Abstract
Background
The prognostic value of Pseudomonas aeruginosa serology for antibiotic therapy in cystic fibrosis patients is not well understood.
Methods
Using five antigens from two ELISAs, we assessed whether positive serology in CF patients participating in the multi-center Early Pseudomonas Infection in Children (EPIC) trial would predict treatment failure, time to pulmonary exacerbation and risk for recurrent P. aeruginosa isolation post eradication.
Results
Baseline positive P. aeruginosa serology was not significantly associated with failure of initial P. aeruginosa eradication measured at week 10 (adjusted for baseline culture) but seropositivity to the antigens alkaline protease and exotoxin A was significantly associated with increased risk for recurrent P. aeruginosa isolation during the 60 week post eradication follow-up period (p=0.003 and p=0.001 respectively). There was no association between baseline seropositivity and time to pulmonary exacerbation.
Conclusion
P. aeruginosa serology may complement culture results in clinicians’ efforts to successfully monitor recurrence of early P. aeruginosa in CF patients.
Keywords: Pseudomonas, Serology, Treatment, Infection, EPIC
1. Introduction
Pseudomonas (P.) aeruginosa remains the major pathogen in patients with cystic fibrosis (CF). However, studies using several antibiotic regimens have demonstrated that eradication of the pathogen from the patients’ airways is possible when therapy is initiated in the acute phase of infection early after isolation. This strategy has been associated with considerably delaying the onset of chronic pulmonary P. aeruginosa infection in CF patients, decreasing the progression of lung disease, and reducing the cost of antibiotic intervention [1, 2]. A prerequisite for applying this therapeutic strategy is close monitoring of P. aeruginosa isolation in CF subjects based on the assumption that the therapeutic window is small and the switch of non-mucoid P. aeruginosa to biofilm formation would render eradication of the pathogen difficult or impossible [3]. Detection is generally accomplished by, oropharyngeal (OP) cough swabs, induced sputum or bronchoalveolar lavage (BAL) specimens [4, 5]. In the US, the standard of care for detection of initial P. aeruginosa infection in pre-expectorating patients is to collect routine OP cultures at least every 3 months [6]; Furthermore, while OP cultures are easy to perform on a frequent basis, they appear to only moderately reflect lower airway infection [7]. Serological tests have been used to diagnose P. aeruginosa, particularly in patients who do not produce sputum (reviewed in [3, 8]). However, this method has not been unequivocally accepted due in part to differences in patient population, study design, and cut-off antibody titers among various studies [9–12].
P. aeruginosa serology has been demonstrated to be an adjunct method for assessment of early eradication therapy [2, 8, 13], because bacterial culturing, particularly from nasopharyngeal aspirates and OP cough swab specimens, is subject to sampling error, and imperfect diagnostic accuracy [7]. Highly sensitive ELISAs for detection of serum antibodies against P. aeruginosa antigens in immunocompetent CF patients may complement less than perfect surveillance methods such as OP swabs. Several assay systems have been developed, for different P. aeruginosa antigens such as P. aeruginosa whole cell proteins and exotoxin A [14, 15], exotoxin A, elastase and alkaline protease [2, 8, 13, 16], and components of the type III secretion system [17, 18].
Elevated antibodies among young subjects at the time of initial P. aeruginosa positive OP culture may be a useful tool for CF clinicians and researchers monitoring patients at risk for subsequent infection. Here we demonstrate the relationship between culture and serologic data available in subjects with newly acquired P. aeruginosa infection participating in the multi-center Early Pseudomonas Infection in Children (EPIC) trial [19] and determine if baseline P. aeruginosa serology would predict treatment failure, time to pulmonary exacerbation and risk for re-isolation of P. aeruginosa post eradication.
2. Methods
2.1. Subjects and serologic analysis
Subjects with CF, recruited for the EPIC Clinical trial, were 1–12 years of age and had P. aeruginosa isolated within six months prior to study entry [19]. The study consisted of an initial 10 week treatment period (in an attempt to eradicate P. aeruginosa) plus 60 weeks of follow up. Serum specimens, collected during the EPIC trial at weeks 0, 22, 46 and 70, were used in the present study to assess antibody titers against P. aeruginosa. Data from up to eight respiratory cultures were available from weeks 0, 3, 10, 22, 34, 46, 58 and 70, to compare serological data to standard of care OP culture results. For serology, the E-15 ELISA kit (Mediagnost, Reutlingen, Germany) tests for antibodies against the P. aeruginosa antigens alkaline protease, elastase, and exotoxin A [8, 16] was used. Additionally, different ELISAs were used, developed at the Medical College of Wisconsin (MCW) (Milwaukee, Wisconsin, USA), to include the pooled P. aeruginosa antigens PopB and ExoS, PAO1 cell lysate preparation [17], and exotoxin A to compare and calibrate to the E-15 ELISA. The technical personnel who carried out the ELISAs were blinded to CF subject data in the EPIC trial. Positive and negative antibody titers were evaluated according to instructions of the manufacturer with regard to the E-15 ELISA, while sero-positivity using the MCW ELISA was defined by antibody titers exceeding a cut-off value of 100. This threshold was selected for all antigens based on earlier results and Receiver Operating Curve (ROC) analysis of pilot data [20].
