Abstract
Background
The liver is an immunological privileged organ – liver allografts are accepted across major histocompatibility complex barriers in many species; however, hepatocyte transplants are acutely rejected, suggesting a role for liver non-parenchymal cells in regulating the immune response. We have shown potent immune regulatory activity of hepatic stellate cells (HSCs) in mice. The aim of this study was to examine the immune regulatory activity of human HSCs.
Methods
HSCs were isolated from normal human livers for analyses of their impact on T cell response.
Results
HSCs expressed low HLA-DR and co-stimulatory molecules CD40, CD80, but constitutively expressed high levels of CD54. IFNγ stimulated HSCs to express B7-H1 in a dose dependent manner, and produce the suppressive cytokines IL-6, IL-10 and TGFβ, but not affect expression of HLA-DR, CD40 and CD80. Human HSCs did not stimulate allogeneic T cell proliferative response, indicating they are not professional APC. HSCs markedly inhibited T cell response elicited by either allogeneic APC or CD3/CD28 beads, which was associated with increases in activated CD4 and CD8 T cell apoptosis. Addition of anti-B7-H1 blocking Ab significantly reversed the inhibitory effect.
Conclusions
Human HSCs demonstrate potent immune regulatory activity via B7-H1-mediated induction of apoptosis in activated T cells. Understanding of the involved mechanisms may lead to development of novel therapeutic approaches for treatment of liver diseases.
Keywords: Hepatic stellate cell, Immune regulation, T cell response, Apoptosis, B7-H1
Introduction
The liver is known for its immune tolerogenic property, including: 1) spontaneous acceptance of liver transplants in many animal models [1–3]. In humans, total weaning off immunosuppression achieves in about one fourth of liver transplant recipients [4–7]; 2) transplantation across ABO barriers leads to accelerated rejection in kidneys but is rarely in livers [8]; 3) accelerated rejection caused by antibodies observed commonly for kidney transplants, is rare in the liver grafts [9, 10]. Moreover, induction of tolerance by the oral administration of antigens has also been attributed to the liver, since similar tolerance can be induced through direct delivery of antigen via the portal vein, but not via the intravenous route [11–13]. Hepatic tolerance may also contribute to the liver’s vulnerability to chronic infections, such as hepatitis B, hepatitis C and parasitic infections [14]. It has been postulated that regulation of immune response in the liver is necessary because of the propensity for overwhelming reactivity to the food and microbial antigens from the gut [15].
We have demonstrated that the liver transplant tolerance in mice is not attributable to the deletion of specific T cell clones since the T cells isolated from the recipients respond well to donor antigen stimulation in vitro [16]. Histology shows abundance of T cell infiltrates during the first week after transplantation, however they are gradually diminished via apoptotic death thereafter [17–19]. After injection of specific antigen into TCR transgenic mice, accumulation followed by apoptotic deletion of specific CD8+ T cells was observed in the liver [20], suggesting that there is a mechanism residing within the liver responsible for its immune modulation. We noted that although liver allografts in many species are spontaneously accepted, hepatocyte transplants are acutely rejected, suggesting a role for liver non-parenchymal cells (NPC) in regulation of the immune response. We have identified in mice that hepatic stellate cells (HSCs) are immunosuppressive [21]. HSCs mixed with islet allografts for transplantation under renal capsule achieves islet long-term survival in 60% recipients without requirement of immunosuppression [22], which is mediated by induction of CD8+ T cell apoptosis, generation of myeloid-derived suppressor cells [23,24] and Foxp3+ regulatory T cells [25]. These findings hold great potential for clinical application. However, all studies so far were conducted in animal models. In this study, we investigated human HSCs, and demonstrated vigorously suppressive effect on T cell response via induction of apoptosis in activated T cells which was mediated by B7-H1 (PDL-1) expressed on HSCs.
