Abstract
Background
A second allogeneic transplant following a prior allogeneic (allo-allo) or autologous (auto-allo) hematopoietic cell transplantation (HCT) is usually performed for graft failure, disease recurrence, secondary malignancy and as planned auto-allo transplant for some diseases.
Methods
We sought to describe the costs of second allogeneic HCT and evaluate their relationship with patient characteristics and post-transplant complications. Clinical information and medical costs for the first 100 days after transplantation of 245 patients (allo-allo: 55, auto-allo: 190) who underwent a second HCT between 2004 and 2010 were collected.
Results
Median costs of the second allogeneic HCT were $151,000 (range $62,000–405,000) for the allo-allo group and $109,000 (range $26,000–490,000) for the auto-allo. Median length of hospital stay was 23 days (range 0 - 76) for the allo-allo group and 9 days (range 0–96) for the auto-allo group. Only the year of transplant and post-transplant complications were significantly associated with costs in both groups when both pre- and post-transplant variables were considered. The overall costs of the second HCT were higher than the first in the allo-allo group. For the auto-allo group, there was no difference between the costs whether done as a planned tandem or as salvage for relapse.
Conclusions
Our results suggest that second allogeneic HCT is costly, particularly if it follows a prior allogeneic transplant, and is driven by the costs of complications.
Keywords: Hematopoietic cell transplantation, economics, costs, cost-effectiveness, Pharmacoeconomics
INTRODUCTION
In the past, allogeneic transplants subsequent to a previous allogeneic (allo-allo) or autologous (auto-allo) transplant were used exclusively as a therapeutic treatment for graft failure, relapse of disease or occasionally the development of a different malignancy after the initial hematopoietic cell transplantation (HCT).(1) The last decade has also seen a substantial increase in the number of planned tandem auto-allo transplants for the treatment of myeloma and lymphoma, even though the efficacy of this strategy is still a matter of great controversy, especially in myeloma.(2) According to government statistics, HCT generated the greatest percentage increase in total hospital costs from 2004 to 2007, due primarily to an increase in the cumulative number of hospital stays.(3) The increase in number of hospital stays is likely because the procedure is more widely available due to reduced intensity conditioning regimens and better supportive care. This has led to inclusion of older and sicker individuals and the ability to perform multiple transplants in patients. The implementation of the Affordable Care Act may further accelerate the trend since it prohibits the health plans from setting lifetime dollar limits on the benefits, which may have previously limited second transplants. Approximately 15,000 allo-allo and 8300 auto-allo transplants were reported to the Center for International Blood and Marrow Transplant Registry between 2000 to 2010 (personal communication, Tanya Pedersen, CIBMTR, January 2012).
Multiple studies examining patient outcomes after a second allogeneic transplant reported an overall survival in the range of 20 to 40% at 3 years.(4–14) An exception has been planned tandem auto-allo transplants which have shown more favorable complete response rates and survival, though a recent phase III trial in myeloma showed an efficacy comparable to tandem autologous HCT.(15–18)
The decision to pursue a second allogeneic transplant has clinical as well as financial implications. Messori et al have looked at the cost effectiveness of second allogeneic bone marrow transplantation in patients with relapsed acute leukemia based on published data from January 1985 to June 1998.(19) They showed that second HCT prolonged survival and was cost effective as compared to chemotherapy for relapse after an initial HCT. However the authors did not report a detailed analysis of costs in their report. Our study aimed to evaluate the clinical and economic outcomes of a second allogeneic HCT following a prior autologous or allogeneic transplant. We specifically sought to identify the main factors that are associated with costs in a modern cohort of patients.
RESULTS
Clinical characteristics of the study cohort
Table 1 summarizes the demographic, disease and transplant-related characteristics of the 245 patients. Median age at second HCT was 50 years (range, 21–68 years) in the allo-allo group and 51 years (range 18–72 years) in the auto-allo group. Three fifths of the study population was male in both groups. Median follow-up period from HCT was 19.8 months (range, 0.5–61.2 months).
Table 1.
