Abstract
Protein entrapment and high-performance affinity chromatography were used with zonal elution to examine the changes in binding that occurred for site-specific probes and various sulfonylurea drugs with normal and glycated forms of human serum albumin (HSA). Samples of this protein in a soluble form were physically entrapped within porous silica particles by using glycogen-capped hydrazide-activated silica; these supports were then placed into 1.0 cm × 2.1 mm inner diameter columns. Initial zonal elution studies were performed using (R)-warfarin and L-tryptophan as probes for Sudlow sites I and II (i.e., the major drug binding sites of HSA), giving quantitative measures of binding affinities in good agreement with literature values. It was also found for solutes with multisite binding to the same proteins, such as many sulfonylurea drugs, that this method could be used to estimate the global affinity of the solute for the entrapped protein. This entrapment and zonal approach provided retention information with precisions of ±0.1–3.3% (± one standard deviation) and elution within 0.50–3.00 min for solutes with binding affinities of 1 × 104–3 × 105 M−1. Each entrapped-protein column was used for many binding studies, which decreased the cost and amount of protein needed per injection (e.g., the equivalent of only 125–145 pmol of immobilized HSA or glycated HSA per injection over 60 sample application cycles). This method can be adapted for use with other proteins and solutes and should be valuable in high-throughput screening or quantitative studies of drug–protein binding or related biointeractions.
Keywords: Entrapment, High-performance affinity chromatography, Human serum albumin, Glycation, Drug-protein binding
Introduction
Diabetes is currently the seventh leading cause of death in the USA. This disease is characterized by an elevated level of glucose in blood and exists in two main forms: type I (juvenile or insulin-dependent) diabetes and type II (non-insulin-dependent or adult onset diabetes) [1]. One side effect of this disease is the glycation, or nonenzymatic addition, of reducing sugars such as glucose to amine groups on proteins [2–4]. One protein in blood that is affected by glycation is human serum albumin (HSA) [2–8]. HSA is not only the most abundant protein in human plasma, but it also serves as an important carrier protein for many drugs, fatty acids, and other solutes [9–12]. This protein has two major binding sites for drugs, which are commonly referred to as Sudlow sites I and II [10–12]. It is known Sudlow sites I and II, or residues near these sites, may be altered as a result of glycation [2–8, 13–15]. It has also been determined that this process could potentially alter the binding of some solutes with HSA [2–4,16], including several sulfonylurea drugs used to treat type II diabetes [17–21].
The purpose of this study is to examine a new combination of tools for quickly examining the overall binding of HSA to drugs and solutes and to determine how such binding might be affected by glycation. This approach is based on the use of entrapped proteins and high-performance affinity chromatography (HPAC). HPAC is a separation method in which a biologically related binding agent is used as the stationary phase on a high-performance liquid chromatography (HPLC) support such as porous silica [22–25]. This approach has been shown in many past reports to be a useful method for studying and characterizing the binding of solutes with biological agents [22, 26], including the interactions of drugs and other solutes with proteins such as HSA [23–25]. Advantages of HPAC include its speed, precision, ease of automation, and ability to reuse the same protein preparation for many experiments [23, 25].
In most studies with HPAC, proteins and other binding agents are covalently immobilized onto the surface of a support. However, this immobilization process can lead to a decrease or loss of activity for the binding agent owing to multisite attachment, improper orientation, or steric effects [26,27]. Recently, a noncovalent immobilization technique has been described for the physical entrapment of soluble proteins such as HSA within the pores or near the surface of silica supports [28]. This approach, as illustrated in Fig. 1, makes use of large particles of mildly oxidized glycogen that combine with hydrazide-activated silica and entrap soluble proteins on the support while still allowing access of small solutes and binding targets to these proteins. This method has been shown in prior work with frontal analysis and a site-selective probe for Sudlow site I, i.e., (S)-warfarin, to produce immobilized normal HSA with essentially full activity and binding constants that are in good agreement with values that are seen for soluble HSA [28].
Fig. 1.

Entrapment of a protein by reaction of hydrazide-activated silica with mildly oxidized glycogen. Details on this approach are provided in the text
This study will examine the combined use of HPAC columns and protein entrapment to compare the overall binding of representative drugs and probe compounds with normal HSA and glycated HSA. The emphasis will be on the use of such columns in zonal elution studies [23–25] to provide a relatively fast means for examining the binding of the entrapped proteins to injected targets. This method will first be evaluated by using it to compare the binding of normal HSA versus glycated HSA to (R)-warfarin and L-tryptophan (i.e., site-selective probes for Sudlow sites I and II, respectively) [10, 29, 30]. This approach will then be extended to studies examining the overall binding of several sulfonylurea drugs to the same protein samples. These results will be compared with those obtained by other methods for similar systems and should provide valuable data regarding the use of protein entrapment and HPAC for comparing the binding properties of normal and modified proteins. The same data should help illustrate the potential for this combined approach in high-throughput screening of drug–protein interactions.
Experimental
Chemicals
p-Periodic acid (periodic acid reagent, or H5IO6), glycogen (from bovine liver), HSA (Cohn fraction V, 99% globulin free, 99% fatty acid free), glycated HSA (95% lyophilized, lot no. 115K6108, prepared in vitro and containing 1.8 mol hexose per mole of HSA), (R)-warfarin, L-tryptophan, acetohexamide, gliclazide, and tolbutamide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nucleosil Si-300 silica (300-Å pore size, 7-μm particle diameter) was purchased from Macherey-Nagel (Düren, Germany). Reagents for the micro bicinchoninic acid (BCA) protein assay were from Pierce (Rockford, IL, USA). Other chemicals were of the purest grades available. Econo-Pac 10 DG disposable 10-mL desalting columns were obtained from Bio-Rad Laboratories (Hercules, CA, USA). All solutions were prepared using water from a NANO pure purification system (Barnstead, Dubuque, IA, USA) and filtered through 0.20-μm GNWP nylon membranes from Millipore (Billerica, MA, USA).