Results
Phenotypic Analysis of human HSCs
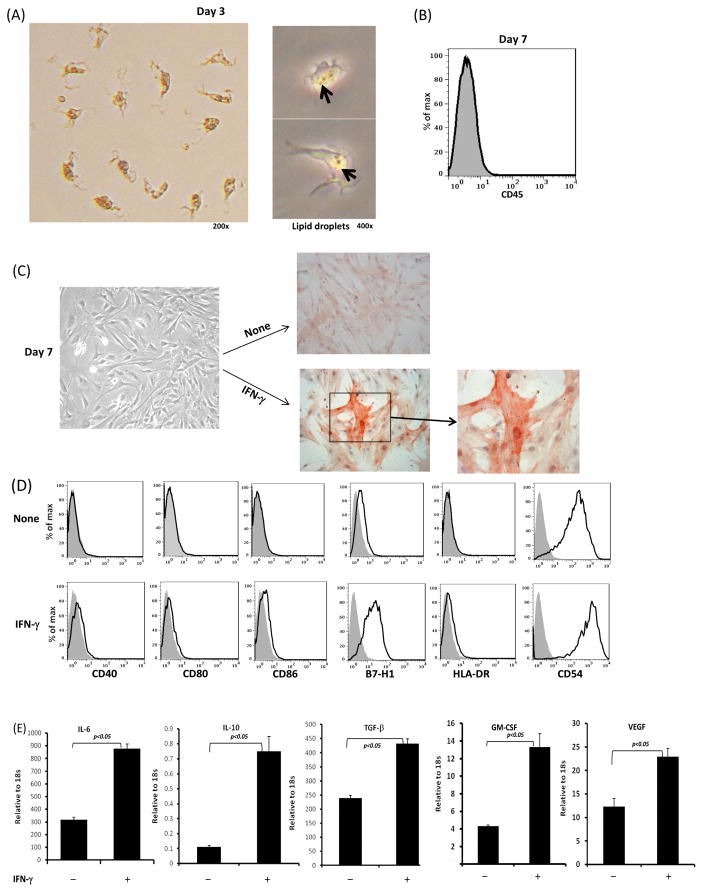
The HSCs used in this study was isolated from normal human liver tissue and enriched by gradient centrifugation. This is feasible because HSCs contain lipid droplets, becoming the least dense fraction and floating away from other cells [27]. The HSCs cultured for 3 days were spindle shaped, still containing multiple lipid droplets (Fig. 1A). Following culture for 7 days, the cultured cells showed no contamination with leukocytes (CD45+) (Fig. 1B), and HSCs were transformed to fibroblast-like morphology and became α-smooth muscle actin (SMA) positive (Fig. 1C). Cell viability was greater than 90% as determined by trypan blue exclusion. The purity of HSCs after 7 days culture was >95% as determined by α-SMA immunostaining. To test the response of HSCs to stimulation of IFN-γ, an important inflammatory cytokine mainly produced by effector T cells, HSCs were exposed to IFN-γ (100U/ml) for the last 18 hours of culture, showing marked upregulation of α-SMA (Fig. 1C). Without IFN-γ stimulation, human HSCs expressed very low co-stimulatory molecules CD40, CD80, CD86, B7-H1 and HLA-DR, while HSCs constitutively expressed adhesion molecule ICAM-1. Exposure to IFN-γ slightly up-regulated expression of CD80, CD40, CD86 and HLA-DR, but markedly enhanced expression of B7-H1 and CD54 (ICAM-1) (Fig. 1D). Cytokine expression analyzed by quantitative (q) polymerase chain reaction (PCR), showed that IFN-γ stimulation markedly increased expression of many inhibitory cytokines, including IL-6, IL-10, TGF-β, as well as GM-CSF and VEGF (Fig. 1E). Exposure to IFN-γ at 100U/ml for 18 hours reached maximum effect, since increase in incubation time up to 48 hours or concentration up to 1000U/ml did not further enhance expression of these cytokines (data not shown).
Figure 1. Phenotypic analysis of human HSCs.
Cells were isolated from the tissue of a donor liver the size of which needed to be reduced for transplantation, enriched for HSCs through percoll gradient centrifugation, and cultured in uncoated plastic plates as described in Materials and Methods. (A) Images of HSCs at day 3 of culture. The pictures in right panel were taken through the phase contrast microscope, showing the intra-cellular lipid droplets. (B) HSCs at day 7 of culture show without contamination of CD45+ hematopoietic cells as determined by flow analysis (histogram). Shaded profile represents isotype control. (C) INF-γ stimulation enhances HSCs activation. HSCs were exposure to INF-γ (100U/μl) for the last 18 hours of culture (7 days). Expression of α-SMA was determined by immunohistochemical staining (red). (D) Expression of surface key molecules on HSCs determined by flow analysis with or without INF-γ stimulation. (E) Expression of key cytokine mRNA in HSCs. RNA was isolated from the cells harvested after culture for 7 days with or without IFN-γ stimulation. Expression of cytokines was determined by q-PCR. The data are representative of three separate experiments.