Baseline clinical characteristics
| Characteristic | Allo-Allo (n = 55) | Auto-Allo (n = 190) |
|---|---|---|
|
| ||
| Age (years) | ||
| Median (range) | 50 (21–68) | 51 (18–72) |
|
| ||
| Males | 34 (62) | 114 (60) |
|
| ||
| Diagnosis | ||
| AML/MDS/MPD | 38 (69) | 21 (11) |
| ALL | 7 (13) | 2 (1) |
| NHL/HD/CLL | 6 (11) | 108 (57) |
| Multiple Myeloma | 0 | 59 (31) |
| Other | 4 (7) | 0 |
|
| ||
| Reason for second transplant | ||
| Tandem | 0 | 80 (42) |
| Relapse | 36 (65) | 102 (54) |
| Graft failure | 14 (25) | 0 |
| Other reasons including second malignancy | 5 (9) | 8 (4) |
|
| ||
| Disease status (3 missing) | ||
| Relapse | 22 (40) | 134 (71) |
| Remission | 33 (60) | 56 (29) |
|
| ||
| Donor | ||
| Matched related | 10 (18) | 72 (38) |
| Matched unrelated | 31 (56) | 63 (33) |
| Mismatched | 14 (25) | 55 (29) |
|
| ||
| Conditioning | ||
| High intensity | 22 (40) | 52 (27) |
| Reduced intensity/Non-myeloablative | 33 (60) | 138 (73) |
|
| ||
| Stem cell source | ||
| PBSC | 44 (80) | 160 (84) |
| BM | 5 (9) | 28 (15) |
| Cord | 6 (11) | 2 (1) |
|
| ||
| CMV serostatus (7 missing) | ||
| D+/R− | 5 (10) | 22 (12) |
| D+/R+ | 16 (32) | 50 (27) |
| D−/R− | 13 (26) | 57 (30) |
| D−/R+ | 16 (32) | 58 (31) |
|
| ||
| Months between transplants | ||
| Median (range) | 12.8 (1.5–186.2) | 9.6 (1.3–167.8) |
Abbreviations: HCT, Hematopoietic cell transplantation; Allo-allo, 2 allogeneic transplants; Auto-allo, autologous transplant followed by allogeneic transplant; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MPD, myeloproliferative disease; ALL, acute lymphoid leukemia; NHL, non-Hodgkin’s lymphoma; HD, Hodgkin’s disease; CLL, chronic lymphocytic leukemia; PBSC, peripheral blood stem cell; BM, bone marrow; CMV, Cytomegalovirus; D, donor; R, recipient
Clinical Events and Outcomes
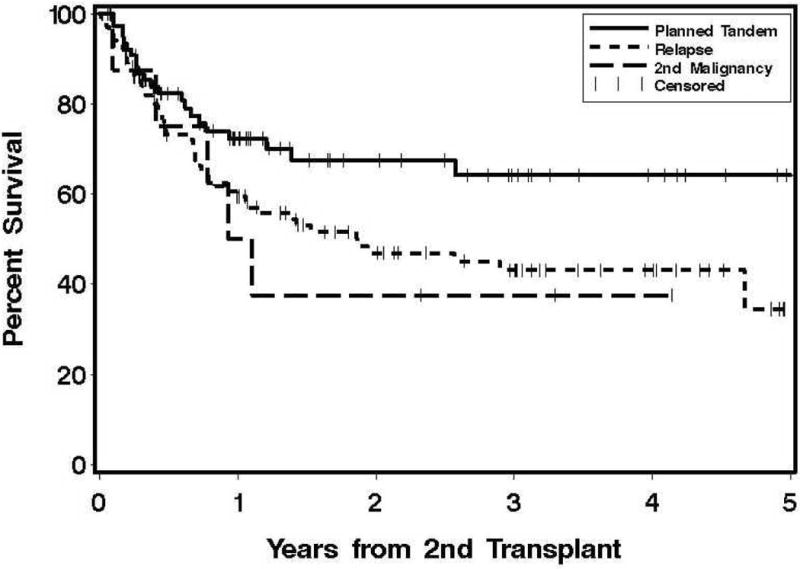
Engraftment occurred in 230 out of 245 patients and the median time to an absolute neutrophil count of ≥ 500/μl was 15 days (range, 6–49 days). The other clinical outcomes of GVHD, organ toxicity, relapse and causes of death for the first 100 days are shown in Table 2. One year transplant-related mortality in this cohort was 15% and the relapse rate was 36%. Figure 1 show the overall survival curves for the allo-allo and auto-allo groups according to the reason for second transplant.
Table 2.
Clinical outcomes in first 100 days after second HCT
| Variable N (%) | Allo-allo (n = 55) | Auto-allo (n = 190) |
|---|---|---|
|
| ||
| Cumulative incidence of relapse | 14 (25) | 40 (21) |
|
| ||
| Cumulative incidence of grades II-IV acute GVHD | 34 (62) | 128 (67) |
|
| ||
| Regimen related toxicity (≥ grade 3)/ Infections | ||
| Pulmonary | 10 (18) | 29 (15) |
| Veno-occlusive disease/sinusoidal obstructive syndrome | 8 (15) | 10 (5) |
| Renal/bladder | 9 (16) | 79 (42) |
| Infections | 29 (53) | 117 (62) |
|
| ||
| Number of deaths | 13 (24) | 24 (13) |
|
| ||
| Cause of death1 | ||
| Relapse | 3 (23) | 3 (33) |
| Graft-vs.-Host disease | 1 (7) | 5 (20) |
| Infection | 5 (38) | 6 (25) |
| Other | 5 (38) | 9 (38) |
Abbreviations: HCT, Hematopoietic cell transplantation; Allo-allo, 2 allogeneic transplants; Auto-allo, autologous transplant followed by allogeneic transplant
Sum of percentages > 100% since multiple causes of death were listed for some patients
Figure 1. Overall survival according to the indication for second hematopoietic cell transplant.