Instrumentation
The chromatographic system consisted of a JASCO (Tokyo, Japan) PU-980i intelligent HPLC isocratic pump, a Rheodyne (Cotati, CA, USA) Advantage PF ten-port valve, and a JASCO UV-975 UV/vis detector. The detection wavelengths were as follows: 304 nm, (R)-warfarin; 280 nm, L-tryptophan; 205 nm, sodium nitrate; 248 nm, acetohexamide; 226 nm, gliclazide; and 250 nm, tolbutamide. Data were collected using an interface and software from National Instruments (Austin, TX, USA). The temperature of the columns and mobile phases was controlled using a PolyScience (Buffalo Grove, IL, USA) circulating water bath and a water jacket from Alltech (Deerfield, IL, USA). All columns were packed using an HPLC column slurry packer from Alltech. The chromatographic data were analyzed using Peakfit 4.12 (Jandel Scientific Software, San Rafael, CA, USA).
Methods
The overall scheme used for protein entrapment was based on a procedure reported in [28] using normal HSA as a model; the same method was found to entrap glycated HSA in preliminary studies in this prior report. The oxidation of glycogen was performed in pH 5.0 buffer containing 20 mM sodium acetate and 15 mM sodium chloride. A 4.0 mL solution was prepared by dissolving 135 mg of periodic acid and 17 mg of glycogen in this buffer. The resulting solution was covered in aluminum foil, because of the light sensitivity of periodic acid, and was shaken at room temperature for 12–24 h. After oxidation, the glycogen was separated from the remaining periodic acid by using an Econo-Pac 10DG disposable desalting column and pH 5.0, 0.10 M potassium phosphate buffer as the mobile phase. The resulting solution of oxidized glycogen had a concentration of approximately 4.2 mg/mL, a final volume of around 4.0 mL, and approximately 0.5% oxidation of the glucose units in glycogen [28]. This solution was stored in pH 5.0, 0.10 M phosphate buffer at 4 °C.
The normal HSA and glycated HSA supports were prepared under the same immobilization conditions. A 50 mg/mL stock solution of each protein was first prepared in pH 5.0, 0.10 M potassium phosphate buffer. The starting support, Nucleosil Si-300, was converted into a hydrazide-activated form according to a previous procedure [31]. To create the entrapped-protein support, 80 mg of hydrazide-activated silica was combined with 160 μL of the protein solution. This mixture was sonicated under a vacuum for 15 min to remove any air trapped within the support’s pores. A 380-μL portion of the oxidized glycogen solution was then added, and this mixture was shaken at room temperature for 12 h. During the final hour of the reaction, 200 μL of a 2 mg/mL oxalic dihydrazide solution in pH 5.0, 0.10 M phosphate buffer was added to the reaction mixture to combine with any remaining aldehyde groups on the glycogen. After immobilization, the support was washed several times with pH 7.4, 0.067 M potassium phosphate buffer. A control support was prepared in the same manner by using only pH 5.0, 0.10 M phosphate buffer in place of the protein solution during the entrapment step [28]. The protein content of each final support was determined in triplicate by using a micro BCA assay, with soluble HSA or glycated HSA being used as the standard and the control support being used as a blank.
All supports were downward slurry-packed into separate 1.0 cm × 2.1 mm diameter stainless steel columns at 27.6 MPa (4,000 psi) using pH 7.4, 0.067 M potassium phosphate buffer as the packing solution. The columns were stored at 4 °C in this packing solution. The sample solutions for the chromatographic studies were prepared in pH 7.4, 0.067 M potassium phosphate buffer, which was also used as the mobile phase. Stock solutions with a concentration of 30 μM (R)-warfarin, or 100 μM L-tryptophan, acetohexamide, gliclazide, or tolbutamide were prepared in the same pH 7.4 buffer and diluted with this pH 7.4 buffer to give 10 μM working samples for these analytes; these sample concentrations were found to be sufficiently low compared with the column’s overall binding capacity to provide linear elution conditions during the retention factor measurements.
A 20-μL volume of each 10 μM sample solution was injected in triplicate onto the entrapped-protein columns and the control column at 37 °C. Flow rates of 0.1–1.0 mL/min were used to examine the binding of each analyte to the columns. The void time of each column was determined by making triplicate 20-μL injections of 25 μM sodium nitrate in the presence of pH 7.4, 0.067 M potassium phosphate buffer at all the flow rates tested. Sodium nitrate has routinely been used in the past as a void volume marker for columns containing covalently immobilized HSA and glycated HSA, as well as with columns containing hydrazide-activated silica, and has been found to give good agreement with calculated void volumes based on the known packing densities and porosities of the corresponding supports [19–21, 23, 25, 28–31]. The void time of the system was determined by repeating the injections of sodium nitrate but using a zero-volume union in place of the column. A fit to an exponentially modified Gaussian curve was used to determine the central moment of each peak for the calculation of retention factors.