HSCs stimulate low T cell response
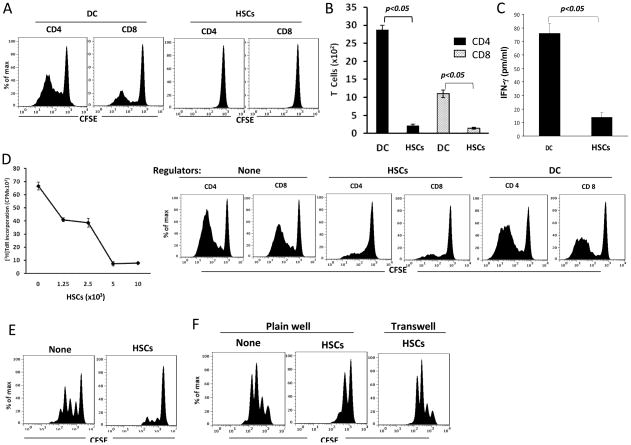
To test the immune stimulatory activity of human HSCs, CFSE labeled PBMC-derived T cells were cultured with irradiated allogeneic DCs or HSCs at a 10:1 ratio for 5 days. CFSE dilution data showed that compared to professional antigen presenting cells (APC) DCs, HSCs stimulated low proliferative response in both CD4+ and CD8+ T cells (Fig. 2A). This trend was also reflected by counting the absolute number of CD4+ and CD8+ cells at the end of culture (Fig. 2B). The production of IFN-γ in culture supernatant in HSC group were markedly lower than DC group (Fig. 2C), indicating that HSCs stimulate low Th1 response. These data suggest that, unlike the professional APC, HSCs have very low allostimulatory activity.
Figure 2. Human HSCs markedly inhibit T cell response.
(A) Human HSCs stimulate low T cell proliferative response. CFSE-labeled PBMC-derived T cells were cultured with irradiated stimulators (allogeneic DC or HSCs) at a stimulator: responder ratio of 1:10 for 5 days. Proliferative response was analyzed by CFSE dilution assay gated in CD4 and CD8 cell populations. (B) Less CD4 and CD8 T cells are generated following HSCs stimulation. Cells in each well were counted at the end of cultures. The numbers of CD4 and CD8 T cells were calculated based on flow analysis. (C) Less IFN-γ is produced by T cells stimulated by HSCs. Supernatant was collected at the end of culture, and the IFN-γ levels were measured by cytometric bead array (CBA, BD biosciences) assay. (D) HSCs inhibit T cell responses. Graded number of irradiated HSCs or DC (as regulators) were added at beginning into a one-way MLR culture in which T cell proliferative response was elicited by allogeneic DC at DC:T ratio of 1:10 for 5 days. T cell proliferative response was examined by thymidine up-take assay (left panel) or by CFSE dilution assay (right panels). (E) HSCs inhibited T cell response elicited by TCR ligation. Irradiated HSCs were added at the beginning into a culture (3 days) of CFSE-labeled T cells (at a ratio of 1:10), proliferation of which were elicited by anti-CD3/CD28 beads. T cell proliferative response was determined by CFSE dilution assay gated in CD3+ cells. (F) HSCs induced T cell suppression is mediated by cell-cell contact. To avoid cell-cell contact, transwell was used, in which irradiated HSCs were separated with CFSE-labeled T cells (stimulated by anti-CD3.CD29 beads). T cell proliferative response was determined by CFSE dilution assay gated in CD3+ cells. The data are representative of three separate experiments.
Human HSCs demonstrate potent immunosuppressive activity
The immune regulatory activity of HSCs was tested by addition of graded numbers of HSCs into a one-way MLR culture in which CFSE labeled PBMC-derived T cells and irradiated allogeneic DCs served as responders and stimulators, respectively. Thymidine uptake results showed that addition of HSCs significantly inhibited T cell proliferative response in a dose dependent manner – achieved ~85% inhibition when HSC:T ratio was increased to 1:10 (Fig. 2D). CFSE dilution analyses similarly demonstrated that HSCs inhibited the proliferative response in both CD4+ and CD8+ cell populations. This was not due to over crowding of the cells in the culture since addition the same number of DCs showed no inhibitory effect (Fig. 2D). To determine whether HSCs directly suppressed T cell function or via inhibition of APC, HSCs were added into the culture of T cells whose proliferative response was stimulated by anti-CD3/CD28-coated beads. HSCs maintained their ability to suppress T cell proliferative responses in the absence of DCs (Fig. 2E), suggesting that HSCs can directly inhibit T cell activation. To examine the involvement of cell-cell contact, the culture was performed in a transwell where HSCs were separated by a pored membrane to avoid direct contact with T cells. HSCs largely lost their ability to suppress T cell proliferative response in the transwell culture (Fig. 2F), indicating that the inhibition is dependent on cell-cell direct contact.