2.2. Statistical analysis
Individual antigen titers were summarized and plotted by concurrent culture status. Concordance, defined as the proportion of samples that were both sero-positive and culture positive or sero-negative and culture negative, and diagnostic properties, including sensitivity, specificity, positive predictive value, and negative predictive value, were calculated for the E-15 and MCW combined ELISAs (if any one antigen in the ELISA is positive, the combined ELISA is positive) and for each antigen individually. P. aeruginosa positivity was plotted over time for the E-15 and MCW ELISAs in addition to culture. Modified Leeds criteria were used to group subjects according to P. aeruginosa culture positivity over the course of the 70 week study [21]: P. aeruginosa free, intermittent P. aeruginosa (at least one positive but less than half of the subject’s cultures) or chronic P. aeruginosa (50% or more of a subject’s cultures are P. aeruginosa positive). Antigen titers were plotted as a function of these categories. Odds ratio and 95% confidence intervals were calculated to evaluate the relationship between sero-positivity at baseline, week 0, and at eradication of the pathogen at week 10 (unable to isolate with OP swab culture). In subjects who were culture negative at week 10, time to next P. aeruginosa isolation, determined by culture, was analyzed as a function of seropositivity at baseline using time-to-event analyses and the log-rank test. Likewise, time to pulmonary exacerbation was analyzed as a function of baseline sero-positivity. Analyses were performed using R 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
There were 304 CF subjects in the EPIC trial [19]. From 303 subjects, serum and culture data were available for P. aeruginosa serology analysis, resulting in 975 paired serum/culture specimens over the four time points at weeks 0, 22, 46 and 70 (see Supplement Fig. 1). Among those with baseline serum, 238 (92.9%) had week 10 presence or absence of P. aeruginosa by culture to determine the success of antibiotic treatment in eradicating the pathogen (unable to isolate with OP swab culture). Baseline characteristics of the entire subject cohort and the week 10 P. aeruginosa culture status have been reported elsewhere [19, 22].
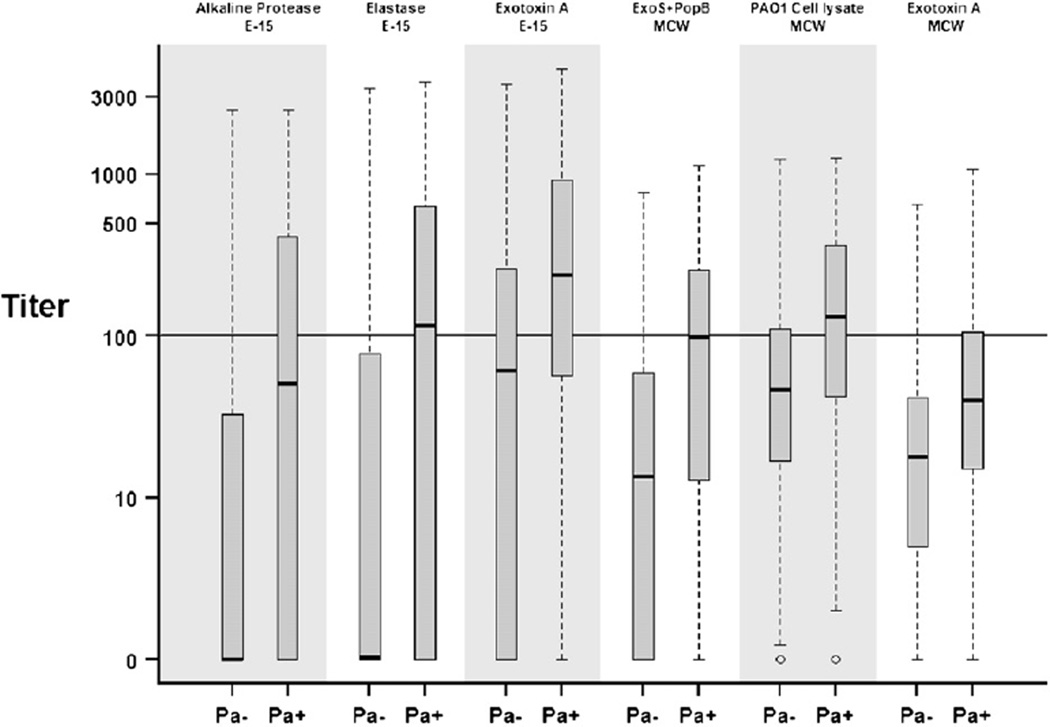
Fig. 1.