Results and discussion
General properties of entrapped HSA and glycated HSA columns
The entrapment supports were found through a BCA protein assay to contain 37 (± 1) mg HSA per gram of silica, or 32 (± 2) mg glycated HSA per gram of silica, where the values listed in parentheses throughout this article represent a range of ± one standard deviation. These supports were packed into 1.0 cm × 2.1 mm inner diameter columns, resulting in a total protein content in terms of moles (mLtotal) of 8.7 nmol or 7.5 nmol (i.e., 500 μg or 580 μg) for the HSA and glycated HSA (note these values were estimated by using a packing density of 0.45 g/cm3 for the support material, as reported by the manufacturer, and a molar mass for HSA and glycated HSA of 66.5 kDa). The columns were found to be stable and give reproducible retention factors over the course of more than 60 sample injection cycles and approximately 4 months of operation. Because each entrapped protein sample was used for many binding studies, this resulted in a decrease in the cost and overall amount of protein needed per injection. For instance, the equivalent of only 125–145 pmol (8.3–9.7 μg) of immobilized HSA or glycated HSA was used per injection when employed over the course of 60 application cycles.
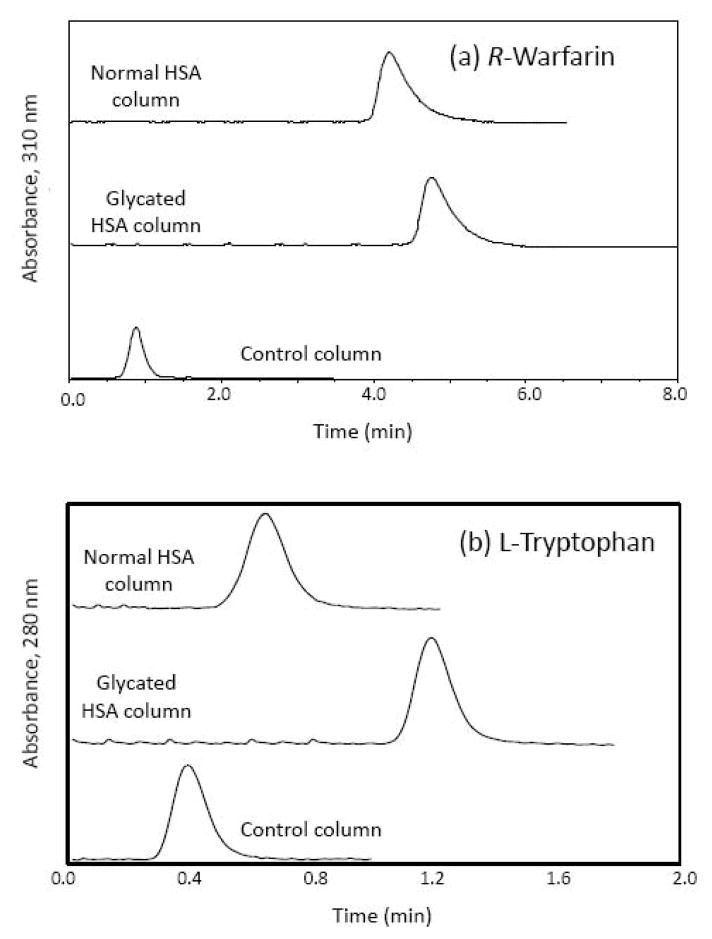
These columns were initially evaluated by using zonal elution as a rapid means for obtaining estimates of binding affinities for injected probe compounds or solutes with the entrapped proteins. Figure 2 provides some typical chromatograms that were obtained for (R)-warfarin and L-tryptophan on these columns. When compared with the control column containing no protein, both the normal HSA column and te glycated HSA column showed markedly increased retention for both probe compounds. (R)-Warfarin, which has an association equilibrium constant of roughly 2 × 105–3 × 105 M−1 for normal HSA [29], was eluted within 5–6 min at 0.5 mL/min from both types of entrapped-protein columns and within 2.5–3 min from the same columns at 1.0 mL/min. L-Tryptophan, which has a tenfold lower association equilibrium constant of approximately 1 × 104–2 × 104 M−1 for normal HSA [30], was eluted in only 1.0–1.5 min at 0.5 mL/min from both types of entrapped-protein columns and within 0.50–0.75 min at 1.0 mL/min. Reproducible retention factors and stable binding characteristics were obtained on these columns for triplicate injections obtained over five flow rates ranging from 0.1 to 1.0 mL/min, with retention factors showing only random variations of ±4% overall for (R)-warfarin and ±11% for L-tryptophan. The precisions of the retention factors measured at individual flow rates for the same solutes ranged from ±0.4 to ±1.1% and from ±0.6 to ±3.3%, respectively, with the precision increasing as slower flow rates and longer residence times were used.
Fig. 2.
Chromatograms obtained for the injection of a (R)-warfarin or b L-tryptophan as a site-selective probe onto a control column containing no entrapped protein or columns containing entrapped normal human serum albumin (HSA) or glycated HSA. These results were obtained at 0.50 mL/min. Other conditions are given in the text
Binding of (R)-warfarin with entrapped HSA and glycated HSA
The next part of this study used HPAC and the columns containing entrapped samples of normal HSA or glycated HSA to evaluate the binding of these columns to (R)-warfarin as a probe for Sudlow site I (i.e., the warfarin–azapropazone site) [10, 29, 32]. This drug (see Fig. S1 for the structure) has been previously shown to bind to both normal and glycated HSA in a site-selective manner. The association equilibrium constants at pH 7.4 and 37 °C for these interactions have been estimated by various methods for normal HSA [2, 29, 30, 32–34] and by frontal analysis for in vitro glycated HSA [2], thus providing reference values for this current study for the evaluation of the entrapped-protein columns.