HSCs inhibit T cell response via induction of T cell apoptosis
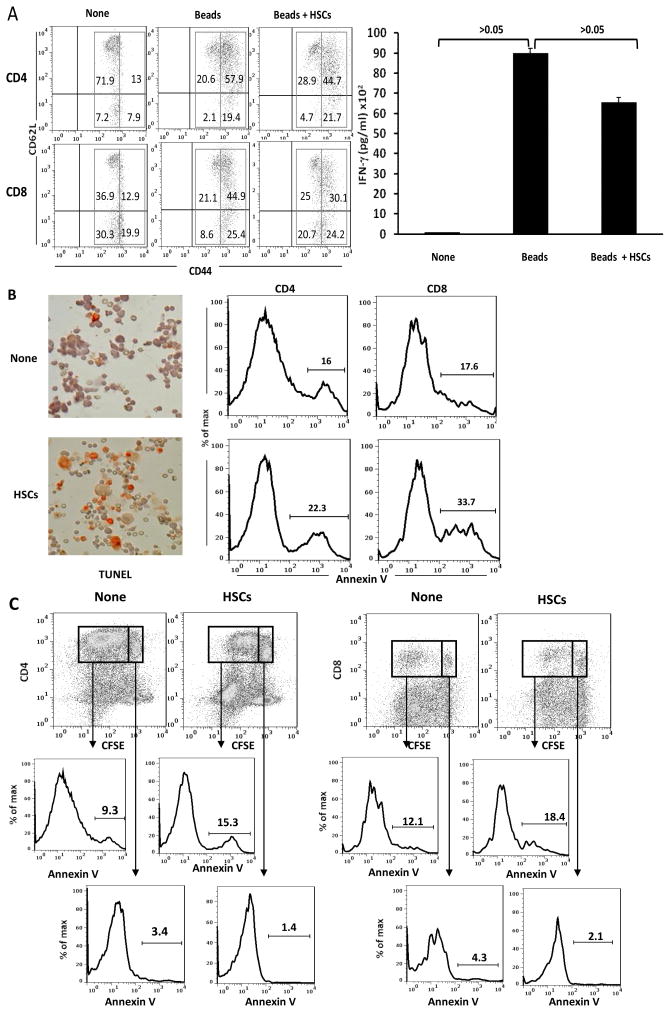
To understand the mechanisms involved in HSCs-induced T cell suppression, we first examined how the presence of HSCs impacted on expression of T cell activation markers (upregulation of CD44 and downregulation of CD62L) and production of IFN-γ. As shown in Fig. 3A, both CD4+ and CD8+ T cells isolated from healthy volunteers (group none) expressed intermediate levels of CD44; most of them were CD62L high and produced little IFN-γ (right panel) while following stimulation by anti-CD3/CD28 beads, expression of CD44 on CD4+ and CD8+ T cells was markedly increased; expression of CD62L was inhibited, and production of IFN-γ was dramatically enhanced, indicating vigorous T cell activation. Addition of HSCs did not affect much expression of T cell activation markers (CD44 and CD62L), but modestly reduced IFN-γ secretion (Fig. 3A, right panel), suggesting HSCs do not impede T cells activation. The modest reduction of IFN-γ production can be explained by the following observations - greater numbers of TUNEL positive cells were identified in T cells in HSCs group, compared to that in no HSCs controls (Fig. 3B, left panels). This was confirmed by flow analysis for annexin V expression. The presence of HSCs enhanced the incidence of annexin V positive population in both CD4+ and CD8+ T cells (Fig. 3B, right panels). Analysis of dividing and non-dividing cell populations (indicated by CFSE dilution assay) revealed that most of annexin V positive T cells were identified in dividing cells (Fig. 3C), suggesting that HSCs preferentially induce apoptosis in activated T cells.
Figure 3. HSCs inhibit T cell response via induction of B7-H1-mediated apoptotic death.
Irradiated human HSCs were added (at a ratio of 1:10) at the beginning into a one-way MLR culture in which the proliferation of PBMC-derived T cells was elicited by anti-CD3/CD28 beads. (A) HSCs do not suppress T cell activation. Expression of CD44 and CD62L were analyzed on naïve CD4 and CD8 T cells (None), stimulated by anti-CD3/CD28 beads or plus addition of HSCs by flow cytometry. The concentration of IFN-γ in supernatant was measured by CBA assay. Naïve T cells expressed intermediate levels of CD44. TCR stimulation markedly enhanced expression of CD44, but modestly inhibited expression of CD62L on both CD4 and CD8 T cells, and markedly increased IFN-γ production. Addition of HSCs did not affect these parameters of activation. The number is percentage of positive cells in each gated area. (B) HSCs induce T cell apoptosis. Apoptosis of the T cells were determined by staining of TUNEL (cytospin histochemistry), as well as by expression of annexin V on T cells (flow analysis gated in CD3+ cells). The number is percentage of annexin V+ T cells. (C) HSCs preferentially induce apoptosis in activated T cells. Expression of annexin V was analyzed in dividing and non-dividing CD4 and CD8 T cells. The number is percentage of annexin V+ cells.