Distribution of individual antigen titers by concurrent culture status for all 975 serum samples. Pa+ indicates P. aeruginosa positive, Pa− indicates P. aeruginosa negative. Hash lines are the median values, boxes represent the 25th and 75th percentiles (inter-quartile range), whiskers extend to minimum and maximum or 1.5× the inter-quartile range (whichever is smaller), outliers are marked as circles.
3.1. P. aeruginosa serology and culture data
To investigate whether serological results from the individual antigens used in the ELISAs would correlate with the P. aeruginosa culture status of the CF subject, ELISA results for each antigen were pooled over all time points and compared with the P. aeruginosa culture status. In an attempt to maximize the detection of serum antibodies directed against P. aeruginosa, we used in our analysis a broad panel of P. aeruginosa antigens, including the extracellular toxins alkaline protease, elastase, exotoxin A and exotoxin S, the pore protein PopB and a PAO1 cell lysate preparation. Sera from CF subjects who were P. aeruginosa culture-positive revealed higher antibody titers than sera from P. aeruginosa culture-negative CF subjects (Fig. 1). The spread of titer values was highest in E-15 ELISA alkaline protease, elastase, and exotoxin A, while the distribution of titer values in the MCW antigens was narrower.
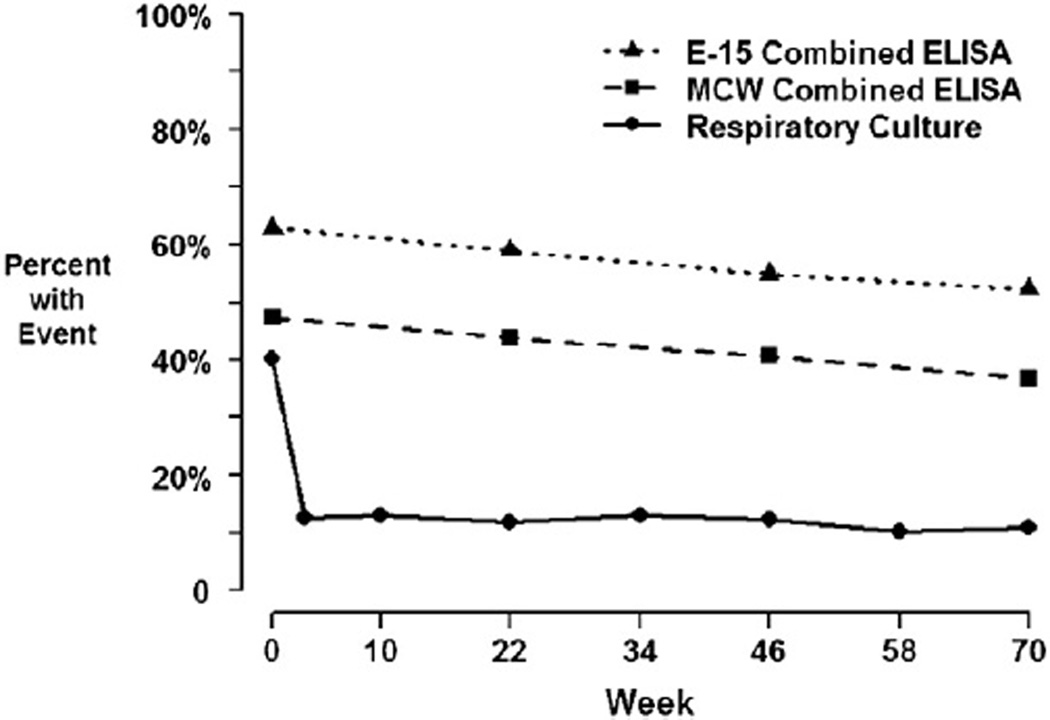
To assess whether eradication of P. aeruginosa from OP swabs during the EPIC trial period, would be reflected by a similar loss of sero-positivity, results of the E-15 and MCW ELISAs were plotted over the investigation period and compared with data from P. aeruginosa cultures. While P. aeruginosa culture positivity sharply declined from 40% at baseline to approximately 12% at subsequent visits in accordance with antibiotic treatment, antibody titers against P. aeruginosa antigens used in the E-15 and MCW ELISAs declined only slightly from 63% and 47% at baseline to 52% and 38% at week 70, respectively (Fig. 2).
Fig. 2.
Culture positivity and seropositivity over time. The solid black line shows the proportion culture positive for P. aeruginosa across each visit among the 303 subjects with available serology. The dashed lines show the proportion of samples seropositive at each visit for each combined ELISA. A combined ELISA is defined as seropositive if any one of the three antigens in an ELISA has titer at least 100.