In the zonal elution approach that was used in this study, the specific retention factor (k) was determined for an injected drug or solute on a given protein column after correcting for the effect of any nonspecific interactions with the support on the total retention, e.g., a correction for (R)-warfarin which was only 2.5–2.6% of the total retention seen on the entrapped protein columns; this approach was adequate for the current study, but a more thorough analysis of the nonspecific interactions could be obtained in future work through frontal analysis. The use of the specific retention factor provided a relatively fast and easy way of measuring and comparing the level of binding for each injected solute on the entrapped-protein columns. For more detailed studies of binding, the resulting value of k due to the entrapped protein could also be directly related to the global affinity constant (nKa′) of the solute with the immobilized protein, as represented by the general expression in Eq. 1 for any group of independent binding sites [23, 25]:
| (1) |
where mLtotal again represents the total number of moles of protein, and VM is the column void volume [23].
It is important to note in Eq. 1 that nKa′ is a number-weighted sum of the association equilibrium constants for all binding sites of the solute on the immobilized protein, as indicated by Eq. 2 for a system with multiple, independent binding sites [23,25]:
| (2) |
In Eq. 2, ni represents the relative number of moles of binding site i for a given solute per mole of protein, and Kai is the association equilibrium constant for the same site and solute. If only a single type of binding site for a drug or solute is present and essentially all of the immobilized protein is present in an active form (i.e., as could occur for an entrapped but noncovalently immobilized protein), Eq. 1 can be rewritten as Eq. 3:
| (3) |
In this specific case, n = 1 and nKa′ is now given by Ka, the association equilibrium constant for the solute at its binding site on the protein.
Table 1 summarizes the specific retention factors that were measured for (R)-warfarin on the columns containing entrapped normal HSA and glycated HSA. To correct for differences in the protein content of these supports and to allow a direct comparison of their retention values, the specific retention factors were normalized by dividing them by each support’s total protein content [35, 36]. When these normalized retention factors were compared, no significant difference at the 95% confidence level (i.e., the confidence level used for all comparisons in this study) was seen in the values for (R)-warfarin on the columns containing normal HSA and glycated HSA. These results indicated the level of glycation present in this case did not have an observable effect on the binding of (R)-warfarin with HSA at Sudlow site I. These results are consistent with previous data and conclusions obtained when frontal analysis was used to compare covalently immobilized normal HSA with in vitro glycated HSA that had levels of modification of the glycated HSA similar or equivalent to those used in the current study [2].
Table 1.
Retention factors and global affinity constants measured for (R)-warfarin and L-tryptophan on columns containing entrapped normal human serum albumin (HSA) or glycated HSA
| Type of HSA and solute | Specific retention factor, ka | Normalized retention factor, k/(protein content)b | Global affinity, nKa′ (M−1)c |
|---|---|---|---|
| Normal HSA | |||
| (R)-Warfarin | 84.7 (± 0.5) | 2.29 (± 0.06) | 2.9 (± 0.1) × 105 |
| L-Tryptophan | 5.7 (± 0.2) | 0.15 (± 0.01) | 1.8 (± 0.1) × 104 |
| Glycated HSA | |||
| (R)-Warfarin | 78.5 (± 0.8) | 2.45 (± 0.08) | 3.1 (± 0.1) × 105 |
| L-Tryptophan | 14.22 (± 0.03) | 0.44 (± 0.01) | 5.5 (± 0.2) × 104 |
The values in parentheses represent a range of ± one standard deviation, as based on triplicate measurements made over five flow rates (n = 15). As indicated in the text, the same range of flow rates was used to initially evaluate the reproducibility and stability of these columns over a range of operating conditions but gave no significant differences in the measured retention factors.
The specific retention factor was obtained by taking the difference between the overall retention factor on an entrapped-protein column and the retention factor due to nonspecific interactions measured for the same solute on the control column, with the latter value being 2.13 (± 0.05) for (R)-warfarin and 0.77 (± 0.03) for L-tryptophan.
The normalized retention factors were calculated by dividing the specific retention factors by the measured total protein content for each given support, using values of 37 (± 1) mg HSA per gram or 32 (± 2) mg glycated HSA per gram.
The value of nKa′ was estimated by using Eq. 1 along with the specific retention factor (k) for each solute, the measured void volume (VM), and the number of moles of protein in the column (mLtotal). The value used for VM was 29 μL and mLtotal was 8.7 nmol or 7.5 nmol for the normal HSA or glycated HSA columns, respectively; details on the determination of VM and mLtotal are provided in the text.
Using the fact that (R)-warfarin has only one major binding site on normal HSA and glycated HSA, and that most of these entrapped proteins should have been active, we used the retention data to further estimate and compare the association equilibrium constants for this interaction. These results are also provided in Table 1. The Ka of 2.9 (± 0.1) × 105 that was calculated from the zonal elution data for (R)-warfarin with normal HSA was in good agreement with previously reported values of 2.1× 105–3.3 × 105 M−1 for this interaction under the same temperature conditions [2, 29, 30, 32–34]. The Ka of 3.1 (± 0.1) × 105 M−1 obtained for (R)-warfarin with the glycated HSA was also consistent with prior estimates of 2.3 (± 0.3) × 105–to 2.7 (± 0.3) × 105 M−1 that have been made by frontal analysis for similar preparations of this protein [2]. In addition, the calculated Ka values did not show any significant difference between the normal HSA and glycated HSA columns, as noted in the prior studies [2].
