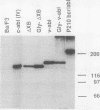
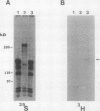
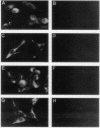
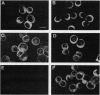
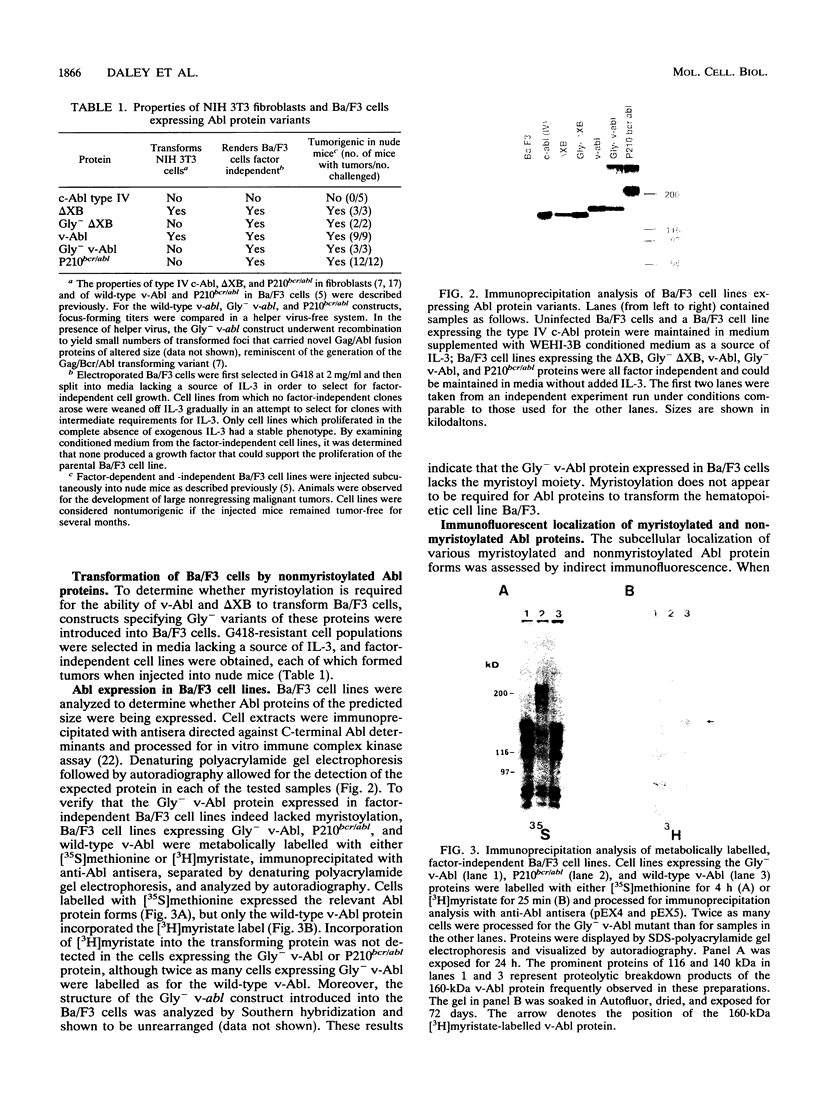
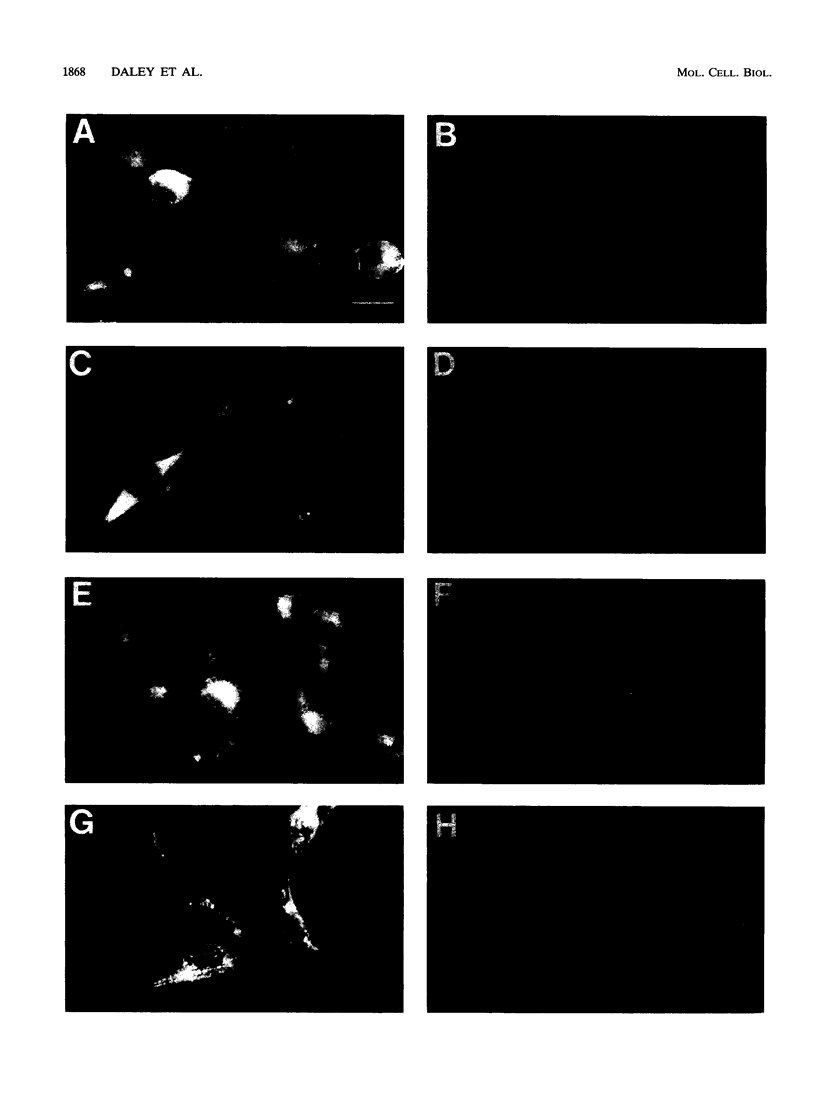
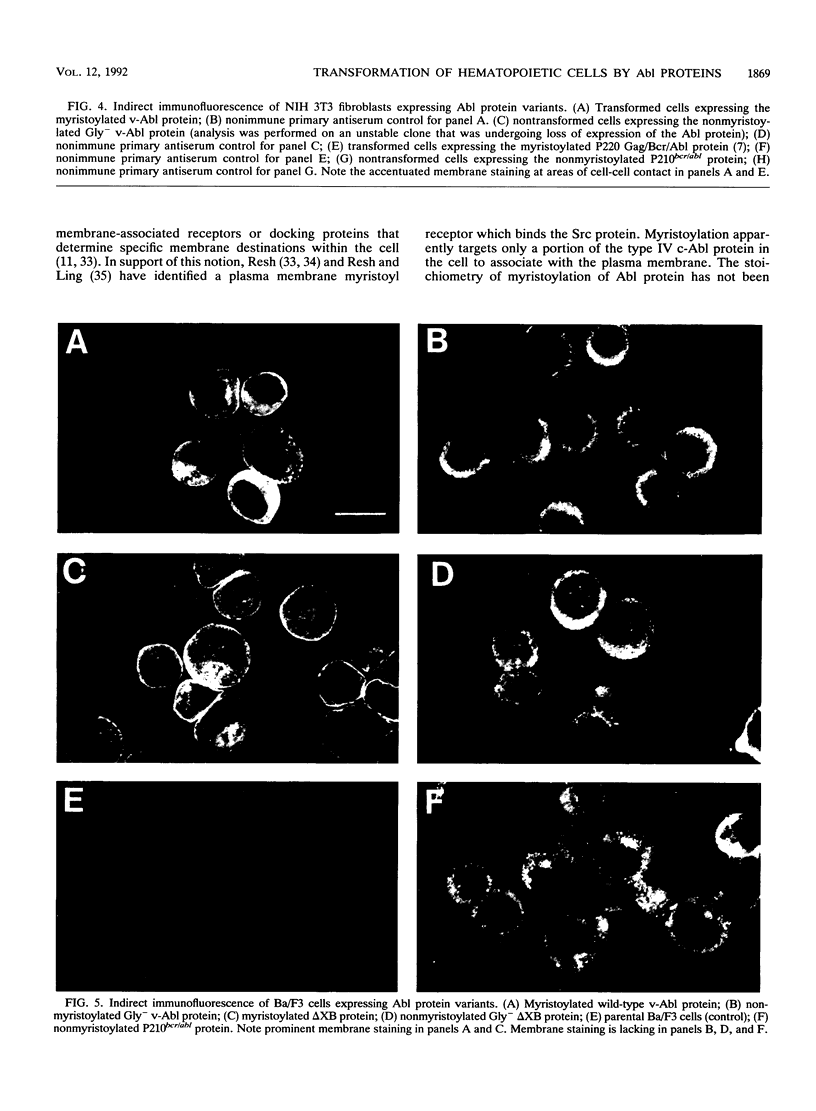
Abstract
N-terminal myristoylation can promote the association of proteins with the plasma membrane, a property that is required for oncogenic variants of Src and Abl to transform fibroblastic cell types. The P210bcr/abl protein of chronic myelogenous leukemia cells is not myristoylated and does not stably transform NIH 3T3 fibroblasts; however, it will transform lymphoid and myeloid cell types in vitro and in vivo, suggesting that myristoylation is not required for Abl variants to transform hematopoietic cells. To test this hypothesis, we introduced point mutations that disrupt myristoylation into two activated Abl proteins, v-Abl and a deletion mutant of c-Abl (delta XB), and examined their ability to transform an interleukin-3-dependent lymphoblastoid cell line, Ba/F3. Neither of the nonmyristoylated Abl proteins transformed NIH 3T3 fibroblasts, but like P210bcr/abl, both were capable of transforming the Ba/F3 cells to factor independence and tumorigenicity. Nonmyristoylated Abl variants did not associate with the plasma membrane in the transformed Ba/F3 cells. These results demonstrate that Abl proteins can transform hematopoietic cells in the absence of membrane association and suggest that distinct functions of Abl are required for transformation of fibroblast and hematopoietic cell types.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buss J. E., Der C. J., Solski P. A. The six amino-terminal amino acids of p60src are sufficient to cause myristylation of p21v-ras. Mol Cell Biol. 1988 Sep;8(9):3960–3963. doi: 10.1128/mcb.8.9.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calothy G., Laugier D., Cross F. R., Jove R., Hanafusa T., Hanafusa H. The membrane-binding domain and myristylation of p60v-src are not essential for stimulation of cell proliferation. J Virol. 1987 May;61(5):1678–1681. doi: 10.1128/jvi.61.5.1678-1681.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9312–9316. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Ben-Neriah Y. Implicating the bcr/abl gene in the pathogenesis of Philadelphia chromosome-positive human leukemia. Adv Cancer Res. 1991;57:151–184. doi: 10.1016/s0065-230x(08)60998-7. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., McLaughlin J., Witte O. N., Baltimore D. The CML-specific P210 bcr/abl protein, unlike v-abl, does not transform NIH/3T3 fibroblasts. Science. 1987 Jul 31;237(4814):532–535. doi: 10.1126/science.2440107. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Elefanty A. G., Hariharan I. K., Cory S. bcr-abl, the hallmark of chronic myeloid leukaemia in man, induces multiple haemopoietic neoplasms in mice. EMBO J. 1990 Apr;9(4):1069–1078. doi: 10.1002/j.1460-2075.1990.tb08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz W. M., Berger P., Wang J. Y. Deletion of an N-terminal regulatory domain of the c-abl tyrosine kinase activates its oncogenic potential. EMBO J. 1989 Jan;8(1):137–147. doi: 10.1002/j.1460-2075.1989.tb03358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard C., Arnold S. T., Felsted R. L. High affinity binding of an N-terminal myristoylated p60src peptide. J Biol Chem. 1989 Sep 15;264(26):15173–15176. [PubMed] [Google Scholar]
- Hamaguchi M., Matsuda M., Hanafusa H. A glycoprotein in the plasma membrane matrix as a major potential substrate of p60v-src. Mol Cell Biol. 1990 Feb;10(2):830–836. doi: 10.1128/mcb.10.2.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Hariharan I. K., Harris A. W., Crawford M., Abud H., Webb E., Cory S., Adams J. M. A bcr-v-abl oncogene induces lymphomas in transgenic mice. Mol Cell Biol. 1989 Jul;9(7):2798–2805. doi: 10.1128/mcb.9.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jackson P., Baltimore D. N-terminal mutations activate the leukemogenic potential of the myristoylated form of c-abl. EMBO J. 1989 Feb;8(2):449–456. doi: 10.1002/j.1460-2075.1989.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Rous sarcoma virus transforming protein lacking myristic acid phosphorylates known polypeptide substrates without inducing transformation. Cell. 1986 Apr 11;45(1):105–112. doi: 10.1016/0092-8674(86)90542-8. [DOI] [PubMed] [Google Scholar]