The role for B7-H1 on HSCs in immunosuppressive activity
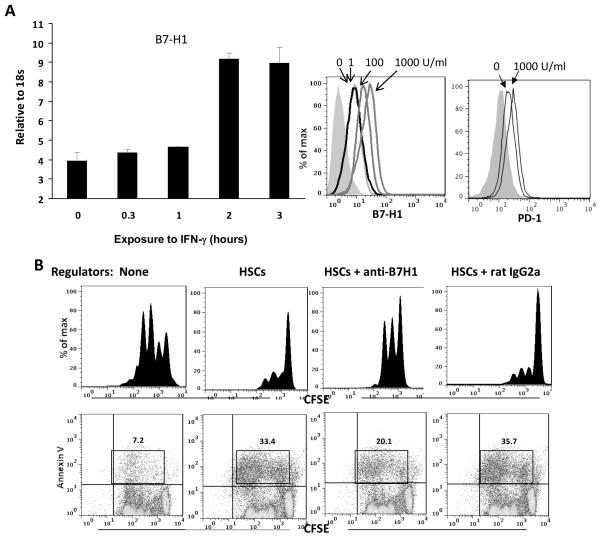
B7-H1, a member of the B7 family, negatively regulates immune response [28]. Its receptor, PD-1, can be induced on T cells and B cells upon activation [29]. Exposure of human HSCs to IFN-γ enhanced surface expression of B7-H1 in a time and dose dependent manner (Fig. 4A), suggesting that expression of B7-H1 on HSCs can be induced in inflammatory environments. However, expression of PD-1 on HSCs was not enhanced following exposure to IFN-γ, indicating that expression of B7-H1 and PD-1 on HSCs is not correlated. To determine the role of B7-H1 expressed on human HSCs in exerting immunosuppressive activity, graded concentrations of anti–human B7-H1 blocking mAb were added into the cultures where T cell proliferative response was inhibited by addition of HSCs. When the concentration of anti-B7-H1 mAb was increased up to 15 μg/ml, the inhibitory effect of HSCs on T cell proliferative response was markedly reversed (Fig. 4B), suggesting that the inhibition is mediated by B7-H1 ligation. This was associated with reduced annexin V expression on T cells, particularly, in dividing cell population (Fig. 3), indicating that HSCs inhibit T cell response via induction of activated T cell apoptosis which was dependent on B7-H1 ligation. These data suggest a critical role of B7-H1 on human HSCs in executing immune regulatory activity.
Figure 4. Regulation of B7-H1 expression on HSCs and its role in induction of T cell apoptosis.
(A) Expression of B7-H1 on HSCs is enhanced by IFN-γ stimulation. HSCs were incubated with IFN-γ (100 U/ml) for various (indicated) durations. B7-H1 expression was analyzed by q-PCR (left panel). HSCs were exposed to graded concentrations of IFN-γ for 18 hours and stained with anti–B7-H1 or anti-PD-1 mAb for flow analysis, and demonstrated as histograms. The filled area represents isotype control (right panel). (B) HSCs-induced T cell inhibition is mediated by B7-H1 ligation. Inhibition of (1×105) were added to HSCs (1×104) and cultured for 3 days. Irradiated HSCs were added at the beginning of a CFSE labeled PBMC-derived T cell culture (at a ratio of 1:10), the proliferation of which was elicited by anti-CD3/CD28 beads. T cell proliferative response was determined by CFSE dilution assay gated in CD3+ cells. Apoptotic activity was analyzed by staining for annexin V expression. The number is percentage of annexin V+ dividing cells. Data are representative of three separated experiments.
Discussion
While there has been extensive work done on the role of HSCs in vitamin A metabolism and fibrosis, there has been recent accumulating evidence suggesting that HSCs also actively participate in regulating immune response via immensely inhibiting T cell responses in the liver. However, so far, all studies were conducted in the rodent models [21, 22, 30–32]. In this study, we provided evidence that human HSCs did not stimulate allogeneic T cell proliferative response, indicating they are unlikely to be the professional APC. However, they demonstrated potent immune inhibitory activity. Thus, addition of HSCs into a one-way MLR culture effectively inhibited T cell proliferative response, achieving ~85% inhibition at an HSC:T ratio as low as 1:10 (Fig. 2D). Another important finding of this study is that the inhibition of T cell response by HSCs is not via directly suppressing T cell activation, as the presence of HSCs did not affect the expression of T cell activation marker molecules. Our data suggested that HSCs inhibited immune response by the induction of apoptosis of the activated T cells because HSCs preferentially enhanced apoptotic activity in the dividing T cells but not the non-dividing cells (Fig. 3C).
HSCs were reported to present antigens as the professional APC [33], and assumed to directly induce Foxp3+ Treg cells [34], because HSCs produce TGF-β and release retinoic acid, both of which are implicated in the induction of Treg cells by the mucosal DC [35]. However, recent studies, using highly sorted HSCs, found that HSCs can not present antigen to naïve T cells, or directly induce Foxp3+ Treg cell differentiation or expansion [36]. Our data generated in mouse studies are consistent with these observations. HSCs have limited ability to activate naïve T cells [21] and directly induce Treg cells [25]. HSCs actually are potent inducers of MDSC which subsequently enhance Treg activity in vivo [23, 37]. Human HSCs tested in the present study also show no APC activity, but can potently inhibit T cell response in an MLR culture. These controversial results may reflect the deviations in cell purity and experimental settings.