Binding of L-tryptophan with entrapped HSA and glycated HSA
The same columns as described in the previous section were examined for their binding to L-tryptophan as a probe for Sudlow site II (i.e., the indole–benzodiazepine site) [10, 30, 37]. Like (R)-warfarin, this solute (see Fig. S1 for the structure) is known to bind to both normal and glycated HSA in a site-selective manner [2, 10, 30]. The association equilibrium constant for the binding of L-tryptophan with normal HSA has also been determined by various techniques at 37 °C [30, 34, 37, 38], and the binding of this solute under the same conditions with in vitro glycated HSA has been previously examined by frontal analysis [2], again providing values that could be used to evaluate the performance of the entrapped-protein columns.
The results from the zonal elution studies that were obtained for L-tryptophan are included in Table 1. Unlike the data acquired for (R)-warfarin, the normalized retention factors that were calculated for L-tryptophan showed a large change when going from normal HSA to glycated HSA, even after a correction had been made for differences in the total protein content of the columns. The normalized retention factor for L-tryptophan on the entrapped glycated HSA column was roughly three times higher than that for L-tryptophan on the column containing normal HSA. This difference was significant at the 95% confidence level. The value of nKa′ between these two columns increased by the same factor and was again significant at the 95% confidence level. These results clearly indicated that the glycation of HSA did affect the binding of L-tryptophan to Sudlow site II, as was concluded in a previous study [2].
Given the fact that L-tryptophan has only one major binding site on HSA, the global affinity measured for this solute was assumed to represent the association equilibrium constant for this interaction. The average Ka of 1.8 (± 0.1) × 104 M−1 that was estimated by this approach for L-tryptophan with the entrapped normal HSA was in good agreement with previous values of 1.1 × 104–2.4 × 104 M−1 that have been determined by other methods for this interaction at 37 °C [2, 30, 34, 37–39]. The Ka of 5.5 (± 0.2) × 104 M−1 measured for L-tryptophan with the entrapped glycated HSA also fit within the range of 5.2 × 104–6.4 × 104 M−1 that has previously been measured by frontal analysis at 37 °C with in vitro glycated HSA preparations having similar levels of modification [2].
Binding of sulfonylurea drugs to entrapped HSA and glycated HSA
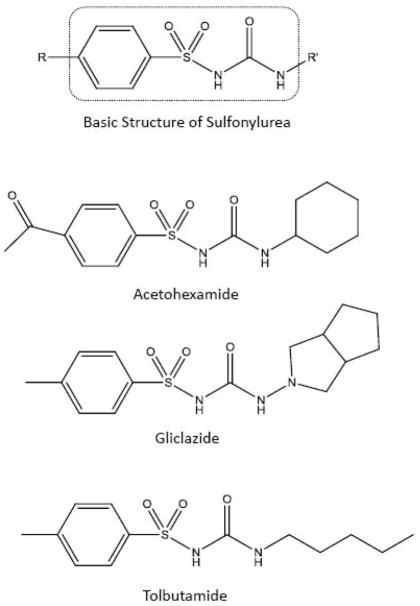
The final stage of this work used the entrapped HSA and glycated HSA columns to screen and compare the binding of various sulfonylurea drugs to these proteins. The sulfonylurea drugs considered in this study were acetohexamide, gliclazide, and tolbutamide (see the structures in Fig. 3). Unlike (R)-warfarin and L-tryptophan, these sulfonylurea drugs are known to bind to both Sudlow site I and Sudlow site II of normal HSA and glycated HSA, as well having a set of weaker interaction sites with these proteins [2, 17, 19–21]. Thus, for these drugs the use of retention factors to describe protein interactions would be expected to reflect the contributions of multiple binding regions, as indicated by Eqs. 1 and 2, rather than a single binding site, as described by Eq. 3.
Fig. 3.
Basic structure of a sulfonylurea drug and the structures of the specific sulfonylurea drugs that were examined in this study
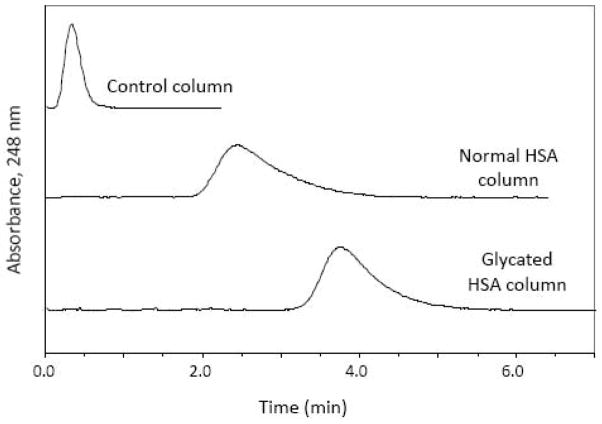
Some typical chromatograms obtained in these studies are shown in Fig. 4. As noted earlier with (R)-warfarin and L-tryptophan, each of the sulfonylurea drugs exhibited a large difference in retention between the entrapped-protein columns and the control column. These drugs were eluted within 1.1–2.5 min at 0.5 mL/min and within 0.6–1.3 min at 1.0 mL/min from the columns containing the entrapped proteins. The measured retention factors had good reproducibility over flow rates ranging from at least 0.1 to 1.0 mL/min, with values varying by only ±3%, ±6%, or ±8% for acetohexamide, gliclazide, and tolbutamide, respectively. The retention factors measured at individual flow rates for the same drugs ranged from ±0.1 to ±0.6%, from ±0.1 to ±1.7%, and from ±0.2 to ±0.7%, respectively, and tended to improve as the flow rate was decreased and the residence time was increased.