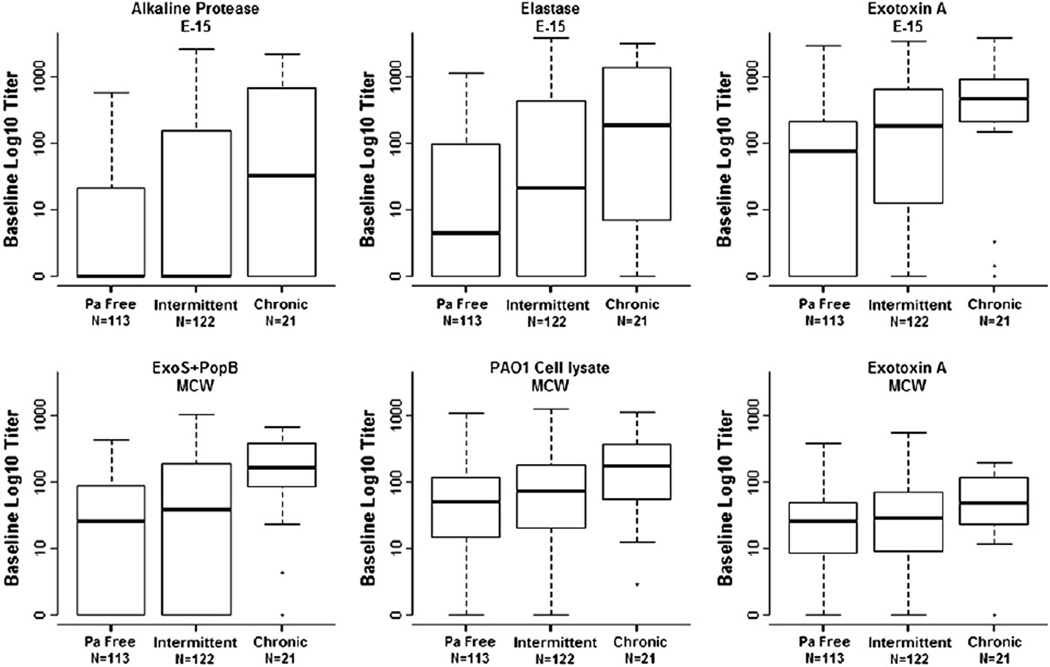
Next we assessed whether the ELISAs might be used to differentiate between P. aeruginosa free, intermittent P. aeruginosa, and chronic P. aeruginosa, as defined by the modified Leeds criteria [21]. Participants who were chronically P. aeruginosa culture positive had generally higher baseline serology than those who were only intermittently culture positive and those who never turned P. aeruginosa culture positive by the end of the study (Fig. 3). Persistence of high titers over the course of the study was observed in individuals with chronic P. aeruginosa infection (Supplement Fig. 2), and at week 70, titers from chronically P. aeruginosa culture positive subjects were significantly higher than those who remained free of P. aeruginosa for all 6 antibodies against their antigens (p-values < 0.01).
Fig. 3.
Baseline serology titers by respiratory culture status using modified Leeds criteria. Subjects were classified as ‘Pa free’ if they were clear of P. aeruginosa from baseline through end of study, ‘Intermittent’ if they had less than half of all available cultures testing positive for P. aeruginosa, and ‘Chronic’ if they had half or more of all available cultures testing positive for P. aeruginosa. Hash lines are the median values, boxes represent the 25th and 75th percentiles (inter-quartile range), whiskers extend to minimum and maximum or 1.5× the inter-quartile range (whichever is smaller), outliers are marked as circles.
Pooled concordance across the four time points ranged from 0.60 to 0.79 between the individual antigens and respiratory P. aeruginosa culture results (Table 1). Among the discordant pairs, serum antibody titers were more often positive than P. aeruginosa cultures with regard to the E-15 antigens alkaline protease (144/253), elastase (169/264), exotoxin A (328/393) and the MCW antigens PopB+ExoS (130/232) and PAO1 cell lysate (217/296). Assuming the respiratory P. aeruginosa culture as standard of care, the sensitivities for the individual antigens were low, ranging from 0.26 to 0.67. In comparison, specificity of the individual antigens was relatively high, ranging from 0.58 to 0.99, indicating that a large fraction of samples had titers less than the reciprocal cut-off titer of 100 among culture negative events. The positive predictive values for the single ELISA antigens were low (<0.5), whereas the negative predictive values were moderately high (0.83–0.88). Combining antigens, as exemplified in the E-15 ELISA improved the sensitivity (E-15: 0.78; MCW: 0.69) while decreasing the specificity (E-15: 0.48; MCW: 0.69).
