Abstract
Purpose
To analyze the minimum inhibitory concentration (MIC) of isolates from fungal keratitis to natamycin and voriconazole, and to assess the relationship between organism, MIC, and clinical outcome.
Methods
Data were collected as part of a randomized, controlled, double-masked clinical trial. Main outcome measures included best spectacle-corrected visual acuity (BSCVA), infiltrate/scar size, time to re-epithelialization, and perforation. Speciation and analysis of MIC to natamycin and voriconazole was done according to NCCLS standards. The relationship between MIC and organism, organism and outcome measure, and each outcome measure and MIC was assessed.
Results
Of 120 samples obtained in the trial, 84 isolates had an identifiable organism and were available for further analyses. Fusarium spp and Aspergillus spp were the most commonly-isolated organisms. MIC was significantly different across the groups of organisms (P=0.0001). A higher MIC was significantly associated with an increased likelihood of perforation (OR 2.03, 95%CI 1.02 to 4.04, P=0.04). There was no significant association between MIC and 3-week visual acuity (0.058, 95%CI -0.01 to 0.13, P=0.11), 3-month visual acuity (0.01, 95%CI -0.08 to 1.04, P=0.79), 3-week infiltrate/scar size (0.12, 95% CI -0.02 to 0.27, P=0.10), 3-month infiltrate/scar size (0.12, 95%CI -0.02 to 0.25, P=0.09), or time to re-epithelialization (HR 1.19, 95%CI 0.98 to 1.45, P=0.08).
Conclusion
A higher MIC was associated with an increased odds of perforation. The results of this study suggest that resistance to antifungal medication may be associated with worse outcomes in fungal keratitis.
Keywords: fungus, keratitis, susceptibility, voriconazole, natamycin
INTRODUCTION
Fungal keratitis is an important cause of visual loss globally.1 Some locations report that as much as 50% of their corneal ulcers are fungal in etiology.2-5 Fungal corneal ulcers are difficult to treat and typically cause more severe vision impairment than bacterial corneal ulcers.6 Treatment of fungal corneal ulcers is largely empirical, and the role of susceptibility testing in guiding treatment decisions is unclear. An important question in the treatment of fungal corneal ulcers is therefore whether antifungal susceptibilities of fungal isolates correlate with clinical outcomes. Previous reports indicate that minimum inhibitory concentrations (MICs) may be correlated with clinical outcomes in ocular bacterial and fungal infections, however larger, prospective studies are required to confirm this.7-9
Triazoles, including voriconazole, have good in vitro activity against isolates from fungal corneal ulcers, but there are conflicting reports about their activity against Fusarium spp.10,11 In this study we report the in vitro activity of natamycin and voriconazole against clinical isolates collected as part of a randomized, controlled clinical trial12, and investigate how organism and MIC correlate with acuity, scar size, re-epithelialization time, and perforation.
METHODS
The Mycotic Ulcer Treatment Trial Therapeutic Exploratory Study was a randomized, controlled, double-masked clinical trial investigating whether natamycin or voriconazole, with or without repeated scraping, results in better visual outcomes three months after presentation. Specific methods for the trial have been described previously.12 Ethics approval was obtained from the University of California, San Francisco Committee on Human Research, Dartmouth Medical School, and Aravind Eye Care System—Madurai.
Main outcome measures for this study included best spectacle-corrected visual acuity at 3 weeks and 3 months from enrollment, infiltrate/scar size at 3 weeks and 3 months, time to re-epithelialization, and proportion of patients with a corneal perforation. Patients were followed every 3 days (+/- 1 day) until re-epithelialization. BSCVA was assessed at enrollment, 3 weeks, and 3 months by masked refractionists using an EDTRS chart. Infiltrate/scar size and epithelial defect size were measured as the longest dimension followed by the longest perpendicular to that dimension by masked examiners on slit lamp examination at each study visit. Re-epithelialization was defined as an epithelial defect <0.5 mm with administration of fluorescein. The depth of the infiltrate was assessed by slit lamp examination and was divided into one of four categories: no infiltrate/scar, >0-33%, >33-67%, or >67% depth. Perforation was defined as a corneal ulcer that extends through the full thickness of the cornea, extending into the anterior chamber. Specific signs of perforation, such as a flat anterior chamber, focal iris (especially an iris plugging a corneal hole), IOP ≤4 mmHg, and a positive Seidel’s test, were assessed at each study visit. All patients were assessed for adverse events, including corneal perforation, at each study visit. Patients with an existing perforation or impending perforation were excluded from the trial. Impending perforation was defined as presence of a descemetocoele. Prior to the start of the study, all observers and refractionists were trained in specific methods for the study and certified. Every tenth patient had a repeat examination performed by a second observer. All observers were masked to MIC measurements, as examinations and visual acuity measurements were performed prior to the availability of MIC results. Laboratory personnel were masked to examination and visual acuity results.