Fig. 4.
Chromatograms obtained for acetohexamide on a control column containing no entrapped protein or on columns containing entrapped normal HSA or glycated HSA. These results were obtained at 0.50 mL/min. Other conditions are given in the text
Table 2 provides the specific retention factors that were measured for each of the sulfonylurea drugs on the normal HSA and glycated HSA columns. Normalized retention factors that corrected for differences in the protein content of these columns were also calculated (see the footnote in Table 2). These sulfonylurea drugs showed an increase of 55–77% in the normalized retention factors for the glycated HSA column compared with the normal HSA column. This increase in the normalized retention factor was in agreement with previously reported trends noted for these sulfonylureas when covalently immobilized samples of normal HSA and a preparation of in vitro glycated HSA similar to that used in the current study were used [18]. These trends also agreed with those seen in separate ultrafiltration studies using physiological levels of normal HSA and in vitro glycated HSA [20, 21, 40].
Table 2.
Retention factors and global affinity constants measured for sulfonylurea drugs on columns containing entrapped normal HSA or glycated HSA
| Type of HSA and drug | Specific retention factor (k) | Measured global affinity, nKa′ (M−1)a | Estimated global affinity, nKa′ (M−1)b |
|---|---|---|---|
| Normal HSA | |||
| Acetohexamide | 46.5 (± 0.1) | 1.6 (± 0.1) × 105 | 1.6 × 105–1.7 × 105 |
| Gliclazide | 17.1 (±0.2) | 5.8 (± 0.2) × 104 | 5.2 × 104–7.9 × 104 |
| Tolbutamide | 30.8 (± 0.1) | 1.0 (± 0.1) × 105 | 1.0 × 105–1.1 × 105 |
| Glycated HSA | |||
| Acetohexamide | 62.7 (± 0.1) | 2.5 (± 0.1) × 105 | 1.6 × 105–1.8 × 105 |
| Gliclazide | 23.4 (± 0.1) | 9.1 (± 0.3) × 104 | 6 × 104–12 × 104 |
| Tolbutamide | 47.0 (± 0.2) | 1.9 (±0.1) × 105 | 1.1 × 105–1.5 × 105 |
The values in parentheses represent a range of ± one standard deviation, as based on triplicate measurements made over five flow rates (n = 15). The specific retention factors and values for nKa′ were determined in the same manner as described in Table 1. In obtaining the specific retention factors, we found the retention factor measured for nonspecific interactions by these drugs on the control column were 0.42 (± 0.04) for acetohexamide, 0.95 (± 0.10) for gliclazide, and 0.39 (± 0.04) for tolbutamide.
The nKa′ values measured for the normal HSA column were determined by using the following normalized retention factors: 1.26 (± 0.03) for acetohexamide, 0.46 (± 0.01) for gliclazide, and 0.83 (± 0.02) tolbutamide. The nKa′ values for the glycated HSA column were found by using the following normalized retention factors: 1.96 (± 0.06) for acetohexamide, 0.73 (± 0.02) for gliclazide, and 1.47 (± 0.05) for tolbutamide.
These retention data were further used to estimate the global affinities of each sulfonylurea drug with the entrapped samples of HSA and glycated HSA, as given in Table 2. In this case, it was known from prior studies that all of these drugs had two sets of binding sites on HSA (i.e., moderate-to-high affinity sites and weak binding regions), with most of this binding being attributed to one or two moderate-to-high affinity sites [17, 19–21]. Thus, the global affinities shown in Table 2 would be predicted from Eq. 2 to represent a number-weighted sum of the association equilibrium constants for these sites. Note that zonal elution competition studies with probes such as (R)-warfarin and L-tryptophan could also be employed to obtain information on the affinities at specific binding sites [17, 19–21, 23]. The relative trends shown in Table 2 for these results were the same as noted for the normalized retention factors, as would be expected from Eq. 1 because these two sets of values were directly proportional to each other.
The global affinities given in Table 2 compared well with those predicted by Eqs. 1 and 2 when calculating such results using previous binding capacities and association equilibrium constants obtained by frontal analysis, or a combination of these results with site-selective binding constants that were obtained by zonal elution at the higher-affinity sites for sulfonylurea drugs with covalently immobilized samples of normal HSA and in vitro glycated HSA [17, 19–21]. In each case, most of the calculated global affinity (i.e., 92–99% for acetohexamide, 82–92% for gliclazide, and 92–98% for tolbutamide) was due to the contributions of the moderate-to-high binding regions for these drugs with HSA (i.e., Sudlow sites I and II).
Because the zonal elution approach used in this study employed direct measurements of retention to estimate global affinities, the precision of this approach was much better than when combining association equilibrium constants and binding capacity results to calculate global affinities. For instance, the global affinities obtained by using data from prior frontal analysis and zonal elution studies [17, 19–21] had typical final precisions of ±20–50%, as based on error propagation. In contrast, the global affinities in Table 2 that were measured directly by zonal elution for the entrapped-protein columns had precisions of ±3–10%.