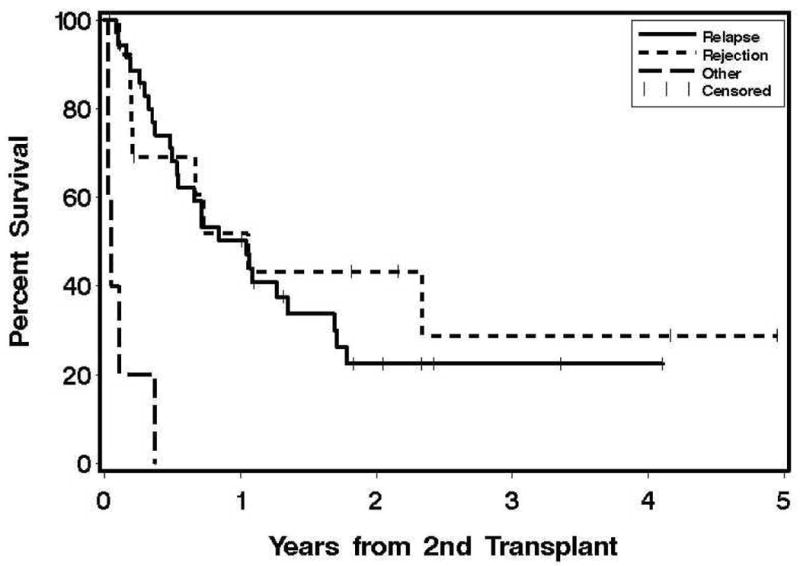
A) Second allogeneic HCT following a prior allogeneic transplant
B) Second allogeneic HCT following a prior autologous transplant
Costs and predictors of costs
Table 3A shows the median costs for the first 100 days (30 days for the autologous HCT) after the first and second HCT. In the subset of the allo-allo group for which we had data for both transplants, the second allogeneic HCT was more expensive than the first (median 156,000 vs. 132,000; p = 0.03). Various categories of the costs are reported in table 3B.
Table 3A.
Costs, length of stay and cost-categories
Costs and length of stay for the first 100 days after the first and second HCT
| Allo-allo | Auto- allo | |||
|---|---|---|---|---|
| 1st Allo (n = 42) | 2nd Allo (n = 55) | Auto1 (n = 119) | Allo (n = 190) | |
| Median total costs, $1000’s (range) | 132 (25–279) | 151 (62–405) | 72 (28–167) | 109 (26–490) |
| Median Inpatient costs, $1000’s (range) | 76 (0–278) | 92 (0–371) | 43 (0–128) | 22 (0–433) |
| Median Outpatient costs, $1000’s (range) | 60 (1–155) | 68 (0–194) | 29 (3–88) | 72 (1–199) |
| Median hospital stay, days (range) | 25 (0–47) | 23 (0–76) | 15 (0–34) | 9 (0–96) |
For first 30 days after the autologous HCT
Abbreviations: Allo-allo, 2 allogeneic transplants; Auto-allo, autologous transplant followed by allogeneic transplant
Table 3B.
Categories of costs (percent of total costs) in the allo-allo and auto-allo group
| Allo-allo | Auto-allo | |
|---|---|---|
| Outpatient | 12% | 19% |
| Inpatient | 29% | 21% |
| Pharmacy | 20% | 19% |
| Laboratory/ pathology | 15% | 21% |
| Radiology/ diagnostics | 3% | 6% |
| Transfusion | 11% | 7% |
| Miscellaneous | 10% | 7% |
Abbreviations: Allo-allo, 2 allogeneic transplants; Auto-allo, autologous transplant followed by allogeneic transplant
In the multivariate analyses for the allo-allo group, there was no association of costs with age, disease, disease status, or conditioning regimen though graft failure as a reason for HCT was associated with higher costs when only pre-transplantation variables were considered. Use of myeloablative conditioning and unrelated or mismatched donors was a significant cost driver in the auto-allo group, but not in allo-allo group, in the model using only pre-transplant variables. Only mismatched donors remained significant when both pre and post-transplantation variables were considered. Among the post-transplant complications, acute GVHD, pulmonary complications and infection were associated with significantly increased costs in both groups while renal complications were significant only for the allo-allo group. Interestingly, while the year of transplant was associated with a 14% decrease in costs per year in the allo-allo group, a 4% increase per year was seen in the auto-allo group. (Table 4A and B)
Table 4A.