Table 1.
Frequency and diagnostic properties of individual and combined ELISAs.
|
P.aeruginosa |
Culture |
Sensitivity | Specificity | PPV a | NPV b | Concordance c | ||
|---|---|---|---|---|---|---|---|---|
| Antigen | − | + | ||||||
| Alkaline protease d | − | 633 | 109 | 0.45 | 0.81 | 0.38 | 0.85 | 0.74 |
| + | 144 | 89 | ||||||
| Elastase d | − | 608 | 95 | 0.52 | 0.78 | 0.38 | 0.86 | 0.73 |
| + | 169 | 103 | ||||||
| Exotoxin A d | − | 449 | 65 | 0.67 | 0.58 | 0.29 | 0.87 | 0.60 |
| + | 328 | 133 | ||||||
| PopB+ExoS e | − | 647 | 102 | 0.48 | 0.83 | 0.42 | 0.86 | 0.76 |
| + | 130 | 96 | ||||||
| PAO1 cell lysate e | − | 560 | 79 | 0.60 | 0.72 | 0.35 | 0.88 | 0.70 |
| + | 217 | 119 | ||||||
| Exotoxin A a | − | 716 | 146 | 0.26 | 0.92 | 0.46 | 0.83 | 0.79 |
| + | 61 | 52 | ||||||
| E-15 combined d | − | 371 | 44 | 0.78 | 0.48 | 0.28 | 0.89 | 0.54 |
| + | 406 | 154 | ||||||
| MCW combined e | − | 502 | 61 | 0.69 | 0.65 | 0.33 | 0.89 | 0.66 |
| + | 275 | 137 | ||||||
PPV refers to positive predictive value.
NPV refers to negative predictive value.
Concordance is the proportion of all 975 samples that were culture negative/seronegative or culture positive/seropositive.
Component of Mediagnost® E-15 ELISA kit (Reutlingen, Germany).
Studies conducted at Dr. Joseph Barbieri’s laboratory, Medical College of Wisconsin.
3.2. Predictive utility of P. aeruginosa serology
Next we sought to know whether a positive antibody titer against one or several of the P. aeruginosa antigens at baseline in CF subjects was associated with eradication or time to next positive P. aeruginosa culture after successful eradication. Eradication rate of P. aeruginosa at week 10 among those with baseline serology was 207/238 (86.9%). Boxplots of baseline antibody titers for each of the antigens by eradication status at week 10 (Supplement Fig. 3) indicated that there were some slightly increased baseline titers in those who failed to eradicate at week 10, but there was an overlap in the serology values between the two groups and thus not statistically significant. Baseline serology (seropositive or seronegative) in the combined E-15 ELISA was not found to have a significant relationship with initial eradication success after adjusting for baseline culture status (OR=1.97, 95% CI=[0.75, 5.19], p-value=0.17). Similarly, the combined MCW ELISA for baseline serology was not significantly associated with initial eradication after adjusting for baseline culture status (OR=1.2, 95% CI=[0.53, 2.73], p-value=0.66).
Using a Cox Proportional Hazard model, we demonstrate that positive baseline antibody titers against the E-15 ELISA antigens alkaline protease and exotoxin A were both significantly associated with a higher risk of P. aeruginosa reisolation in the 60 weeks after eradication (HR=2.20, 95% CI= [1.30, 3.71], p-value =0.003) and (HR= 2.50, 95% CI= [1.42.4.40], p-value= 0.001), respectively (Table 2). Seropositivity at baseline for all other ELISA antigens and the combined ELISAs were found to increase the risk of P. aeruginosa recurrence but this finding was not statistically significant.
Table 2.
Hazard ratios for risk of P. aeruginosa occurrence post eradication treatment.
| Antigen | Hazard ratio | Hazard ratio 95% CI | p-value | |
|---|---|---|---|---|
| E-15 | Alkaline protease | 2.19 | (1.30, 3.71) | 0.003 |
| Elastase | 1.66 | (0.99, 2.78) | 0.055 | |
| Exotoxin A | 2.50 | (1.42, 4.39) | 0.001 | |
| Combined | 1.68 | (0.96, 2.95) | 0.072 | |
| MCW | PopB+ExoS | 1.51 | (0.89, 2.56) | 0.120 |
| PAO1 cell lysate | 1.61 | (0.97, 2.68) | 0.068 | |
| MCW exotoxin A | 1.69 | (0.89, 3.18) | 0.106 | |
| Combined | 1.38 | (0.83, 2.31) | 0.219 |
Post-eradication is week 10 onward. Each hazard ratio is attributable to baseline seropositivity against the individual antigens, combined E-15 ELISA, and MCW ELISA. A combined ELISA is defined as seropositive if any one of the three antibodies in an ELISA has titer at least 100.
