: 10.1080/10409230600760382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Rye HS. Expansion and compression of a protein folding intermediate by GroEL. Mol Cell. 2004;16:23–34. doi: 10.1016/j.molcel.2004.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Madan D, Rye HS. GroEL stimulates protein folding through forced unfolding. Nat Struct Mol Biol. 2008;15:303–311. doi: 10.1038/nsmb.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- Machida K, Kono-Okada A, Hongo K, Mizobata T, Kawata Y. Hydrophilic residues 526KNDAAD531 in the flexible C-terminal region of the chaperonin GroEL are critical for substrate protein folding within the central cavity. J Biol Chem. 2008 doi: 10.1074/jbc.M708002200. [DOI] [PubMed] [Google Scholar]
- Madan D, Lin Z, Rye HS. Triggering protein folding within the GroEL-GroES complex. J Biol Chem. 2008;283:32003–32013. doi: 10.1074/jbc.M802898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Langer T, Boteva R, Schramel A, Horwich AL, Hartl FU. Chaperonin-mediated protein folding at the surface of groEL through a “molten globule-” like intermediate. Nature. 1991;352:36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Mayhew M, da Silva A, Martin J, Erdjument-Bromage H, Tempst P, Hartl F. Protein folding in the central cavity of the GroEL-GroES chaperonin complex. Nature. 1996;379:420–426. doi: 10.1038/379420a0. [DOI] [PubMed] [Google Scholar]
- McLennan NF, Girshovich AS, Lissin NM, Charters Y, Masters M. The strongly conserved carboxyl-terminus glycine-methionine motif of the Escherichia coli GroEL chaperonin is dispensable. Mol Microbiol. 1993;7:49–58. doi: 10.1111/j.1365-2958.1993.tb01096.x. [DOI] [PubMed] [Google Scholar]
- Penczek PA, Yang C, Frank J, Spahn CMT. Estimation of variance in single-particle reconstruction using the bootstrap technique. J Struct Biol. 2006;154:168–183. doi: 10.1016/j.jsb.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Pettersen E, Goddard T, Huang C, Couch G, Greenblatt D, Meng E, Ferrin T. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Ranson N, Clare D, Farr G, Houldershaw D, Horwich A, Saibil H. Allosteric signaling of ATP hydrolysis in GroEL-GroES complexes. Nat Struct Mol Biol. 2006;13:147–152. doi: 10.1038/nsmb1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman AM, Chen S, White H, Braig K, Saibil HR. The chaperonin ATPase cycle: mechanism of allosteric switching and movements of substrate-binding domains in GroEL. Cell. 1996;87:241–251. doi: 10.1016/s0092-8674(00)81342-2. [DOI] [PubMed] [Google Scholar]
- Rye H. Application of fluorescence resonance energy transfer to the GroEL-GroES chaperonin reaction. Methods. 2001;24:278–288. doi: 10.1006/meth.2001.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye H, Burston S, Fenton W, Beechem J, Xu Z, Sigler P, Horwich A. Distinct actions of cis and trans ATP within the double ring of the chaperonin GroEL [see comments] Nature. 1997;388:792–798. doi: 10.1038/42047. [DOI] [PubMed] [Google Scholar]
- Rye H, Roseman A, Chen S, Furtak K, Fenton W, Saibil H, Horwich A. GroEL-GroES cycling: ATP and nonnative polypeptide direct alternation of folding-active rings. Cell. 1999;97:325–338. doi: 10.1016/s0092-8674(00)80742-4. [DOI] [PubMed] [Google Scholar]
- Scheres SHW, Chen S. Prevention of overfitting in cryo-EM structure determination. Nat Methods. 2012;9:853–854. doi: 10.1038/nmeth.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder GF, Brunger AT, Levitt M. Combining efficient conformational sampling with a deformable elastic network model facilitates structure refinement at low resolution. Structure. 2007;15:1630–1641. doi: 10.1016/j.str.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrer H, Buchner J. How GroES regulates binding of nonnative protein to GroEL. J Biol Chem. 1997;272:14080–14086. doi: 10.1074/jbc.272.22.14080. [DOI] [PubMed] [Google Scholar]
- Tang Y, Chang H, Roeben A, Wischnewski D, Wischnewski N, Kerner M, Hartl F, Hayer-Hartl M. Structural features of the GroEL-GroES nano-cage required for rapid folding of encapsulated protein. Cell. 2006;125:903–914. doi: 10.1016/j.cell.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Todd MJ, Viitanen PV, Lorimer GH. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science. 1994;265:659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- Ueno T, Taguchi H, Tadakuma H, Yoshida M, Funatsu T. GroEL mediates protein folding with a two successive timer mechanism. Mol Cell. 2004;14:423–434. doi: 10.1016/s1097-2765(04)00261-8. [DOI] [PubMed] [Google Scholar]
- van der Vies S, Viitanen P, Gatenby A, Lorimer G, Jaenicke R. Conformational states of ribulosebisphosphate carboxylase and their interaction with chaperonin 60. Biochemistry. 1992;31:3635–3644. doi: 10.1021/bi00129a012. [DOI] [PubMed] [Google Scholar]
- Viitanen P, Donaldson G, Lorimer G, Lubben T, Gatenby A. Complex interactions between the chaperonin 60 molecular chaperone and dihydrofolate reductase. Biochemistry. 1991;30:9716–9723. doi: 10.1021/bi00104a021. [DOI] [PubMed] [Google Scholar]
- Weissman J, Hohl C, Kovalenko O, Kashi Y, Chen S, Braig K, Saibil H, Fenton W, Horwich A. Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell. 1995;83:577–587. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Weissman J, Kashi Y, Fenton W, Horwich A. GroEL-mediated protein folding proceeds by multiple rounds of binding and release of nonnative forms. Cell. 1994;78:693–702. doi: 10.1016/0092-8674(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Weissman J, Rye H, Fenton W, Beechem J, Horwich A. Characterization of the active intermediate of a GroEL-GroES-mediated protein folding reaction. Cell. 1996;84:481–490. doi: 10.1016/s0092-8674(00)81293-3. [DOI] [PubMed] [Google Scholar]
- Xu Z, Horwich A, Sigler P. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex [see comments] Nature. 1997;388:741–750. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- Yifrach O, Horovitz A. Nested cooperativity in the ATPase activity of the oligomeric chaperonin GroEL. Biochemistry. 1995;34:5303–5308. doi: 10.1021/bi00016a001. [DOI] [PubMed] [Google Scholar]
- Yifrach O, Horovitz A. Coupling between protein folding and allostery in the GroE chaperonin system. Proc Natl Acad Sci U S A. 2000;97:1521–4. doi: 10.1073/pnas.040449997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.