Linear regression for costs
Allogeneic HCT following a prior allogeneic transplant
| Variable | Baseline model | Full model | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cost multiplier | 95% CI | P value | Cost multiplier | 95% CI | P value | |
| Pre-transplant factors | ||||||
|
| ||||||
| Age | ||||||
| < 50 | 1.0 | 1.0 | ||||
| ≥ 50 | 1.01 | 0.77–1.32 | 0.96 | 1.03 | 0.80–1.33 | 0.81 |
|
| ||||||
| Disease | ||||||
| Acute leukemia/MDS | 1.0 | 1.0 | ||||
| Lymphoma | 1.07 | 0.68–1.68 | 0.77 | 0.89 | 0.59–1.34 | 0.57 |
| Other | 0.61 | 0.33–1.11 | 0.11 | 0.67 | 0.39–1.15 | 0.15 |
|
| ||||||
| Disease status | ||||||
| Remission | 1.0 | 1.0 | ||||
| Relapse | 0.89 | 0.64–1.25 | 0.51 | 0.84 | 0.61–1.15 | 0.29 |
|
| ||||||
| Graft source | ||||||
| PBSC | 1.0 | 1.0 | ||||
| BM/Cord | 1.27 | 0.83–1.94 | 0.28 | 1.36 | 0.92–1.99 | 0.13 |
|
| ||||||
| Conditioning | ||||||
| NMA | 1.0 | 1.0 | ||||
| MA | 1.39 | 0.96–2.03 | 0.09 | 1.23 | 0.88–1.72 | 0.24 |
|
| ||||||
| Reason for transplant | ||||||
| Relapse | 1.0 | 1.0 | ||||
| Rejection/failure | 1.41 | 1.01–1.97 | 0.05 | 1.25 | 0.93–1.69 | 0.14 |
| Other | 1.00 | 0.57–1.76 | 0.99 | 0.96 | 0.54–1.72 | 0.90 |
|
| ||||||
| Year of transplant | ||||||
| Per year | 0.96 | 0.86–1.07 | 0.45 | 0.86 | 0.78–0.95 | 0.005 |
|
| ||||||
| Donor type | ||||||
| Matched related | 1.0 | 1.0 | ||||
| Matched unrelated | 0.97 | 0.68–1.37 | 0.86 | 0.91 | 0.67–1.22 | 0.52 |
| Mismatched (related and unrelated) | 1.00 | 0.63–1.59 | 0.99 | 0.77 | 0.51–1.18 | 0.24 |
|
| ||||||
| Patient CMV serostatus | ||||||
| Negative | 1.0 | 1.0 | ||||
| Positive | 1.01 | 0.76–1.35 | 0.94 | 0.87 | 0.67–1.13 | 0.30 |
|
| ||||||
| Post-transplant complications | ||||||
|
| ||||||
| Death | 0.70 | 0.46–1.06 | 0.10 | |||
| Relapse | 1.20 | 0.90–1.60 | 0.23 | |||
| GVHD (II-IV) | 1.42 | 1.09–1.84 | 0.01 | |||
| SOS | 0.92 | 0.63–1.33 | 0.66 | |||
| Pulmonary | 1.55 | 1.12–2.16 | 0.01 | |||
| Renal | 1.90 | 1.16–3.10 | 0.02 | |||
| Infection | 1.42 | 1.10–1.85 | 0.01 | |||
Abbreviations: MDS, myelodysplastic syndrome; PBSC, peripheral blood stem cells; BM, bone marrow; NMA, non-myeloablative; MA, myeloablative; CMV, cytomegalovirus; GVHD, graft vs. host disease; SOS, sinusoidal obstruction syndrome
Table 4B.
Allogeneic HCT following a prior autologous transplant
| Variable | Baseline model | Full model | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cost multiplier | 95% CI | P value | Cost multiplier | 95% CI | P value | |
|
| ||||||
| Pre-transplant factors | ||||||
| Age | ||||||
| < 50 | 1.0 | 1.0 | ||||
| ≥ 50 | 1.04 | 0.91–1.18 | 0.58 | 1.02 | 0.91–1.15 | 0.68 |
|
| ||||||
| Disease | ||||||
| Lymphoma | 1.0 | 1.0 | ||||
| Acute leukemia/MDS | 1.05 | 0.81–1.35 | 0.71 | 1.09 | 0.87–1.36 | 0.47 |
| Myeloma | 0.87 | 0.74–1.02 | 0.09 | 0.94 | 0.82–1.09 | 0.44 |
|
| ||||||
| Disease status | ||||||
| Remission | 1.0 | 1.0 | ||||
| Relapse | 1.07 | 0.91–1.25 | 0.40 | 1.03 | 0.90–1.19 | 0.66 |
|
| ||||||
| Graft source | ||||||
| PBSC | 1.0 | 1.0 | ||||
| BM/Cord | 0.97 | 0.73–1.28 | 0.81 | 0.92 | 0.72–1.18 | 0.52 |
|
| ||||||
| Conditioning | ||||||
| NMA | 1.0 | 1.0 | ||||
| MA | 1.23 | 1.00–1.51 | 0.05 | 1.13 | 0.94–1.35 | 0.21 |
|
| ||||||
| Reason for transplant | ||||||
| Relapse | 1.0 | 1.0 | ||||
| Tandem auto-allo | 0.93 | 0.80–1.09 | 0.37 | 0.93 | 0.81–1.06 | 0.26 |
| Secondary malignancy | 0.78 | 0.53–1.14 | 0.20 | 0.82 | 0.59–1.15 | 0.25 |
|
| ||||||
| Year of transplant | ||||||
| Per year | 1.05 | 1.01–1.10 | 0.02 | 1.04 | 1.01–1.08 | 0.03 |
|
| ||||||
| Donor type | ||||||
| Matched related | 1.0 | 1.0 | ||||
| Matched unrelated | 1.23 | 1.05–1.45 | 0.01 | 1.12 | 0.97–1.29 | 0.12 |
| Mismatched (related and unrelated) | 1.34 | 1.10–1.62 | 0.004 | 1.19 | 1.00–1.42 | 0.05 |
|
| ||||||
| Patient CMV serostatus | ||||||