The liver has been shown to be a site for attracting and eliminating activated T cells following systemic T cell activation [38, 39]. The data generated in this study suggest that HSCs may be one of the important cell types in the liver that contribute to regulation of the T cell response via induction of apoptotic death of activated T cells. We have analyzed several death molecules in mouse HSCs. HSCs express very low FasL, TNF-α, TRAIL and its receptor DR5. Following IFN-γ stimulation, only TRAIL expression was slightly upregulated, while TNF-α and FasL remained low [40]. Considering that hepatocytes express high Fas, and are extremely sensitive to FasL mediated injury - injection of anti-Fas Ab results in massive hepatocyte damage [41], HSCs that locate close to hepatocytes are smart enough to utilize B7-H1, instead of Fas pathway, in induction of apoptosis in activated T cells in the liver. We also showed that after exposure to the inflammatory cytokine IFN-γ, HSCs upregulate expression of B7-H1, inhibitory cytokines and adhesion molecule ICAM-1. It is plausible to speculate that in inflammatory conditions such as hepatitis, liver transplantation, etc., HSCs attract activated lymphocytes through adhesion molecules to interact with them to down-regulate the immune response, in which B7-H1 plays a critical role since blocking B7-H1 ligation by specific mAb markedly reverses the HCS-mediated suppressive effect. B7-H1 is a member of the immunoglobulin super-family and is known to deliver negative co-stimulatory signals to T cells [42]. The data generated in this study suggest that B7-H1 on HSCs inhibits T cell response via induction of activated T cell apoptotic death. These results agree with what have been shown in murine models; mice deficient in B7-H1 results in the accumulation of CD8+ T cells in the liver [43]. In humans, the role of B7-H1 in transplantation is not as clearly defined, However, it has been shown that expression of B7-H1 is abundant in human carcinomas of lung, ovary and colon and in melanomas; cancer cell-associated B7-H1 increases apoptosis of antigen-specific human T cell clones in vitro. In addition, expression of B7-H1 on mouse P815 tumor increases apoptosis of activated tumor-reactive T cells and promotes the growth of tumors in vivo [44].
It is noted that the addition of anti–B7-H1 mAb only partially reverses the inhibition of T cell response and apoptotic activity, indicating that the molecules other than B7-H1 may also be involved in these inhibitory process. ICAM-1 is unlikely responsible, because blockade of ICAM-1 by specific mAb did not abrogate T cell apoptosis (data not shown). In addition, other cells in the liver may also participate in the B7-H1-mediated immune regulation. B7-H1 has been shown to be responsible for induction of CD8+ T cell hyporesponsiveness by liver sinusoidal endothelial cells (LSEC) since B7-H1 deficient LSEC largely lose their capacity of inhibiting CD8+ T cell response [45].
HSCs are well known to participate in the process of liver fibrosis and cirrhosis following chronic injury such as hepatitis B or hepatitis C that distorts the normal hepatic structure with fatal complications, including portal hypertension, GI bleeding and ascites. The data demonstrated in this study suggest that the participation of HSCs in liver fibrosis and cirrhosis may also involve some immunological mechanisms, which have not been extensively studied.
Materials and Methods
Isolation and culture of human HSCs
The research was conducted with the approval of the Institutional Review Board. Human liver tissue was obtained from the donor livers when the size needed to be reduced for transplantation, meshed, and agitated in collagenase IV (1 mg/ml) at 37°C for 30 minutes. Cells were filtered through a nylon mesh. HSCs were enriched via Percoll gradient centrifugation as previously described [26]. The isolated HSCs were cultured (105/ml) in an uncoated plastic flask (Nunclon, Roskilde, Denmark) with RPMI-1640 (Mediatech Inc.) supplemented with 10% fetal calf serum in 5% CO2 in air at 37°C for 7 days (unless specified otherwise). The ages of the liver donors ranged from 19 to 52. Only those samples that showed normal lobular hepatic architecture without histological evidence of fibrosis/cirrhosis were utilized. Cold ischemia time ranged from 5.5 – 8 hrs. HSCs were harvested immediately upon receipt of the samples. HLA typing was reviewed for all donors to ensure that the stimulators and responders were fully allogeneic.