- Kaplan J. M., Varmus H. E., Bishop J. M. The src protein contains multiple domains for specific attachment to membranes. Mol Cell Biol. 1990 Mar;10(3):1000–1009. doi: 10.1128/mcb.10.3.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher M. A., McLaughlin J., Witte O. N., Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Detection of c-abl tyrosine kinase activity in vitro permits direct comparison of normal and altered abl gene products. Mol Cell Biol. 1985 Nov;5(11):3116–3123. doi: 10.1128/mcb.5.11.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo T. G., Pendergast A. M., Muller A. J., Witte O. N. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990 Mar 2;247(4946):1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- Lugo T. G., Witte O. N. The BCR-ABL oncogene transforms Rat-1 cells and cooperates with v-myc. Mol Cell Biol. 1989 Mar;9(3):1263–1270. doi: 10.1128/mcb.9.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Courtneidge S. A. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985 May;4(5):1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Nabel G., Palacios R., Baltimore D. Abelson virus abrogation of interleukin-3 dependence in a lymphoid cell line. Mol Cell Biol. 1986 Nov;6(11):4133–4135. doi: 10.1128/mcb.6.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter J. R., Wang J. Y. Activation of tyrosinase kinase and microfilament-binding functions of c-abl by bcr sequences in bcr/abl fusion proteins. Mol Cell Biol. 1991 Mar;11(3):1553–1565. doi: 10.1128/mcb.11.3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Spizz G. Fatty acylation of cellular proteins. Temporal and subcellular differences between palmitate and myristate acylation. J Biol Chem. 1986 Feb 15;261(5):2458–2466. [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. Fine structural mapping of a critical NH2-terminal region of p60src. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1623–1627. doi: 10.1073/pnas.82.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Livneh E., Ullrich A., Schlessinger J. Mutations in the cytoplasmic domain of EGF receptor affect EGF binding and receptor internalization. EMBO J. 1986 Sep;5(9):2179–2190. doi: 10.1002/j.1460-2075.1986.tb04482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Ling H. P. Identification of a 32K plasma membrane protein that binds to the myristylated amino-terminal sequence of p60v-src. Nature. 