| Negative | 1.0 | 1.0 | ||||
| Positive | 1.10 | 0.96–1.25 | 0.17 | 1.01 | 0.89–1.13 | 0.91 |
|
| ||||||
| Post-transplant complications | ||||||
|
| ||||||
| Death | 1.06 | 0.87–1.29 | 0.55 | |||
| Relapse | 1.02 | 0.89–1.18 | 0.73 | |||
| GVHD (II-IV) | 1.15 | 1.02–1.30 | 0.03 | |||
| SOS | 0.93 | 0.71–1.22 | 0.61 | |||
| Pulmonary | 1.42 | 1.19–1.71 | 0.0002 | |||
| Renal | 1.01 | 0.90–1.14 | 0.82 | |||
| Infection | 1.38 | 1.21–1.57 | < 0.0001 | |||
Abbreviations: MDS, myelodysplastic syndrome; PBSC, peripheral blood stem cells; BM, bone marrow; NMA, non-myeloablative; MA, myeloablative; auto-allo, autologous transplant followed by allogeneic transplant; CMV, cytomegalovirus; GVHD, graft vs. host disease; SOS, sinusoidal obstruction syndrome
We also analyzed the costs in the auto-allo group (n = 110) after excluding the planned tandem auto-allo transplants and found that the magnitude and direction of the effects was similar but some previously significant findings were no longer significant, likely due to the loss of power associated with the smaller sample size. The sole substantive difference was that transplant costs for myeloma were higher when planned tandem transplants were excluded, though not statistically significant in either case. The costs and predictors of costs for the auto-allo group after excluding the planned tandem transplants are shown in the supplemental digital content table 1 and 2.
DISCUSSION
Second allogeneic HCT has been used as a therapeutic strategy to address relapse, graft failure or second malignancy after a prior HCT, or to try to achieve better disease control as part of planned tandem transplant after a prior autologous procedure. Our study evaluated two distinct cohorts of second allogeneic HCT (allo-allo and auto-allo) for costs of second HCT and described their association with pre-transplant characteristics and post-transplant complications. Similar to what has been shown for first HCT; we found that baseline patient characteristics do not help predict the costs except for mismatched donor transplants which were associated with higher costs in the auto-allo group. Additionally, post-transplant complications are significant cost drivers for second HCT in both the groups.(20) The outcomes of a second allogeneic HCT after a prior allogeneic HCT have generally been quite disappointing.(4) High transplant related mortality (TRM), ranging from 30 to 60%, is reportedly due to post-transplant infections and regimen related toxicity which is higher than with first transplants.(7, 8, 11, 12, 21) Our study shows that these complications also increase the cost of the procedure significantly. In the allo-allo group, the second HCT was more expensive than the first allogeneic procedure (p = 0.03), even though the overall costs for the second HCT are still in the range of reported costs in prior studies.(22) There was no evidence that this cost difference was dependent on the interval between the two transplants being less than or greater than 100 days (p = 0.88).
Our study also described the outcomes and costs of allogeneic HCT following an autologous HCT. Similar to the allo-allo group, the overall costs for the autologous and allogeneic HCT are in the range of what has been described in the literature.(22) Interestingly, while there was a difference in costs between second HCT when done as a planned tandem auto-allo or for relapse of disease after a prior autologous HCT in univariate analysis (data not shown), this distinction lost its significance in the multivariate analysis, likely due to adjustment for patient and disease related characteristics. The cost multipliers were similar in magnitude and direction for most variables (except for pulmonary complications and multiple myeloma) when the planned tandem auto-allo transplants were excluded.