Antibodies and flow cytometry
Mouse anti-human monoclonal antibodies (mAb) anti-CD3, CD4, CD8, CD54, CD80, CD40, CD42, CD62L, CD86 or HLA-DR were purchased from BD PharMingen (San Diego, CA), anti–B7-H1 from eBioscience (San Diego, CA). The appropriate isotype control antibodies were used in all experiments. For carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling, cells (107/ml) were incubated with 6.5 μmol/l CFSE (Molecular Probes, Eugene, OR) from a 10-mmol/l stock solution (in dimethylsulfoxide) for 10 minutes at 37°C. Flow analysis was performed on a FACSCalibur flow cytomer (BD Biosciences, Mountain View, CA), using FlowJo software.
Mixed lymphocyte reaction (MLR) assay
Peripheral blood mononuclear cells (PBMC) were collected from healthy human volunteers, and T cells were enriched using the Pan T Cell isolation Kit (Miltenyi Biotec, Auburn, CA). CFSE labeled human T cells (2 × 105/well in 100 μl) were incubated for 3 days with anti-CD3/CD28 coated beads (Invitrogen, Grand Island, NY) or culture with γ-irradiated (20–50 Gy; X-ray source) allogeneic stimulators, such as PBMC-derived dendritic cells (DCs) or HSCs for 5 days, at an indicated ratio in triplicate in 96-well round-bottom microculture plates (Corning Inc., Corning, NY) in RPMI-1640 complete medium in 5% CO2 in air. T cell proliferation was measured by thymidine uptaking or CFSE dilution analysis. IFN-γ concentration in the culture supernatant was measured by using Cytokine Bead Array (CBA) kit (BD Biosciences) according to the manufacturer’s instruction. To examine the inhibitory activity, γ-irradiated (50 Gy) human HSCs were added at the beginning into a one-way MLR culture in which T cell proliferative response was elicited by DCs at a HSC:DC ratio of 1:1.
TUNEL staining
Cell suspensions were fixed with 3.7% (W/V) formaldehyde in methanol for 10 minutes, dropped onto a slide, dried at room temperature, and stained for TUNEL using an in situ apoptosis detection kit (Intergen, Norcross, GA) according to the manufacturer’s instructions.
Culture of human DCs
PBMC (2×106 cells/well) obtained from the blood of healthy volunteers via elutriation method were cultured in RPMI-1640 medium containing 10% fetal calf serum in the presence of human recombinant granulocyte-macrophage colony-stimulating factor [granulocyte-macrophage colony-stimulating factor (GM-CSF), 1000U/mL, Schering-Plough, Kenilworth, NJ] and interleukin (IL)-4 (500U/mL) for 5 days. The floating cells were harvested and used in the experiments.
Statistical Analysis
The statistical significance of the parametric data was determined using the Student t test; p values of less than 0.05 were considered statistically significant.
Acknowledgments
This study was partially supported by NIH grants: DK084192 (LL) and AI090468 (SQ), and funds from the National Center for Research Resources, CTSA, Cleveland, Ohio (RC).
Abbreviations
- APC
antigen-presenting cells
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DCs
dendritic cells
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HSCs
hepatic stellate cells
- IL
interleukin
- IFN
interferon
- MLR
mixed lymphocyte reaction
- mAb
monoclonal antibody
- NPC
non parenchymal cell
- PBMC
peripheral blood mononuclear cells
- PCR
polymerase chain reaction
- SMA
smooth muscle actin
Footnotes
R.C. participated in research design, performed most experiments, analyzed the data, and wrote the manuscript; H-S.C. performed part of cellular immunology tests; L.W. performed molecular biology experiments; J.J.F. senior discussant and contributed to experimental design; L.L. and S.Q. Participated research design and data analyses, wrote and finalized the manuscript, sponsored the project.