Microbiology
Corneal scrapings were obtained from all patients who were eligible for the trial, and Gram stains and potassium hydroxide (KOH) wet mounts were performed. Patients were considered for inclusion in the trial if they had a KOH wet mount positive for fungus and a Gram stain negative for bacteria. Scrapings were also inoculated onto sheep’s blood agar, chocolate agar, and either potato dextrose agar or Sabouraud’s agar for fungal and bacterial cultures. Fungal cultures were determined to be positive if there was growth on two or more media or if there was moderate to heavy growth on one medium. Fungal identification was performed using gross and microscopic characteristics, as previously described.5,13
Antifungal susceptibility testing for natamycin and voriconazole was performed on all samples that had a positive fungal culture according to standardized methods outlined in the Clinical and Laboratory Standards Institute document M38-A2.10,14 Minimum inhibitory concentration (MIC) was defined as the lowest concentration that exhibited a 100% visual reduction in turbidity when compared to the control tube for natamycin, and an 80% reduction in turbidity for voriconazole.14 Only natamycin and voriconazole were analyzed, as these were the treatments studies in the clinical trial. MIC50 and MIC90 were estimated as the median and 90th percentile (PERCENTILE function in Microsoft Excel; Microsoft Inc, Redmond, WA).
Statistical Analyses
A log2-transformation of MIC was used for all statistical models. The association between MIC and best-spectacle corrected visual acuity (BSCVA) at 3 weeks and 3 months was analyzed with linear regression controlling for baseline visual acuity and treatment arm. Infiltrate/scar size at 3 weeks and 3 months was analyzed with linear regression controlling for baseline scar size and treatment arm. Perforation was analyzed by logistic regression controlling for enrollment depth of the ulcer and treatment arm. Time to re-epithelialization was analyzed with a Cox proportional hazards model, controlling for baseline epithelial defect size, treatment assignment, and whether or not the patient had rescraping. Organism and MIC were analyzed with a one-way ANOVA. Organism and outcomes were analyzed with one-way ANOVA for continuous outcomes (BSCVA and infiltrate/scar size), log-rank test for time to re-epithelialization, and Fisher’s exact test for perforation. Means for continuous variables were compared with a t-test. All statistical analyses were conducted using Stata 10.0 (Stata Corporation, College Park, TX, USA). Isolates with more than one organism identified were excluded from the analysis (N=2).
RESULTS
Of the 120 patients enrolled in the trial, 101 (84%) had a positive fungal culture available for genotyping and analysis of minimum inhibitory concentration (MIC). Of these, 84 samples had an identifiable organism with more than one observation per organism and could be used for calculation of MIC50 and MIC90. Thirteen patients (16%) were on a topical antifungal at presentation: 9 were on topical natamycin (11%) and 4 were on topical fluconazole (5%). Of the 84 patients with identifiable organisms, 52 (62%) were judged to have an enrollment depth of 0-33%, 21 (25%) had enrollment depth of 33-67%, and 11 (13%) had an enrollment depth of greater than 67%. The most common genus of fungus was Fusarium (N=44), followed by Aspergillus (N=17) (Table 1). Because the samples were collected as part of a clinical trial, prospectively-collected clinical data was available for all isolates. While there were no statistically significant differences across groups of organism, Aspergillus spp had the worst visual acuity at 3 weeks and 3 months, with Aspergillus flavus having worse visual acuity at 3 months than Aspergillus fumigatus. Aspergillus spp also had the largest infiltrate/scar size at 3 months. Aspergillus fumigatus had the highest rate of perforations and the longest time to re-epithelialization (Table 1). Patients with isolates that did not grow on culture had significantly worse mean BSCVA than patients whose isolates did grow on culture at baseline (1.2 logMAR vs. 0.88 logMAR, P=0.02), three weeks (1.14 logMAR vs. 0.67 logMAR, P=0.01), and three months (0.82 logMAR vs. 0.49 logMAR, P=0.04).