A cost-effectiveness analysis using the upper range of published cost data from 1992 to 1996 for HCT showed an acceptable cost profile for second HCT done for relapse of leukemia as compared to chemotherapy.(19) The sensitivity analysis in this study explored the consequences of varying HCT costs and found that the cost per life year gained remained close to the acceptable threshold of $50,000 per quality adjusted life year gained if the transplant costs were ≤ $150,000 ($184,688 in 2010 dollars). A more recent descriptive cost study by Svahn et al showed that re-transplantation is associated with higher mean costs of the first HCT itself (relative hazard 1.21, p = 0.001) , though the costs for second transplant were not separated from the first transplant.(23) This is similar to the solid organ literature where re-transplantation has also been shown to be associated with greater costs and worse survival, raising important ethical questions due to depletion of already limited supply of organs.(24) While the ethical dilemma is not quite as poignant in the field of blood and marrow transplantation since the graft is usually not a limited resource, the question about high costs, optimum use of societal resources and ethics of second HCT has been raised by investigators.(6, 25)
In this era of increasing health care costs, consideration of second HCT especially for relapse should be made based on clinical effectiveness and economic impact of the projected outcome to optimize the utility of our scarce resources. This is especially relevant if non-transplant options may be available with no convincing evidence for one approach being better than others. Similarly, for the tandem auto-allo transplants, doing them outside a clinical study may result in widespread use before evidence of their effectiveness is available. This risks a similar experience as with the use of high dose therapy and autologous transplants for breast cancer, where public opinion, political influence and the threat of litigation resulted in coverage of this procedure by insurance companies even though eventually definitive clinical trial data showed no overall benefit and greater toxicity.(26)
Our study does have some limitations. The study is from a single center and the sample size was limited in the allo-allo group though still bigger than most single center studies reporting on clinical outcomes of second HCT. This may have prevented our ability to detect any associations between costs and clinical characteristics. We were missing some details that would have been helpful, such as information about co-morbidities and uniform follow-up cost data beyond day 100, since most patients return to the care of their local physician beyond 100 days. It is unknown if the rate of complications beyond 100 days is higher for second HCT since that would imply higher downstream costs also. We did not conduct a cost-effectiveness analysis because we could not analyze costs of patients who were managed without a second transplant. Also, there are no randomized trial data available to estimate survival with and without a second transplant. Modeling survival estimates from published literature would be dubious because of the limited follow-up and only small amount of information being available. Because this was a retrospective study, we did not have any information about financial burden of the second HCT on patients and their families. Finally, the cost estimates reflect resource utilizations at our center and may not be representative because of differences in practice patterns, patient mix and accounting methodologies at different centers.
Despite the above limitations, our study provides valuable information that can be used to design economic analyses comparing prevention or treatment of relapse, graft failure or second malignancy vs. second HCT. This study is one of the first to provide economic data on the tandem auto-allo transplants to build a framework for the economic assessment of this strategy. It provides estimates of direct medical costs to help design a cost-effectiveness analysis comparing the planned approach to salvage HCT for relapse. Finally, it characterizes the factors that are associated with higher costs of second HCT to help design interventions that would help improve clinical outcomes while helping to contain the costs. Efforts to improve the clinical outcomes further by decreasing the regimen related toxicity and recurrence rates of second transplant can not only help lower costs, but also improve the cost-effectiveness of the procedure further.
Our results suggest that the short-term costs of second transplants appear higher than those of first transplants especially for allo-allo transplants and the clinical outcomes have much room for improvement. Additional work is needed with larger numbers of patients to confirm the characteristics that would predict the group with the best clinical and economic outcomes. A careful assessment of both benefits and costs of expensive medical interventions such as second HCT is paramount for providing high-value and high-quality care and allow optimum allocation of the finite resources.
PATIENTS AND METHODS
Patients
This retrospective study included 245 patients who underwent an allogeneic transplant following a prior autologous or allogeneic HCT between 2004 and 2010 at a single large center. Patients gave consent allowing the use of medical records for research, and the Institutional Review Board at the Fred Hutchinson Cancer Research Center (FHCRC) approved the study.
Eighty four patients received their first transplant at another center, so the details and costs of the prior procedure were not available. In the allo-allo group, the second HCT was performed for recurrence of the original disease in 65% of patients and for graft failure in 25%. Fifty four percent of patients in the auto-allo group received the second HCT for relapsed disease, and 42% were planned tandem auto-allo transplants. For the remaining patients in both groups, the second HCT was done for a different indication than the original diagnosis (e.g., aplastic anemia after initial transplant for acute myeloid leukemia, treatment related myelodysplastic syndrome/ acute myeloid leukemia following an autologous HCT). The median time between the first and second transplantations was 12.8 months (range, 1.5–186.2) in the allo-allo group and 9.6 months (range, 1.3–167.8 months) in the auto-allo group.
Conditioning, GVHD prophylaxis and supportive care for the second HCT
Myeloablative conditioning was used more frequently for the second HCT in the allo-allo group as compared to the auto-allo group (40% vs. 27%, p = 0.07) Patients with aplastic anemia (AA) received cyclophosphamide and antithymocyte globulin. Graft vs. host disease (GVHD) prophylaxis was per protocol and antimicrobial prophylaxis, blood product and nutritional support were provided per institutional guidelines.
Most non-myeloablative/ reduced intensity transplants were performed as outpatients with hospital admission only for cell infusion (if mandated by insurance or for stem cell products arriving when the outpatient clinic was closed). Patient were admitted to the hospital primarily for management of complications such as febrile neutropenia, severe GVHD requiring parenteral nutrition and iv medications, severe pain requiring intravenous narcotics, inability to maintain oral intake etc.