A similar question was whether a positive antibody titer against one or several of the P. aeruginosa antigens at baseline in CF subjects in whom P. aeruginosa was successfully eradicated would be associated with a shorter time to next acute pulmonary exacerbation; the primary clinical outcome of the EPIC trial. Results based on the E-15 ELISA revealed that sero-positive CF subjects did not have a different risk for pulmonary exacerbation requiring antibiotics or hospitalization than those who were sero-negative at baseline (CI=1.1, 95% CI=[0.77, 1.57], p-value=0.61).
4. Discussion
Our study of serology in the EPIC trial examines the utility of blood antigen detection as a prognostic marker of subsequent OP culture isolation after initial eradication of Pa from upper respiratory tract swabs. In addition, the use of serology as a prognostic marker of key clinical outcomes such as pulmonary exacerbations was evaluated. We report that elevation of 2 antigens, exo A and alkaline protease, prior to eradication therapy in CF subjects in whom P. aeruginosa was successfully eradicated, was significantly associated with a higher risk of P. aeruginosa re-isolation during the 60 week follow-up period. Serology was not predictive, however, of pulmonary exacerbation during this time period. As displayed in Fig. 2, it is evident that OP swabs and Pa serology have different time courses in response to early Pa infection. Antibody response against P. aeruginosa was high initially, and serum antibody titers remained elevated over the course of the study period, while P. aeruginosa culture positivity dropped significantly at the end of the first treatment course It is therefore not surprising that only a moderate rate of concordance was observed between serology and culture collected at the same time,. It is well established [7, 9] that OP culture is by no means a perfect diagnostic indicator for lower or upper airway P. aeruginosa infection; however in the US it is the standard of care [6]. The very fact that serology does not exactly mimic culture results, highlights the complementary potential of P. aeruginosa serology in CF.
The diagnostic and concordance results alone make it impossible to determine if serology characterizes residual Pa infection, precursor to infection, or if the discordance is a product of the limitations of OP. Host response to initial P. aeruginosa infection appears to be variable in cystic fibrosis. Our results confer that early P. aeruginosa infection does not always elicit a serologic response [9], and conversely, serology often perpetuates when respiratory culture is Pa negative—the latter being more often the case. The sustained elevation of serology when OP culture positivity appears to wane may be due to slow disappearance of P. aeruginosa-positive plasma cells after the eradication of the antigenic stimulus in the organism or due to persistence of P. aeruginosa antigens in obstructed airways of CF subjects despite negative P. aeruginosa culture results.
In previous studies [2, 8, 13] a P. aeruginosa ELISA had been used to monitor the success of the antibiotic intervention in CF subjects for eradication of early P. aeruginosa infection based upon the return of elevated serum antibodies toward normal levels. We could not confirm these findings in the present study as there was no statistically significant association between baseline serology and eradication rate at week 10 after adjusting for baseline culture status. We note above, however, that positive antibody titers against 2 antigens, alkaline protease and exotoxin A, were significantly associated with a higher risk of P. aeruginosa re-isolation. These data suggest that previous P. aeruginosa isolation may predispose a CF patient to a subsequent P. aeruginosa isolation episode in a shorter time period. Thus, CF patients with new acquisition of P. aeruginosa and positive serology may benefit from closer monitoring (by culture or culture-independent techniques [23–25]) than those with negative serology, enhancing the ability to quickly treat recurrent infection. Because serology appears to contain information not captured by OP culture, or BAL [9] culture, we hypothesize that a combination of detection methods may yield better diagnosis and prognosis than does each independently [23–25]. The design of the EPIC study did not provide the ideal setting to answer questions about optimal surveillance algorithms using OP and serology in combination. Rather, the results compel the CF community to consider evaluation of potential protocols through prospective trials — which may include serology, OP, BAL, sputum, or polymerase chain reaction genomic identification methods. Two and three stage screening programs, where no one diagnostic tool is sufficient, can provide effective monitoring [26, 27]. The combination of screening tools in other disease settings such as cervical [28, 29] and prostate [30, 31] cancer yields accurate disease detection. Only prospective trials comparing different surveillance algorithms will confirm efficacy and effectiveness on an individual level.
Since all subjects in the EPIC clinical trial had a new or recent P. aeruginosa positive respiratory culture and the time period of the trial was relatively short, a longitudinal analysis of the correlation between the time period of P. aeruginosa culture positivity and serological data in CF subjects was not possible. Serologic data from the observational cohort of the EPIC study are currently being analyzed and will provide further insights into the longitudinal relationship between P. aeruginosa serology and P. aeruginosa culture results.