References
- 1.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;233:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 2.Kamada N, Brons G, Davies HS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429–431. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos HC, Reyes J, Abu-Elmagd K, et al. Weaning of immunosuppression in long-term liver transplant recipients. Transplantation. 1995;59:212–217. doi: 10.1097/00007890-199501270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazariegos GV, Reyes J, Marino IR, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243–249. doi: 10.1097/00007890-199701270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devlin J, Doherty D, Thomson L, et al. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology. 1998;27:926–933. doi: 10.1002/hep.510270406. [DOI] [PubMed] [Google Scholar]
- 7.Orlando G, Soker S, Wood K. Operational tolerance after liver transplantation. J Hepatology. 2009;50:1247–1257. doi: 10.1016/j.jhep.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990;336:519–523. doi: 10.1016/0140-6736(90)92082-s. [DOI] [PubMed] [Google Scholar]
- 9.Iwatsuki S, Iwaki Y, Kano T, et al. Successful liver transplantation from crossmatch-positive donors. Transplant Proc. 1981;13:286–288. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Koep LJ, Halgrimson CG, et al. Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375–388. [PMC free article] [PubMed] [Google Scholar]
- 11.Ilan Y, Sauter B, Chowdhury NR, et al. Oral tolerization to adenoviral proteins permits repeated adenovirus-mediated gene therapy in rats with pre-existing immunity to adenoviruses. Gastroenterology. 1998;27:1368–1376. doi: 10.1002/hep.510270525. [DOI] [PubMed] [Google Scholar]
- 12.Callery MP, Kamei T, Flye MW. The effect of portacaval shunt on delayed-hypersensitivity responses following antigen feeding. J Surg Res. 1989;46:391–394. doi: 10.1016/0022-4804(89)90208-4. [DOI] [PubMed] [Google Scholar]
- 13.Yu S, Nakafusa Y, Flye MW. Portal vein administration of donor cells promotes peripheral allospecific hyporesponsiveness and graft tolerance. Surgery. 1994;116:229–235. [PubMed] [Google Scholar]
- 14.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 15.Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- 16.Dahmen U, Qian S, Rao AS, et al. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation. 1994;58:1–8. doi: 10.1097/00007890-199407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian S, Lu L, Fu F, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 18.Qian S, Thai NL, Lu L, Fung JJ, Thomason AW. Liver transplant tolerance: mechanistic insights from animal models, with particular reference to the mouse. Transplant Rev. 1997;11:151–164. [Google Scholar]
- 19.Meyer D, Baumgardt S, Loeffeler S, et al. Apoptosis of T lymphocytes in liver and/or small bowel allografts during tolerance induction. Transplantation. 1998;66:1530–1536. doi: 10.1097/00007890-199812150-00018. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Soldevila G, Leeker M, Flavell RA, Crispe IN. Liver is the site of T cell destruction during peripheral deletion. Immunity. 1994;1:741–749. doi: 10.1016/s1074-7613(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 21.Yu MC, Chen CH, Liang X, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–21. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, Kuo LM, Chang Y, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–81. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 23.Chou H-S, Hsieh C-C, Yang H-R, et al. Hepatic stellate cells regulate immune response via induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou H-S, Hsieh C-C, Charles R, et al. Myeloid-derived suppressor cells protect islet transplants via B7-H1 mediated enhancement of T regulatory cells. Transplantation. 2012;93:272–282. doi: 10.1097/TP.0b013e31823ffd39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang G, Yang HR, Wang L, et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86:1492–502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno R, Galastri S, Sacchi P, et al. Gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513–520. doi: 10.1136/gut.2008.163287. [DOI] [PubMed] [Google Scholar]
- 27.Weiskirchen R, Gressner AM. Isolation and culture of hepatic stellate cells. Methods Mol Med. 2005;117:99–113. doi: 10.1385/1-59259-940-0:099. [DOI] [PubMed] [Google Scholar]
- 28.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 29.Zha Y, Blank C, Gajewski TF. Negative regulation of T-cell function by PD-1. Crit Rev Immunol. 2004;24:229–37. doi: 10.1615/critrevimmunol.v24.i4.10. [DOI] [PubMed] [Google Scholar]
- 30.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schildberg FA, Wojtalla A, Siegmund SV, et al. Hepatic stellate cells veto CD8 T cell activation by a CD54-dependent mechanism. Hepatology. 2011:12. doi: 10.1002/hep.24352. [DOI] [PubMed] [Google Scholar]
- 32.Dangi A, Sumpter TL, Kimura S, et al. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J Immunol. 2012;188:3667–3677. doi: 10.4049/jimmunol.1102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winau F, Hegasy G, Weiskirchen R, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Thomson AW, Geller DA, Gandhi C, Murase N, Demetris AJ, Beer-Stolz D. Hepatic antigen-presenting cells and regulation of liver transplant outcome. Immunol Res. 2011;50:221–227. doi: 10.1007/s12026-011-8223-0. [DOI] [PubMed] [Google Scholar]
- 35.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103? DCs induces Foxp3? regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichikawa S, Mucida D, Tyznik AJ, Kronenberg M, Cheroutre H. Hepatic stellate cells function as regulatory bystanders. J Immunol. 2011;186:5549–5555. doi: 10.4049/jimmunol.1003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang HR, Chou HS, Gu X, et al. Mechanistic Insights into Immunomodulation by Hepatic Stellate Cells in Mice: A Critical Role of IFN-γ Signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J Immunol. 1999;63:3202–3210. [PubMed] [Google Scholar]
- 39.Crispe IN, Dao T, Klugewitz K, et al. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang HR, Chou HS, Wang L, et al. A critical role of TRAIL expressed on co-transplanted hepatic stellate cells in prevention of islet allograft rejection. Microsurgery. 2010;30:332–337. doi: 10.1002/micr.20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 42.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunological Reviews. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong H, Gefeng Z, Tamada K, Flies DB, Van Deursen JMA, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8 T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 44.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 45.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008;47:296–305. doi: 10.1002/hep.21965. [DOI] [PubMed] [Google Scholar]