Table 1.
Organism, MIC, and outcome
| Organism | N | Vori cona zole |
Natamycin | Voriconazole | 3 week BSCVA (95% CI)** |
3 month BSCVA (95% CI)** |
3 week infiltrate/ scar size (95% CI)** |
3 month infiltrate/ scar size (95% CI)** |
Perforation (%) |
Time to re- epithelialization, median, days (95% CI) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (μg/ml) |
MIC90 * (μg/ml) |
MIC50 (μg/ml) |
MIC90 * (μg/ml) |
|||||||||
|
Aspergillus
spp 1 |
17 | 7 | 32 | 32 | 1 | 1 | 0.80 (0.45, 1.15) |
0.68 (0.29, 1.06) |
4.50 (3.66, 5.33) |
4.57 (3.67, 5.47) |
4 (25%) | 20.5 (11.5, NE***) |
| A. flavus | 11 | 6 | 32 | 32 | 1 | 1 | 0.85 (0.43, 1.26) |
0.77 (0.31, 1.24) |
4.62 (3.52, 5.71) |
4.79 (3.62, 5.96) |
2 (18%) | 15 (4, NE) |
| A. fumigatus | 5 | 0 | 4 | 8 | 0.5 | 0.5 | 0.85 (0.43, 1.26) |
0.57 (−0.10, 1.24) |
4.65 (3.55, 5.74) |
4.55 (3.43, 5.67) |
2 (40%) | 20.5 (20.5, NE) |
| Fusarium spp | 44 | 23 | 4 | 8 | 4 | 16 | 0.66 (0.42, 0.89) |
0.40 (0.19, 0.61) |
4.20 (3.64, 4.75) |
4.00 (3.44, 4.57) |
7 (16%) | 14 (5, NE) |
| Bipolaris | 6 | 4 | 4 | 4 | 0.5 | 8 | 0.28 (0.07, 0.48) |
0.25 (0.09, 0.41) |
2.99 (1.74, 4.24) |
2.96 (1.93, 3.99) |
2 (33%) | 11.5 (4, NE) |
| Curvularia | 8 | 4 | 4 | 256 | 0.5 | 2 | 0.64 (0.20, 1.08) |
0.58 (0.03, 1.13) |
3.34 (2.74, 4.19) |
3.07 (2.42, 3.73) |
0 (0%) | 13.5 (4, NE) |
| Exserohilum | 4 | 0 | 4 | 4 | 1 | 2 | 0.66 (0.43, 0.90) |
0.51 (−0.47, 1.49) |
3.88 (2.31, 5.44) |
3.77 (1.57, 5.97) |
0 (0%) | 5 (4.5, NE) |
| Lasiodiplodia | 3 | 3 | 4 | 32 | 2 | 4 | 0.68 (−0.14, 1.51) |
0.70 (−0.13, 1.53) |
4.69 (3.62, 5.75) |
4.71 (3.58, 5.83) |
0 (0%) | 7 (7, NE) |
| Scedosporium | 2 | 1 | 8 | 8 | 0.5 | 0.5 | 0.10 **** |
0.0 | 2.86 | 2.86 | 0 (0%) | NE |
| P | 0.21 | <0.001 | <0.001 | 0.84 | 0.82 | 0.55 | 0.39 | 0.60 | 0.73 | |||
| Total | 84 | 42 | 4 | 32 | 2 | 8 | 0.65 (0.49, 0.81) |
0.48 (0.32, 0.63) |
4.07 (3.69, 4.45) |
3.96 (3.56, 4.35) |
13 (15%) | 15 (5, NE) |
There was one Aspergillus terreus organism isolated
Highest value for organisms with fewer than 9 observations
95% confidence intervals estimated by accelerated bootstrap, 1000 resamplings. Infiltrate-scar size is the geometric mean of the largest scar diameter and the largest perpendicular, in mm. BSCVA is logMAR.