From a pathophysiological standpoint, it is still unclear, how the findings in the sero-positive CF cohort with higher risk for earlier P. aeruginosa isolation could be interpreted. Possibly, this group may have previously had a higher number of P. aeruginosa isolations, a higher bacterial burden in the airways, and as a consequence, more lung tissue damage than those CF patients with sero-negative baseline findings. The notion that specific antibodies against P. aeruginosa antigens augment lung inflammation and tissue damage due to immune complex mediated type III reactions, resulting in worse long term prognosis, has been repeatedly demonstrated in the past [32–35].
Ultimately patient experience and clinical endpoints determine the usefulness of any diagnostic tool or monitoring algorithm. There was no statistically significant association between baseline serology with pulmonary exacerbation requiring antibiotics or hospitalization over 60 weeks of follow-up; however our findings and those from other investigators would suggest that P. aeruginosa serology may have diagnostic and prognostic value to CF clinicians in their efforts to early detect re-isolation of P. aeruginosa. Prospective, randomized trials of monitoring algorithms inclusive of serology, and culture, and culture independent methods are needed to evaluate effectiveness of P. aeruginosa monitoring protocols on clinical outcomes in CF.
Supplementary Material
Acknowledgments
This study was supported by CFFT RAMSEY10A0 and CFFT EPIC04K0 from the US Cystic Fibrosis Foundation and NIH P30 DK089507, NIH U01 HL80310, and NIH-R01 AI30162 from The National Institutes of Health.
Footnotes
Presented at the Twenty Fifth Annual North American Cystic Fibrosis Conference, Anaheim, California, November 4, 2011: Pseudomonas aeruginosa serology predicts response to treatment and re-isolation in the EPIC clinical study.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jcf.2012.08.001.
References
- 1.Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol. 1997;23:330–335. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Taccetti G, Campana S, Festini F, Mascherini M, Doring G. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur Respir J. 2005;26:458–461. doi: 10.1183/09031936.05.00009605. [DOI] [PubMed] [Google Scholar]
- 3.Doring G, Hoiby N. Early intervention and prevention of lung disease in cystic fibrosis: a European consensus. J Cyst Fibros. 2004;3:67–91. doi: 10.1016/j.jcf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32:356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 5.Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, et al. Longitudinal assessment of Pseudomonas aeruginosa in young childrenwith cystic fibrosis. J Infect Dis. 2001;183:444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 6.Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Am J Infect Control. 2003;31:S1–S62. [PubMed] [Google Scholar]
- 7.Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castile R, Grimwood K, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28:321–328. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Ratjen F, Walter H, Haug M, Meisner C, Grasemann H, Doring G. Diagnostic value of serum antibodies in early Pseudomonas aeruginosa infection in cystic fibrosis patients. Pediatr Pulmonol. 2007;42:249–255. doi: 10.1002/ppul.20562. [DOI] [PubMed] [Google Scholar]
- 9.Douglas TA, Brennan S, Berry L, Winfield K, Wainwright CE, Grimwood K, et al. Value of serology in predicting Pseudomonas aeruginosa infection in young children with cystic fibrosis. Thorax. 2010;65:985–990. doi: 10.1136/thx.2009.132845. [DOI] [PubMed] [Google Scholar]
- 10.Kappler M, Kraxner A, Reinhardt D, Ganster B, GrieseM, Lang T. Diagnostic and prognostic value of serum antibodies against Pseudomonas aeruginosa in cystic fibrosis. Thorax. 2006;61:684–688. doi: 10.1136/thx.2005.049536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tramper-Stranders GA, van der Ent CK, Slieker MG, Terheggen-Lagro SW, Teding van Berkhout F, Kimpen JL, et al. Diagnostic value of serological tests against Pseudomonas aeruginosa in a large cystic fibrosis population. Thorax. 2006;61:689–693. doi: 10.1136/thx.2005.054726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell PM, Govan JR. Pseudomonas serology: confusion, controversy, and challenges. Thorax. 2006;61:645–647. doi: 10.1136/thx.2006.062612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratjen F, Doring G, Nikolaizik WH. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patientswith cystic fibrosis. Lancet. 2001;358:983–984. doi: 10.1016/S0140-6736(01)06124-4. [DOI] [PubMed] [Google Scholar]
- 14.West SE, Zeng L, Lee B, Kosorok MR, Laxova A, Rock M, et al. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. J Am Med Assoc. 2002;287:2958–2967. doi: 10.1001/jama.287.22.2958. [DOI] [PubMed] [Google Scholar]