Patients receiving myeloablative regimens were admitted prior to the cell infusion or during the conditioning depending on the regimen, and discharge criteria were based on neutrophil engraftment, adequate oral intake, and medical stability.
Cost data
All charges (inpatient and outpatient) and total hospital days for the second HCT from D –7 of transplant to D+100 were retrieved from the administrative database. This information was also retrieved for the first HCT for patients who had received their first HCT at our institution. For the first autologous HCT, D −7 to D+30 was used as the time horizon for costs. If the interval between the two transplants was shorter, the upper limit for the first HCT costs was considered d −8 of the second HCT. Charges were converted to costs using departmental ratios of charges to costs and adjusted to the year 2010 using the medical care component of the consumer price index.(27) This methodology was used since our administrative database employs the traditional system of cost allocation and has only started to implement activity based costing which provides a more accurate estimates of costs. Costs of donor identification and graft procurement were excluded. Professional charges, patient time and productivity costs, costs of prescription medications and direct non-medical costs (e.g. transportation, lodging) were not captured.
Statistical Analysis
Cumulative incidence estimates were calculated for relapse, acute GVHD, and non-hematological toxicity, treating death as a competing risk. Cumulative incidence estimates of non-relapse mortality treated relapse as a competing risk. Overall survival was estimated using the Kaplan-Meier method. A descriptive analysis of the inpatient and outpatient costs for the first 100 days was done using numeric summaries (mean, variance, median, range). Pre-transplant and post-transplant predictors of costs were identified using multiple linear regression with year of transplant, patient (e.g. demographics, disease variables, CMV status) and transplant characteristics (e.g. stem cell source, HLA matching, donor type, conditioning regimen) entered first, and post-transplant complications (severe infections, VOD, pulmonary or renal complications) added second. Separate models were created for the allo-allo and the auto-allo groups.
Since distribution of costs is typically right skewed, the logarithm of costs was used for the multivariate analysis. Results are presented as ‘cost multipliers’ which are the ratios of costs of patients with specific baseline characteristics or experiencing the specific complication compared with those who do not. For example, a cost multiplier of 1.17 for relapse corresponds to a 17% increase in costs of patients who relapsed as compared to those who did not. Separate analyses were done for the auto-allo group after excluding the planned tandem transplants. All reported p-values are two-sided and p-values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
The study was supported by National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000423 and grants CA78904, HL36444, CA18029 from the National Institutes of Health.
We wish to thank Brent Jarosek and Lisa Acomb for providing the costs information.
Abbreviations
- AA
aplastic anemia
- ALL
acute lymphoid leukemia
- AML
acute myeloid leukemia
- BM
bone marrow
- CLL
chronic lymphocytic leukemia
- CMV
Cytomegalovirus
- D
donor
- GVHD
graft vs. host disease
- HCT
Hematopoietic cell transplantation
- HD
Hodgkin’s disease
- MA
myeloablative
- MDS
myelodysplastic syndrome
- MPD
myeloproliferative disease
- NHL
non-Hodgkin’s lymphoma
- NMA
non-myeloablative
- PBSC
peripheral blood stem cell
- R
recipient
- SOS
sinusoidal obstruction syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution: N.K. conceived and designed the research, collected and interpreted the data and wrote the manuscript. B.S. analyzed and interpreted the data. B.M.S. provided study materials and patients, interpreted the data and critically reviewed the manuscript. M.K.C. interpreted the data and critically reviewed the manuscript. S.J.L. conceived and designed the research, interpreted the data and wrote the manuscript. All authors approved the final version.