Another potential advantage of using entrapped proteins to measure global affinities was that little or no loss of activity should have occurred during the immobilization process [26, 28]. This feature is essential if zonal elution data are to be used directly for determining binding constants [23, 25] and has made this approach generally impractical for use with covalent immobilization techniques because the loss of protein activity is common during such coupling methods [23, 25, 26]. For instance, it was estimated from previous frontal analysis data [17, 19–21] that a combined loss in activity of 35–60% for acetohexamide, 45–59% for tolbutamide, and 75–80% for gliclazide occurred at Sudlow sites I and II when normal HSA and glycated HSA that had been covalently immobilized by the Schiff base method were used [17, 19–21]. On the basis of prior work with (R)-warfarin and L-tryptophan, it is expected that a similar or even larger decrease in the activity for HSA might occur when other covalent immobilization methods are used [35, 36].
Concluding remarks
This report examined the use of entrapment and HPAC-based zonal elution studies to examine the changes in binding and overall affinity that occurred for site-specific probes and various sulfonylurea drugs with normal HSA versus glycated HSA. The columns created by entrapment gave retention factors with precisions of ±0.1–3.3% over the course of more than 4 months of operation. The compounds retained on the entrapped-protein columns had elution times of less than 2.5–3.0 min at 1.0 mL/min for solutes with affinities as high as 2× 105–3 × 105 M−1, e.g., as represented by (R)-warfarin on the normal HSA and glycated HSA columns, and less than 0.50–0.75 min for solutes with affinities down to 1× 104–2 × 104 M−1, e.g., as seen for L-tryptophan on the normal HSA column. The ability to use these columns over many experiments produced a system that required the equivalent of less than 125–145 pmol of immobilized protein per injection over 60 application cycles. All of these characteristics are attractive for using such an approach in biointeraction studies and in the high-throughput screening of drug–protein binding.
The ability of this method to provide a rapid, quantitative measure of binding affinity was also examined. In the case of solutes having a single major binding site on HSA, this approach could be used to determine the association equilibrium constant for the injected solutes with the entrapped proteins. For instance, the binding constants determined by this approach for (R)-warfarin at Sudlow site I and for L-tryptophan at Sudlow site II were in good agreement with results reported with other techniques for normal HSA or glycated HSA [2, 29, 30, 32–34, 37–39]. For solutes with multisite binding, this method could be used to estimate the global affinity of the solute for the entrapped protein. This was illustrated by using this approach to examine and compare the global affinities of various sulfonylurea drugs with normal HSA and glycated HSA. The values obtained again showed good agreement with trends and results that were predicted by other techniques and with use of similar protein preparations [17–21, 40].
These results indicate the entrapped-protein columns could be used directly with HPAC and zonal elution to estimate and compare binding constants or global affinities for normal and modified proteins such as HSA and glycated HSA. Obtaining these quantitative estimates of affinities was made possible by the fact the entrapped protein was immobilized in a form that retained all or most of its original activity [28]. This feature, in turn, allowed the value of Ka or nKa′ to be determined from the measured retention factors of solutes if the protein content of the support was also known. This direct approach based on zonal elution was not possible in the past when covalent immobilization methods were used because of the loss of activity that occurs for some of the immobilized protein and the possible presence of different degrees of inactivation at separate regions on an immobilized protein [26]. Instead, retention measurements have been used in the past to make only relative comparisons of activity [18, 23, 35, 36], or more complicated and time-consuming methods based on frontal analysis or zonal elution plus competition experiments have been required to obtain binding constants that can be measured in a manner independent of the actual amount of active binding sites [2, 17, 19–21, 23–25].
The combined speed and ease of using zonal elution with the entrapped forms of proteins should make this approach attractive for clinical and pharmaceutical applications that may benefit from high-throughput screening or quantitative studies of binding between proteins and small soluble targets. For instance, the shifts in normalized retention factors and affinities that were measured when entrapped normal HSA and glycated HSA were used are of potential interest in examination of how glycation may affect the protein binding of sulfonylurea drugs in blood [17–21]. Such information, in turn, may help clinicians to avoid issues with using either too low or too high of a dose for these drugs when treating diabetes, which could lead to the undesired side effects of inadequate glucose control or hypoglycemia, respectively [19, 41]. The same general methods as used in this study could be adapted for other proteins and solutes or for comparing different protein populations and biointeraction systems of interest in clinical, pharmaceutical, or biomedical research.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health under grants R01 GM044931 and R01 DK069629 and was conducted in facilities which were renovated under grant RR015468.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet. Vol. 2001. Centers for Disease Control and Prevention; Atlanta: 2001. [Google Scholar]