1990 Jul 5;346(6279):84–86. doi: 10.1038/346084a0. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Reconstitution of the Rous sarcoma virus transforming protein pp60v-src into phospholipid vesicles. Mol Cell Biol. 1988 May;8(5):1896–1905. doi: 10.1128/mcb.8.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D. Specific and saturable binding of pp60v-src to plasma membranes: evidence for a myristyl-src receptor. Cell. 1989 Jul 28;58(2):281–286. doi: 10.1016/0092-8674(89)90842-8. [DOI] [PubMed] [Google Scholar]
- Reynolds A. B., Roesel D. J., Kanner S. B., Parsons J. T. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989 Feb;9(2):629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Schultz A., Oroszlan S. Myristylation of gag-onc fusion proteins in mammalian transforming retroviruses. Virology. 1984 Mar;133(2):431–437. doi: 10.1016/0042-6822(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Purification and characterization of yeast myristoyl CoA:protein N-myristoyltransferase. Proc Natl Acad Sci U S A. 1987 May;84(9):2708–2712. doi: 10.1073/pnas.84.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Eubanks S. R., Towery D. S., Adams S. P., Glaser L. Amino-terminal processing of proteins by N-myristoylation. Substrate specificity of N-myristoyl transferase. J Biol Chem. 1987 Jan 25;262(3):1030–1036. [PubMed] [Google Scholar]
- Van Etten R. A., Jackson P., Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989 Aug 25;58(4):669–678. doi: 10.1016/0092-8674(89)90102-5. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Daley G. Q., Jackson P., Baltimore D., Cantley L. C. Activation of phosphatidylinositol 3-kinase in cells expressing abl oncogene variants. Mol Cell Biol. 1991 Feb;11(2):1107–1113. doi: 10.1128/mcb.11.2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C., Hu J. S., Olson E. N. Acylation of proteins with myristic acid occurs cotranslationally. Science. 1987 Nov 27;238(4831):1275–1278. doi: 10.1126/science.3685978. [DOI] [PubMed] [Google Scholar]
- Ziegler S. F., Whitlock C. A., Goff S. P., Gifford A., Witte O. N. Lethal effect of the Abelson murine leukemia virus transforming gene product. Cell. 1981 Dec;27(3 Pt 2):477–486. doi: 10.1016/0092-8674(81)90389-5. [DOI] [PubMed] [Google Scholar]