NE: Not estimable
Only 1 observation at 3 weeks and 3 months, no 95% CI
Organism versus MIC
MIC50 and MIC90 for each genus of organism are listed in Table 1. The MIC50 for natamycin was equal to or higher than voriconazole for all organisms; the MIC90 for natamycin was higher for all organisms except Fusarium and Bipolaris spp (Figure 1). The MIC for voriconazole was lowest for Aspergillus spp. Organism and MIC were significantly different across groups of organisms (P=0.0001).
Figure 1.
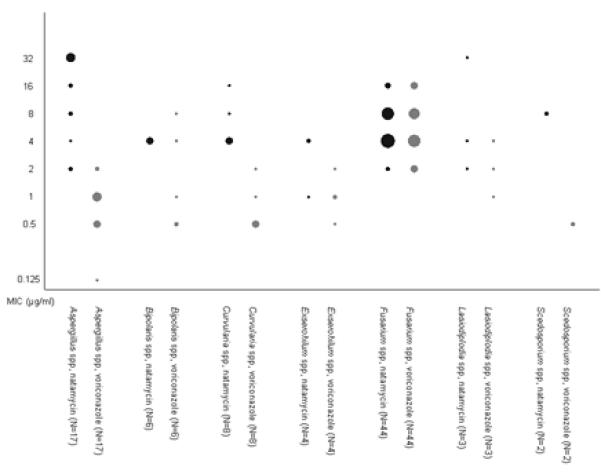
Results of suceptibility testing, by organism and antifungal. MICs to natamycin are represented by black points, and to voriconazole by grey points. The number of cases with a particular MIC is represented by the area of the point.
MIC versus Outcome
Analysis of the association between minimum inhibitory concentration and individual outcomes (BSCVA, infiltrate/scar size, perforation, and time to re-epithelialization) are listed in Table 2. Corneal perforation was the only outcome significantly associated with MIC, with isolates with higher MICs coming from corneal ulcers that were more likely to perforate (OR 2.03, 95%CI 1.02 to 4.04, P=0.04). The median MIC in patients who perforated was 8 μg/ml (interquartile range [IQR] 4 to 8 μg/ml), compared to a median MIC of 4 μg/ml (IQR 2 μg/ml to 8 μg/ml) in patients who did not perforate. Median time to perforation was 17 days (IQR 13 to 19 days).
Table 2.
Minimum inhibitory concentration predicting outcome
| Outcome | Estimated Effect (95% CI) |
P |
|---|---|---|
| 3-week visual acuity1 | 0.058 (−0.01 to 0.13) | 0.11 |
| 3-month visual acuity1 | 0.01 (−0.08 to 1.04) | 0.79 |
| 3-week infiltrate/scar size2 | 0.12 (−0.02 to 0.27) | 0.10 |
| 3-month infiltrate/scar size2 | 0.12 (−0.02 to 0.25) | 0.09 |
| Perforation3 | 2.035 (1.02 to 4.04) | 0.04 |
| Time to re-epithelialization4 | 1.196 (0.98 to 1.45) | 0.08 |
Linear regression with baseline visual acuity and drug as covariates
Linear regression with baseline scar size and drug as covariates
Logistic regression with enrollment depth and drug as covariates
Cox proportional hazards with baseline epithelial defect, drug and scraping as covariates
Odds ratio;
Hazards ratio
DISCUSSION
Fungal keratitis is an increasingly important cause of visual loss globally.6 In tropical climates such as South India, a large portion of cases of keratitis are due to fungus.4,5 While the incidence of fungal keratitis remains relatively low in the United States, the United Kingdom, and other temperate climates, there is some evidence that incidence may be increasing, particularly contact lens-related fungal keratitis.15,16 In this study, the majority of fungal pathogens isolated were Fusarium spp or Aspergillus spp. Previous studies in India have shown that Fusarium and Aspergillus are both common causative organisms of fungal keratitis.2,4,5,10 While fungal keratitis due to yeast may be more common in temperate climates, both Fusarium and Aspergillus are important organisms in filamentous fungal keratitis globally.3,15-20
Different fungal pathogens may result in a more or less severe course of disease. A previous study in South India showed that the genus Aspergillus was significantly associated with primary treatment failure.21 Similarly, in this group of patients, Aspergillus spp produced the worst visual acuity, as well as the largest infiltrate/scar size at 3 months, highest rate of perforation, and longest time to re-epithelialization, however these differences were not statistically significant. Of note, fewer patients with Aspergillus spp were randomized to receive voriconazole than natamycin. In vitro, voriconazole had a lower MIC against Aspergillus spp than natamycin, and appeared to have better activity against Aspergillus spp than any other organism. A larger sample size may be needed to determine if organism is significantly associated with outcomes, and how MIC mediates this association.