Disclosures: The authors of this manuscript have no conflicts of interest to disclose. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
References
- 1.Wolff SN. Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone marrow transplantation. 2002;29(7):545. doi: 10.1038/sj.bmt.1703389. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A. Is there a future for auto-allo HSCT in multiple myeloma? Lancet Oncol. 2011;12(13):1176. doi: 10.1016/S1470-2045(11)70258-3. [DOI] [PubMed] [Google Scholar]
- 3.Stranges ETR, Russo CA, Thomson Reuters. Friedman B. Procedures with the Most Rapidly Increasing Hospital Costs, 2004–2007 HCUP Statistical Brief #82. Agency for Healthcare Research and Quality; Rockville, MD: Dec, 2009. [Google Scholar]
- 4.Arfons LM, Tomblyn M, Rocha V, Lazarus HM. Second hematopoietic stem cell transplantation in myeloid malignancies. Current opinion in hematology. 2009;16(2):112. doi: 10.1097/MOH.0b013e3283257a87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron F, Storb R, Storer BE, et al. Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopoietic cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(25):4150. doi: 10.1200/JCO.2006.06.9914. [DOI] [PubMed] [Google Scholar]
- 6.Bosi A, Laszlo D, Labopin M, et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(16):3675. doi: 10.1200/JCO.2001.19.16.3675. [DOI] [PubMed] [Google Scholar]
- 7.Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone marrow transplantation. 2004;34(8):721. doi: 10.1038/sj.bmt.1704645. [DOI] [PubMed] [Google Scholar]
- 8.Guardiola P, Kuentz M, Garban F, et al. Second early allogeneic stem cell transplantations for graft failure in acute leukaemia, chronic myeloid leukaemia and aplastic anaemia. French Society of Bone Marrow Transplantation. British journal of haematology. 2000;111(1):292. doi: 10.1046/j.1365-2141.2000.02306.x. [DOI] [PubMed] [Google Scholar]
- 9.Gyurkocza B, Cao TM, Storb RF, et al. Salvage allogeneic hematopoietic cell transplantation with fludarabine and low-dose total body irradiation after rejection of first allografts. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(10):1314. doi: 10.1016/j.bbmt.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meshinchi S, Leisenring WM, Carpenter PA, et al. Survival after second hematopoietic stem cell transplantation for recurrent pediatric acute myeloid leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9(11):706. doi: 10.1016/j.bbmt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Michallet M, Tanguy ML, Socie G, et al. Second allogeneic haematopoietic stem cell transplantation in relapsed acute and chronic leukaemias for patients who underwent a first allogeneic bone marrow transplantation: a survey of the Societe Francaise de Greffe de moelle (SFGM) British journal of haematology. 2000;108(2):400. doi: 10.1046/j.1365-2141.2000.01851.x. [DOI] [PubMed] [Google Scholar]
- 12.Platzbecker U, Binder M, Schmid C, Rutt C, Ehninger G, Bornhauser M. Second donation of hematopoietic stem cells from unrelated donors for patients with relapse or graft failure after allogeneic transplantation. Haematologica. 2008;93(8):1276. doi: 10.3324/haematol.12798. [DOI] [PubMed] [Google Scholar]
- 13.Radich JP, Gooley T, Sanders JE, Anasetti C, Chauncey T, Appelbaum FR. Second allogeneic transplantation after failure of first autologous transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2000;6(3):272. doi: 10.1016/s1083-8791(00)70009-7. [DOI] [PubMed] [Google Scholar]
- 14.Freytes CO, Loberiza FR, Rizzo JD, et al. Myeloablative allogeneic hematopoietic stem cell transplantation in patients who experience relapse after autologous stem cell transplantation for lymphoma: a report of the International Bone Marrow Transplant Registry. Blood. 2004;104(12):3797. doi: 10.1182/blood-2004-01-0231. [DOI] [PubMed] [Google Scholar]
- 15.Carella AM, Cavaliere M, Lerma E, et al. Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin’s disease and non-Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(23):3918. doi: 10.1200/JCO.2000.18.23.3918. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. The lancet oncology. 2011;12(13):1195. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroger N, Schwerdtfeger R, Kiehl M, et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood. 2002;100(3):755. doi: 10.1182/blood-2002-01-0131. [DOI] [PubMed] [Google Scholar]
- 18.Maloney DG, Molina AJ, Sahebi F, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102(9):3447. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 19.Messori A, Bosi A, Bacci S, et al. Retrospective survival analysis and cost-effectiveness evaluation of second allogeneic bone marrow transplantation in patients with acute leukemia. Gruppo Italiano Trapianto di Midollo Osseo. Bone marrow transplantation. 1999;23(5):489. doi: 10.1038/sj.bmt.1701600. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Klar N, Weeks JC, Antin JH. Predicting costs of stem-cell transplantation. J Clin Oncol. 2000;18(1):64. doi: 10.1200/JCO.2000.18.1.64. [DOI] [PubMed] [Google Scholar]
- 21.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(7):805. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012;120(8):1545. doi: 10.1182/blood-2012-05-426783. [DOI] [PubMed] [Google Scholar]
- 23.Svahn BM, Remberger M, Alvin O, Karlsson H, Ringden O. Increased costs after allogeneic haematopoietic SCT are associated with major complications and re-transplantation. Bone marrow transplantation. 2012;47(5):706. doi: 10.1038/bmt.2011.162. [DOI] [PubMed] [Google Scholar]
- 24.Azoulay D, Linhares MM, Huguet E, et al. Decision for retransplantation of the liver: an experience- and cost-based analysis. Annals of surgery. 2002;236(6):713. doi: 10.1097/01.SLA.0000036264.66247.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone marrow transplantation. 2009;44(12):769. doi: 10.1038/bmt.2009.300. [DOI] [PubMed] [Google Scholar]
- 26.Mello MM, Brennan TA. The controversy over high-dose chemotherapy with autologous bone marrow transplant for breast cancer. Health Affairs. 2001;20(5):101. doi: 10.1377/hlthaff.20.5.101. [DOI] [PubMed] [Google Scholar]
- 27.Overview of BLS Statistics on Inflation and Prices. Available from: http://www.bls.gov/bls/inflation.htm. Accessed on June 29, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