- 2.Joseph KS, Hage DS. J Pharm Biomed Anal. 2010;53:811–818. doi: 10.1016/j.jpba.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendez DL, Jensen RA, McElroy LA, Pena JM, Esquerra RM. Arch Biochem Biophys. 2005;444:92–99. doi: 10.1016/j.abb.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Nakajou K, Watanabe H, Kragh-Hansen U, Maruyama T, Otagiri M. Biochim Biophys Acta. 2003;1623:88–97. doi: 10.1016/j.bbagen.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Colmenarejo G. Med Res Rev. 2003;23:275–301. doi: 10.1002/med.10039. [DOI] [PubMed] [Google Scholar]
- 6.Koyama H, Sugioka N, Uno A, Mori S, Nakajima K. Biopharm Drug Dispos. 1997;18:791–801. doi: 10.1002/(sici)1099-081x(199712)18:9<791::aid-bdd66>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Iberg N, Fluckiger R. J Biol Chem. 1986;261:13542–13545. [PubMed] [Google Scholar]
- 8.Garlick RL, Mazer JS. J Biol Chem. 1983;258:6142–6146. [PubMed] [Google Scholar]
- 9.Ascoli GA, Domenici E, Bertucci C. Chirality. 2006;18:667–679. doi: 10.1002/chir.20301. [DOI] [PubMed] [Google Scholar]
- 10.Sudlow G, Birkett DJ, Wade DN. Mol Pharmacol. 1976;12:1052–1061. [PubMed] [Google Scholar]
- 11.Sudlow G, Birkett DJ, Wade DS. Mol Pharmacol. 1975;11:824–832. [PubMed] [Google Scholar]
- 12.Peters T., Jr . All about albumin: biochemistry, genetics and medical applications. Academic; San Diego: 1996. [Google Scholar]
- 13.Barnaby OS, Cerny RL, Clarke W, Hage DS. Clin Chim Acta. 2011;412:277–285. doi: 10.1016/j.cca.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnaby OS, Cerny RL, Clarke W, Hage DS. Clin Chim Acta. 2011;412:1606–1615. doi: 10.1016/j.cca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frolov A, Hoffman R. Anal Bioanal Chem. 2010;397:2349–2356. doi: 10.1007/s00216-010-3810-9. [DOI] [PubMed] [Google Scholar]
- 16.Sattarahmady N, Moosavi-Movahedi AA, Ahmad F, Hakimelahi GH, Habib-Rexaei M, Saboury AA, Sheibani N. Biochim Biophys Acta. 2007;1770:933–942. doi: 10.1016/j.bbagen.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Joseph KS, Hage DS. J Chromatogr B. 2010;878:1590–1598. doi: 10.1016/j.jchromb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basiaga SBG, Hage DS. J Chromatogr B. 2010;878:3193–3197. doi: 10.1016/j.jchromb.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda R, Anguizola J, Joseph KS, Hage DS. Anal Bioanal Chem. 2011;401:2811–2819. doi: 10.1007/s00216-011-5382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph KS, Anguizola J, Jackson AJ, Hage DS. J Chromatogr B. 2010;878:2775–2781. doi: 10.1016/j.jchromb.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph KS, Hage DS. J Pharm Biomed Anal. 2011;54:426–432. doi: 10.1016/j.jpba.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustavsson PE, Larsson PO. In: Handbook of affinity chromatography. 2. Hage DS, editor. chap 2 CRC; Boca Raton: 2006. [Google Scholar]
- 23.Schiel JE, Joseph KS, Hage DS. In: Advances in chromatography. Grinsberg N, Grushka E, editors. chap 4 Taylor & Francis; New York: 2009. [Google Scholar]
- 24.Patel S, Wainer IW, Lough WJ. In: Handbook of affinity chromatography. 2. Hage DS, editor. chap 24 CRC; Boca Raton: 2006. [Google Scholar]
- 25.Hage DS, Jackson A, Sobansky MR, Schiel JE, Yoo MJ, Joseph KS. J Sep Sci. 2009;32:835–853. doi: 10.1002/jssc.200800640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hage DS, Kim HS. In: Handbook of affinity chromatography. 2. Hage DS, editor. chap 3 CRC; Boca Raton: 2006. [Google Scholar]
- 27.Walters RR. Anal Chem. 1985;57:1099A–1114A. doi: 10.1021/ac00288a001. [DOI] [PubMed] [Google Scholar]
- 28.Jackson AJ, Xuan H, Hage DS. Anal Biochem. 2010;404:106–108. doi: 10.1016/j.ab.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loun B, Hage DS. Anal Chem. 1994;66:3814–3822. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 30.Loun B, Hage DS. J Chromatogr. 1992;579:225–235. [PubMed] [Google Scholar]
- 31.Ruhn PF, Garver S, Hage DS. J Chromatogr A. 1994;669:9–19. doi: 10.1016/0021-9673(94)80332-3. [DOI] [PubMed] [Google Scholar]
- 32.Miller JHM, Smail GA. J Pharmacy Pharmacol. 1977;29:33P. doi: 10.1111/j.2042-7158.1977.tb11501.x. [DOI] [PubMed] [Google Scholar]
- 33.O’Reilly RA. Mol Pharmacol. 1971;7:209–218. [PubMed] [Google Scholar]
- 34.Lagercrantz C, Larsson T, Denfors I. Comp Biochem Physiol. 1981;69C:375–378. doi: 10.1016/0306-4492(81)90153-2. [DOI] [PubMed] [Google Scholar]
- 35.Mallik R, Jiang T, Hage DS. Anal Chem. 2004;76:7013–7022. doi: 10.1021/ac049001q. [DOI] [PubMed] [Google Scholar]
- 36.Pfaunmiller EL, Hartmann M, Dupper CM, Soman S, Hage DS. J Chromatogr A. 2012;1269:198–207. doi: 10.1016/j.chroma.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMenamy RH, Seder RH. J Biol Chem. 1963;238:3241–3248. [PubMed] [Google Scholar]
- 38.Lagercrantz C, Larsson T, Karlsson H. Anal Biochem. 1979;99:352–364. doi: 10.1016/s0003-2697(79)80019-6. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Hage DS. J Chromatogr A. 1996;725:273–285. doi: 10.1016/0021-9673(95)01009-2. [DOI] [PubMed] [Google Scholar]
- 40.Anguizola JA. MS thesis. University of Nebraska; Lincoln: 2009. Analysis of drug binding to serum proteins in diabetes. [Google Scholar]
- 41.Davis SN. In: Goodman and Gilman’s the pharmacological basis of therapeutics. 11. Brunton LL, Lazo JS, Parker KL, editors. chap 60 McGraw-Hill; New York: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.