Minimum inhibitory concentrations were analyzed only for natamycin and voriconazole, as these were the treatments under study in the clinical trial in which the samples were collected.12 Absolute MICs for natamycin overall were higher for all organisms than MICs for voriconazole, with the exception of Fusarium spp, which had a higher MIC90 for voriconazole. In this study, Fusarium spp had a higher MIC90 for voriconazole than has been previously reported.10,11 These results are not adjusted for available dose, which may change dose-adjusted MICs, since natamycin is commercially available in a 5% concentration, while voriconazole is prepared at a 1% concentration. However, natamycin has been shown to have poor penetration through an intact epithelium, while voriconazole may have better penetration.22-24 These differences in dosage and penetration may change the available dose and affect the efficacy of the medication.
We noted a significant association between corneal perforation and higher MIC. Ulcers caused by organisms that had higher MICs were more likely to perforate. In systemic bacterial infections, in vitro susceptibility is thought to predict the response of bacterial infections according the “90-60 rule”, which states that susceptible organisms respond 90% of the time, and resistant organisms respond 60% of the time.25,26 More recently, a similar predictive utility has been suggested for systemic fungal infections, and susceptibility testing is an increasingly utilized tool for managing these infections.25 A previous study in South India, on a different set of cases, demonstrated that a lower MIC was significantly associated with a good outcome, defined as a healing time of less than three weeks.8 In systemic fungal infections, lower MICs have been shown to correlate with successful outcomes.25,27
In our study, it appeared that a lower MIC was correlated with a successful outcome (no perforation). Visual acuity, infiltrate/scar size and time to re-epithelialization appeared to be improved with lower MICs, however this difference was not significant. Organisms that are more susceptible to antifungal treatment may respond more quickly to these therapies, clearing the infection more quickly and allowing the ulcer to heal, thus reducing the time to re-epithelialization and decreasing the likelihood of perforation. In this study, we assessed how the ulcers responded during the course of treatment by controlling for baseline characteristics. The median time to perforation was 17 days, indicating that these perforations were occurring after several weeks of appropriate therapy. This study therefore suggests that MIC may play a role in whether or not an ulcer progresses to perforation. A previous study of bacterial corneal infections showed that lower MICs were associated with a smaller infiltrate/scar size at three months.7 Although cases with lower MICs tended to have lower infiltrate/scar sizes, this association was not statistically significant in this population. Many factors play a role in the healing of a corneal ulcer, in addition to the effectiveness of the antimicrobial. A larger sample size may be needed to demonstrate whether or not there is a true association between visual acuity and infiltrate/scar size and MIC.