- 15.Hayes D, Jr, Farrell PM, Li Z, West SE. Pseudomonas aeruginosa serological analysis in young children with cystic fibrosis diagnosed through newborn screening. Pediatr Pulmonol. 2010;45:55–61. doi: 10.1002/ppul.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doring G, Meisner C, Stern M. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc Natl Acad Sci U S A. 2007;104:11020–11025. doi: 10.1073/pnas.0702403104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corech R, Rao A, Laxova A, Moss J, Rock MJ, Li Z, et al. Early immune response to the components of the type III system of Pseudomonas aeruginosa in children with cystic fibrosis. J Clin Microbiol. 2005;43:3956–3962. doi: 10.1128/JCM.43.8.3956-3962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milagres LG, Castro TL, Garcia D, Cruz AC, Higa L, Folescu T, et al. Antibody response to Pseudomonas aeruginosa in children with cystic fibrosis. Pediatr Pulmonol. 2009;44:392–401. doi: 10.1002/ppul.21022. [DOI] [PubMed] [Google Scholar]
- 19.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, Khan U, Kulich M, Kronmal R, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165:847–856. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anstead M, Lymp J, Khan U, Barbieri J, Langkamp M, Doring G, et al. Pseudomonas aeruginosa serology predicts response to treatment and re-infection inthe EPIC clinical study (abstract) Pediatr Pulmonol. 2011;S34:303–304. [Google Scholar]
- 21.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 22.Mayer-Hamblett N, Kronmal RA, Gibson RL, Rosenfeld M, Retsch-Bogart G, Treggiari MM, et al. Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatr Pulmonol. 2012;47:125–134. doi: 10.1002/ppul.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billard-Pomares T, Herwegh S, Wizla-Derambure N, Turck D, Courcol R, Husson MO. Application of quantitative PCR to the diagnosis and monitoring of Pseudomonas aeruginosa colonization in 5–18-year-old cystic fibrosis patients. J Med Microbiol. 2011;60:157–161. doi: 10.1099/jmm.0.023838-0. [DOI] [PubMed] [Google Scholar]
- 24.McCulloch E, Lucas C, Ramage G, Williams C. Improved early diagnosis of Pseudomonas aeruginosa by real-time PCR to prevent chronic colonisation in a paediatric cystic fibrosis population. J Cyst Fibros. 2011;10:21–24. doi: 10.1016/j.jcf.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 25.da Silva Filho LV, Tateno AF, Martins KM, Azzuz Chernishev AC, Garcia Dde O, Haug M, et al. The combination of PCR and serology increases the diagnosis of Pseudomonas aeruginosa colonization/infection in cystic fibrosis. Pediatr Pulmonol. 2007;42:938–944. doi: 10.1002/ppul.20686. [DOI] [PubMed] [Google Scholar]
- 26.Shaw PA, Pepe MS, Alonzo TA, Etzioni R. Methods for assessing improvement in specificity when a biomarker is combined with a standard screening test. Stat Biopharm Res. 2009;1:18–25. doi: 10.1198/sbr.2009.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etzioni R. Statistical issues in the evaluation of screening and early detection modalities. Urol Oncol: Semin Orig Investig. 2008;26:308–315. doi: 10.1016/j.urolonc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbyn M, Ronco G, Cuzick J, Wentzensen N, Castle PE. How to evaluate emerging technologies in cervical cancer screening? Int J Cancer. 2009;125:2489–2496. doi: 10.1002/ijc.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etzioni R, Falcon S, Gann PH, Kooperberg CL, Penson DF, Stampfer MJ. Prostate-specific antigen and free prostate-specific antigen in the early detection of prostate cancer: do combination tests improve detection? Cancer Epidemiol Biomarkers Prev. 2004;13:1640–1645. [PubMed] [Google Scholar]
- 31.Etzioni R, Kooperberg C, PepeM, Smith R, Gann PH. Combining biomarkers to detect disease with application to prostate cancer. Biostatistics. 2003;4:523–538. doi: 10.1093/biostatistics/4.4.523. [DOI] [PubMed] [Google Scholar]
- 32.Doring G, Hoiby N. Longitudinal study of immune response to Pseudomonas aeruginosa antigens in cystic fibrosis. Infect Immun. 1983;42:197–201. doi: 10.1128/iai.42.1.197-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler WB, Williams M, Matthews WJ, Jr, Colten HR. Progression of cystic fibrosis lung disease as a function of serum immunoglobulin G levels: a 5-year longitudinal study. J Pediatr. 1984;104:695–699. doi: 10.1016/s0022-3476(84)80946-4. [DOI] [PubMed] [Google Scholar]
- 34.Brett MM, Ghoneim AT, Littlewood JM. Prediction and diagnosis of early Pseudomonas aeruginosa infection in cystic fibrosis: a follow-up study. J Clin Microbiol. 1988;26:1565–1570. doi: 10.1128/jcm.26.8.1565-1570.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proesmans M, Els C, Vermeulen F, De Boeck K. Change in IgG and evolution of lung function in children with cystic fibrosis. J Cyst Fibros. 2011;10:128–131. doi: 10.1016/j.jcf.2010.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.