Although 1% voriconazole (10,000 μg/ml) is released on the corneal surface, concentrations of voriconazole in the cornea are likely to be substantially lower than the concentration of drug as prepared. Aqueous concentrations of voriconazole have been found to be between 0.61 μg/ml and 6.94 μg/ml, depending on dosing schedules and administration.22,24,28 In this study, MIC50 for voriconazole was between 0.5 and 4 μg/ml depending on the organism, which is generally lower than these aqueous concentrations given our dosing schedule for the trial of 1 drop of voriconazole 1% every 1 hour while awake for 1 week, and then 1 drop every 2 hours while awake until 3 weeks from enrollment.12 The association seen between MIC and clinical outcome suggests that aqueous concentrations of drug were similar to what has previously been reported. Corneal penetration of natamycin is poor in the presence of an intact epithelium, although debridement is thought to increase its penetration.23 Voriconazole may penetrate deeper into the corneal stroma, thus achieving therapeutic concentrations more efficiently.28
Limitations of this study include a small sample size for many of the organisms identified. Making comparisons across groups of organisms is difficult when there are small numbers for some of the organisms identified. Secondly, the criterion for a fungal positive ulcer for enrollment in the trial was evidence of filamentous fungus on smear, and therefore not all of the patients had an identifiable organism that grew on culture. Patients with no growth on culture had significantly worse baseline, 3 week, and 3 month visual acuity as well as significantly larger 3 month infiltrate/scar size. It is impossible to know how non-culturable organisms might affect results. We did not collect detailed information on patient compliance to medications in the trial. It is unknown whether patients had different compliance patterns based on the drug they were randomized to, or the course of their ulcer. Any difference in compliance could potentially bias results. Finally, patients with an impending perforation were excluded from the trial. Clinical outcomes for these patients are not known, nor is the MIC to voriconazole or natamycin. However, this study investigated how MIC affected how ulcers responded during the course of treatment. Ulcers with impending perforation would not have added information for that particular outcome, and in fact may have biased the study, since these ulcers had not yet received treatment with either natamycin or voriconazole.
In this study, we describe the organisms collected in a fungal corneal ulcer clinical trial in South India and their susceptibilities to natamycin and voriconazole, the treatments investigated in the trial. MIC and organism were found to be significantly associated. In addition, we found that there is a higher likelihood of perforation in the ulcers with organisms that were more resistant to the antifungal they were treated with. While a larger sample size is needed to confirm these results, as well as to demonstrate if MIC is associated with visual acuity, infiltrate/scar size, and re-epithelialization time, our results suggest that high MIC may be associated with worse prognoses. This has implications for the potential role of MIC data in guiding therapeutic decisions in the management of mycotic keratitis. Our study suggests that resistant strains of fungus lead to poor outcomes in fungal keratitis, emphasizing the need for development and study of new antifungal strategies.
Acknowledgments
Alcon Inc. donated natamycin and Pfizer Inc. donated voriconazole for the study. None of the authors have any financial disclosures related to this manuscript. Funding for this research was from That Man May See and the South Asia Research Fund. The Department of Ophthalmology at UCSF is supported by a core grant from the National Eye Institute, EY02162. Dr. Acharya is supported by a National Eye Institute K23EY017897 grant and a Research to Prevent Blindness Career Development Award. Dr. Lietman is supported by a National Eye Institute grant U10-EY015114 and a Research to Prevent Blindness award. Dr. Porco is supported by That Man May See Foundation at UCSF. The sponsors did not have role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitcher J, Srinivasan M, Upadhyay M. Corneal blindness: a global perspective. Bulletin of the World Health Organization. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhary A, Singh K. Spectrum of fungal keratitis in north India. Cornea. 2005;24:8–15. doi: 10.1097/01.ico.0000126435.25751.20. [DOI] [PubMed] [Google Scholar]
- 3.Laspina F, Samudio M, Cibils D, et al. Epidemiological characteristics of microbiological results on patients with infectious corneal ulcers: a 13-year survey in Paraguay. Graefe’s Arch Clin Exp Ophthalmol. 2004;242:204–209. doi: 10.1007/s00417-003-0808-4. [DOI] [PubMed] [Google Scholar]
- 4.Leck A, Thomas P, Hagan M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan M, Gonzales C, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan M. Fungal keratitis. Current Opinion in Ophthalmology. 2004;15:321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Chen A, Prajna L, Srinivasan M, et al. Does in vitro susceptibility predict clinical outcome in bacterial keratitis? Am J Ophthalmol. 2008;145:409–412. doi: 10.1016/j.ajo.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro B, Lalitha P, Loh A, et al. Susceptibility testing and clinical outcome in fungal keratitis. Br J Ophthalmol. 2010;94:384–385. doi: 10.1136/bjo.2009.158675. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelmus K, Abshire R, Schlech B. Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitis. Arch Ophthalmol. 2003;121:1229–1233. doi: 10.1001/archopht.121.9.1229. [DOI] [PubMed] [Google Scholar]
- 10.Lalitha P, Shapiro B, Srinivasan M, et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125:789–793. doi: 10.1001/archopht.125.6.789. [DOI] [PubMed] [Google Scholar]
- 11.Marangon F, Miller D, Giaconi J, et al. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004;137:820–825. doi: 10.1016/j.ajo.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 12.Prajna N, Mascarenhas J, Krishnan T, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128(6):672–678. doi: 10.1001/archophthalmol.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larone D. Medically Important Fungi: A Guide to Identification. ASM Press; Washington, DC: 2002. [Google Scholar]
- 14.NCCLS . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard NCCLS document M38-A2. Clinical and Laboratory Standards Institute; Wayne, PA: 2002. [Google Scholar]
- 15.Iyer S, Tuli S, Wagoner R. Fungal keratitis: emerging trends and treatment outcomes. Eye & Contact Lens. 2006;32:267–271. doi: 10.1097/01.icl.0000249595.27520.2e. [DOI] [PubMed] [Google Scholar]
- 16.Jurkunas U, Behlau I, Colby K. Fungal keratitis: changing pathogens and risk factors. Cornea. 2009;28:638–643. doi: 10.1097/ICO.0b013e318191695b. [DOI] [PubMed] [Google Scholar]
- 17.Galarreta D, Tuft S, Ramsay A, et al. Fungal keratitis in London: microbiological and clinical evaluation. Cornea. 2007;26:1082–1086. doi: 10.1097/ICO.0b013e318142bff3. [DOI] [PubMed] [Google Scholar]
- 18.Green M, Apel A, Stapleton F. A longitudinal study of trends in keratitis in Australia. Cornea. 2008;27:33–39. doi: 10.1097/ICO.0b013e318156cb1f. [DOI] [PubMed] [Google Scholar]
- 19.Ritterband D, Seedor J, Shah M, et al. Fungal keratitis at the New York Eye and Ear Infirmary. Cornea. 2006;25:264–267. doi: 10.1097/01.ico.0000177423.77648.8d. [DOI] [PubMed] [Google Scholar]
- 20.Xie L, Zhong W, Shi W, et al. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113:1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Lalitha P, Prajna N, Kabra A, et al. Risk factors for treatment outcome in fungal keratitis. Ophthalmology. 2006;113:526–530. doi: 10.1016/j.ophtha.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 22.Lau D, Fedinands M, Leung L, et al. Penetration of voriconazole, 1%, eyedrops into human aqueous humor. Arch Ophthalmol. 2008;126:343–346. doi: 10.1001/archophthalmol.2007.71. [DOI] [PubMed] [Google Scholar]
- 23.O’Day D, Head W, Robinson R, et al. Corneal penetration of topical amphotericin B and natamycin. Curr Eye Res. 1986;5:877–882. doi: 10.3109/02713688609029240. [DOI] [PubMed] [Google Scholar]
- 24.Thiel M, Zinkernagel A, Burhenne J, et al. Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrobial Agents and Chemotherapy. 2007;51:239–244. doi: 10.1128/AAC.00762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rex J, Pfaller M. Has antifungal susceptibility testing come of age? CID. 2002;35:982–989. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein M, Reller L, Murphy J, et al. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Reviews of Infectious Diseases. 1983;5(1):35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- 27.Lass-Florl C, Kofler G, Kropshofer G, et al. In-vitro testing of susceptibility to amphotericin B is a reliable predicotr of clinical outcome in invasive aspergillosis. Journal of Antimicrobial Chemotherapy. 1998;42:497–502. doi: 10.1093/jac/42.4.497. [DOI] [PubMed] [Google Scholar]
- 28.Vemulakonda G, Hariparasad S, Mieler W, et al. Aqueous and vitreous concentrations following topical administration of 1% voriconazole in humans. Arch Ophthalmol. 2008;126:18–22. doi: 10.1001/archophthalmol.2007.8. [DOI] [PubMed] [Google Scholar]


